Antibacterial Activity of Nanoparticles of Garlic (Allium sativum) Extract against Different Bacteria Such as Streptococcus mutans and Poryphormonas gingivalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Bacteria Strains
2.2. Culture Preparation
2.3. Preparation of Ultrasonicated Aqueous Garlic Extract
2.4. Antibacterial Activity by Agar Disc Diffusion
2.5. Antibacterial Activity by Agar Well Diffusion Method
2.6. Post Interaction Antibacterial Activity
2.7. Characterization of Garlic Nanoparticles
2.8. Statistical Analysis
3. Results and Discussion
3.1. Inhibition of Nanoparticles of Garlic Extract on the Different Bacteria
3.2. Antibacterial Activity of Nanoparticles of Garlic Extract on Tested Bacteria by Plate Count Method
3.3. Role of Sonication on Antibacterial Activity of Garlic Extract
3.4. Characterization of Nanoparticles of Garlic Extract by Microplate Spectrophotometer
3.5. Characterization of Nanoparticle of Garlic Extract by FTIR
3.6. Characterization of Nanoparticles of Garlic Extract by TEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, D.I.; Hughes, D. Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol. Rev. 2011, 35, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The Complex Relationship between Virulence and Antibiotic Resistance. Genes 2017, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassoum, O.; Sougou, N.M.; Diongue, M.; Lèye, M.M.M.; Mbodji, M.; Fall, D.; Seck, I.; Faye, A.; Tal-Dia, A. Assessment of General Public’s Knowledge and Opinions towards Antibiotic Use and Bacterial Resistance: A Cross-Sectional Study in an Urban Setting, Rufisque, Senegal. Pharmacy 2018, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.R.; Cho, I.H.; Jeong, B.C.; Lee, S.H. Strategies to minimize antibiotic resistance. Int. J. Environ. Res. Public Health 2013, 10, 4274–4305. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef]
- Vuorela, P.; Leinonen, M.; Saikku, P.; Tammela, P.; Wennberg, T.; Vuorela, H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, B.; Kishor, A.; Singh, S.; Bhat, M.N.; Surmal, O.; Musarella, C.M. Exploring Plant-Based Ethnomedicine and Quantitative Ethnopharmacology: Medicinal Plants Utilized by the Population of Jasrota Hill in Western Himalaya. Sustainability 2020, 12, 7526. [Google Scholar] [CrossRef]
- WHO. Traditional Medicine Strategy 2002–2005; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Karuppiah, P.; Rajaram, S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple drug resistant chemical pathogens. Asian Pac. J. Trop. Biomed. 2012, 2, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Botas, J.; Fernandes, Â.; Barros, L.; Alves, M.J.; Carvalho, A.M.; Ferreira, I.C.F.R. A Comparative Study of Black and White Allium sativum L.: Nutritional Composition and Bioactive Properties. Molecules 2019, 24, 2194. [Google Scholar] [CrossRef] [Green Version]
- Chekki, R.Z.; Snoussi, A.; Hamrouni, I.; Bouzouita, N. Chemical composition, antibacterial and antioxidant activities of Tunisian garlic (Allium sativum) essential oil and ethanol extract. Med. J. Chem. 2014, 3, 947–956. [Google Scholar]
- Gam, D.-H.; Park, J.-H.; Kim, J.-H.; Beak, D.-H.; Kim, J.-W. Effects of Allium sativum Stem Extract on Growth and Migration in Melanoma Cells through Inhibition of VEGF, MMP-2, and MMP-9 Genes Expression. Molecules 2022, 27, 21. [Google Scholar] [CrossRef]
- Patiño-Morales, C.C.; Jaime-Cruz, R.; Sánchez-Gómez, C.; Corona, J.C.; Hernández-Cruz, E.Y.; Kalinova-Jelezova, I.; Pedraza-Chaverri, J.; Maldonado, P.D.; Silva-Islas, C.A.; Salazar-García, M. Antitumor Effects of Natural Compounds Derived from Allium sativum on Neuroblastoma: An Overview. Antioxidants 2022, 11, 48. [Google Scholar] [CrossRef]
- Yetgin, A.; Canli, K.; Altuner, E.M. Comparison of antimicrobial activity of Allium sativum cloves from China and Taşköprü, Turkey. Adv. Pharmacol. Sci. 2018, 2018, 9302840. [Google Scholar] [PubMed] [Green Version]
- Chen, C.; Liu, C.H.; Cai, J.; Zhang, W.; Qi, W.L.; Wang, Z.; Liu, Z.-B.; Yang, Y. Broad-spectrum antimicrobial activity, chemical composition and mechanism of action of garlic (Allium sativum) extracts. Food Control 2018, 86, 117–125. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Fufa, B. Anti-bacterial and anti-fungal properties of garlic extract (Allium sativum): A review. Microbiol. Res. J. Int. 2019, 28, 1–5. [Google Scholar] [CrossRef]
- Kshirsagar, M.M.; Dodamani, A.S.; Karibasappa, G.N.; Vishwakarma, P.K.; Vathar, J.B.; Sonawane, K.R.; Khobragade, V.R. Antibacterial activity of garlic extract on cariogenic bacteria: An in vitro study. Ayu 2018, 39, 165–172. [Google Scholar] [CrossRef]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A.; et al. Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Brajer, M.; Voća, S.; Galić, A.; Radman, S.; Rimac-Brnčić, S.; Xia, Q.; Zhu, Z.; Grimi, N.; Barba, F.J.; et al. ultrasound as a promising tool for the green extraction of specialized metabolites from some culinary spices. Molecules 2021, 26, 1866. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Kimbaris, A.C.; Siatis, N.G.; Daferera, D.J.; Tarantilis, P.A.; Pappas, C.S.; Polissiou, M.G. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason Sonochem. 2006, 13, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Pavlic, B.; Vladic, J.; Ramic, M.; Brindza, J.; Vidovic, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar] [CrossRef]
- Mathialagan, R.; Mansor, N.; Shamsuddin, M.R.; Uemura, Y.; Majeed, Z. Optimisation of ultrasonic-assisted extraction (UAE) of allicin from garlic (Allium sativum L.). Chem. Eng. Trans. 2017, 56, 1747–1752. [Google Scholar]
- Ciric, A.R.; Krajnc, B.; Heath, D.; Ogrinc, N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Gupta, D.; Sen, D.; Bhattacharjee, C. Process intensification on the enhancement of allicin yield from Allium sativum through ultrasound attenuated nonionic micellar extraction. Chem. Eng. Process. Process Intensif. 2021, 169, 108610. [Google Scholar] [CrossRef]
- Salie, F.; Eagles, P.F.; Leng, H.M. Preliminary antimicrobial screening of four South African Asteraceae species. J. Ethnopharmacol. 1996, 52, 27–33. [Google Scholar] [CrossRef]
- Wikler, M.A. Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement; Part M2-A9. M100-S17; C.L.S.I. (Clinical and Laboratory Standard Institute): Pennsylvania, PA, USA, 2007. [Google Scholar]
- Gopal, J.; George, R.P.; Muraleedharan, P.; Khatak, H.S. Photocatalytic inhibition of microbial adhesion by anodized titanium. Biofouling 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Durairaj, S.; Srinivasan, S.; Lakshmana-perumalsamy, P. In vitro Antibacterial Activity and Stability of Garlic Extract at Different pH and Temperature. Electron. J. Biol. 2009, 5, 5–10. [Google Scholar]
- Khashan, A.A. Antibacterial activity of garlic extract (Allium sativum) against Staphylococcus aureus in vitro. GJBB 2014, 3, 346–348. [Google Scholar]
- Petropoulos, S.; Fernandes, Â.; Barros, L.; Ciric, A.; Sokovic, M.; Ferreira, I.C. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem. 2018, 245, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, R.M.; Saleh, A.H.; Ali, K.S. GC-MS analysis and antibacterial activity of garlic extract with antibiotic. J. Med. Plants Stud. 2020, 8, 26–30. [Google Scholar]
- Hoglund, K.B.; Barnett, B.K.; Watson, S.A.; Melgarejo, M.B.; Kang, Y. Activity of bioactive garlic compounds on the oral microbiome: A literature review. Gen. Dent. 2020, 68, 27–33. [Google Scholar]
- Rees, L.P.; Minney, S.F.; Plummer, N.T.; Slater, J.H.; Skyrme, D.A. Aquantitative assessment of the antimicrobial activity of garlic (Allium sativum). World J. Microbiol. Biotechnol. 1993, 9, 303–307. [Google Scholar] [CrossRef]
- Hughes, B.G.; Lawson, L.D. Antimicrobial effects of Allium sativum L. (garlic), Allium ampeloprasum L. (elephant garlic), and Allium cepa L. (onion), garlic compounds and commercial garlic supplement products. Phytother. Res. 1991, 5, 154–158. [Google Scholar] [CrossRef]
- Magryś, A.; Olender, A.; Tchórzewska, D. Antibacterial properties of Allium sativum L. against the most emerging multidrug-resistant bacteria and its synergy with antibiotics. Arch. Microbiol. 2021, 203, 2257–2268. [Google Scholar] [CrossRef]
- Indu, M.N.; Hatha, A.; Abirosh, C.; Harsha, U.; Vivekanandan, G. Antimicrobial activity of some of the south-Indian spices against serotypes of Escherichia coli, Salmonella, Listeria monocytogenes and Aeromonas hydrophila. Braz. J. Microbiol. 2006, 37, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Naureen, I.; Riaz, M.; Anjum, F.; Fatima, H.; Rafiq, M.A. Biofilm Inhibition and Antibacterial Potential of Different Varieties of Garlic (Allium sativum) Against Sinusitis Isolates. Dose-Response 2021, 19, 15593258211050491. [Google Scholar] [CrossRef]
- Gangoué-Piéboji, J.; Eze, N.; Djintchui, A.N.; Ngameni, B.; Tsabang, N.; Pegnyemb, D.E.; Biyiti, L.; Ngassam, P.; Koulla-Shiro, S.; Galleni, M. The in vitro antimicrobial activity of some traditionally used medicinal plants against beta-lactam-resistant bacteria. J. Infect. Dev. Ctries 2009, 3, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Fatemeh, A.M.; Soraya, H.; Mohammad, Y.A.; Jalal, P.; Javad, S. Antibacterial effect of Eucalyptus globulus Labill) and garlic (Allium sativum) extracts on oral Cariogenic bacteria. J. Microbiol. Res. Rev. 2013, 1, 12–17. [Google Scholar]
- Garba, I.; Umar, A.I.; Abdulrahman, A.B.; Tijjani, M.B.; Aliyu, M.S.; Zango, U.U.; Muhammad, A. Phytochemical and antibacterial properties of garlic extracts. Bayero J. Pure Appl. Sci. 2013, 6, 45–48. [Google Scholar] [CrossRef]
- Liaqat, A.; Zahoor, T.; Atif, M.; Muhammad, R. Characterization and antimicrobial potential of bioactive components of sonicated extract from garlic (Allium sativum) against foodborne pathogens. J. Food Process. Preserv. 2019, 43, e13936. [Google Scholar] [CrossRef]
- Alwazni, D.S.; Drwesh, M.F. Comparative study of effect garlic and anion extract on the growth of some gram negative bacteria. Mag. Al-Kufa Univ. Biol. 2010, 2, 238–244. [Google Scholar]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Ilić, D.; Nikolić, V.; Stanković, M.; Nikolić, L.; Stanojević, L.; Mladenović-Ranisavljević, I.; Smelcerović, A. Transformation of synthetic allicin: The influence of ultrasound, microwaves, different solvents and temperatures, and the products isolation. Sci. World J. 2012, 2012, 561823. [Google Scholar] [CrossRef] [Green Version]
- Wanyika, H.; Gachanja, A.; Kenji, G.; Keriko, J.; Mwangi, A. A rapid method based on uv spectrophotometry for quantitative determination of allicin in aqueous garlic extracts. J. Agric. Sci. Technol. 2010, 12, 74–82. [Google Scholar]
- Rastogi, L.; Arunachalam, J. Green synthesis route for the size controlled synthesis of biocompatible gold nanoparticles using aqueous extract of garlic (Allium sativum). Adv. Mater. Lett. 2013, 4, 548–555. [Google Scholar] [CrossRef]
- Rastogi, L.; Arunachalam, J. Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nano particles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mater. Chem. Phys. 2011, 129, 558–563. [Google Scholar] [CrossRef]
- Yulizar, Y.; Harits, A.A.; Abduracman, L. Green synthesis of gold nanoparticles using aqueous garlic (Allium sativum L.) Extract, and its interaction study with melamine. Bull. Chem. React. Eng. Catal. 2017, 12, 212. [Google Scholar] [CrossRef] [Green Version]
- Beato, V.M.; Orgaz, F.; Mansilla, F.; Montaño, A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods Hum. Nutr. 2011, 66, 218–223. [Google Scholar] [CrossRef]
- Stan, M.; Popa, A.; Toloman, D.; Dehelean, A.; Lung, I.; Katona, G. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater. Sci. Semicond. Process. 2015, 39, 23–29. [Google Scholar] [CrossRef]
- Whitesides, G.M. The “right” size in nanobiotechnology. Nat. Biotechnol. 2003, 21, 1161–1165. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Bouqellah, N.A.; Mohamed, M.M.; Ibrahim, Y. Synthesis of eco-friendly silver nanoparticles using Allium sp. and their antimicrobial potential on selected vaginal bacteria. Saudi J. Biol. Sci. 2019, 26, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
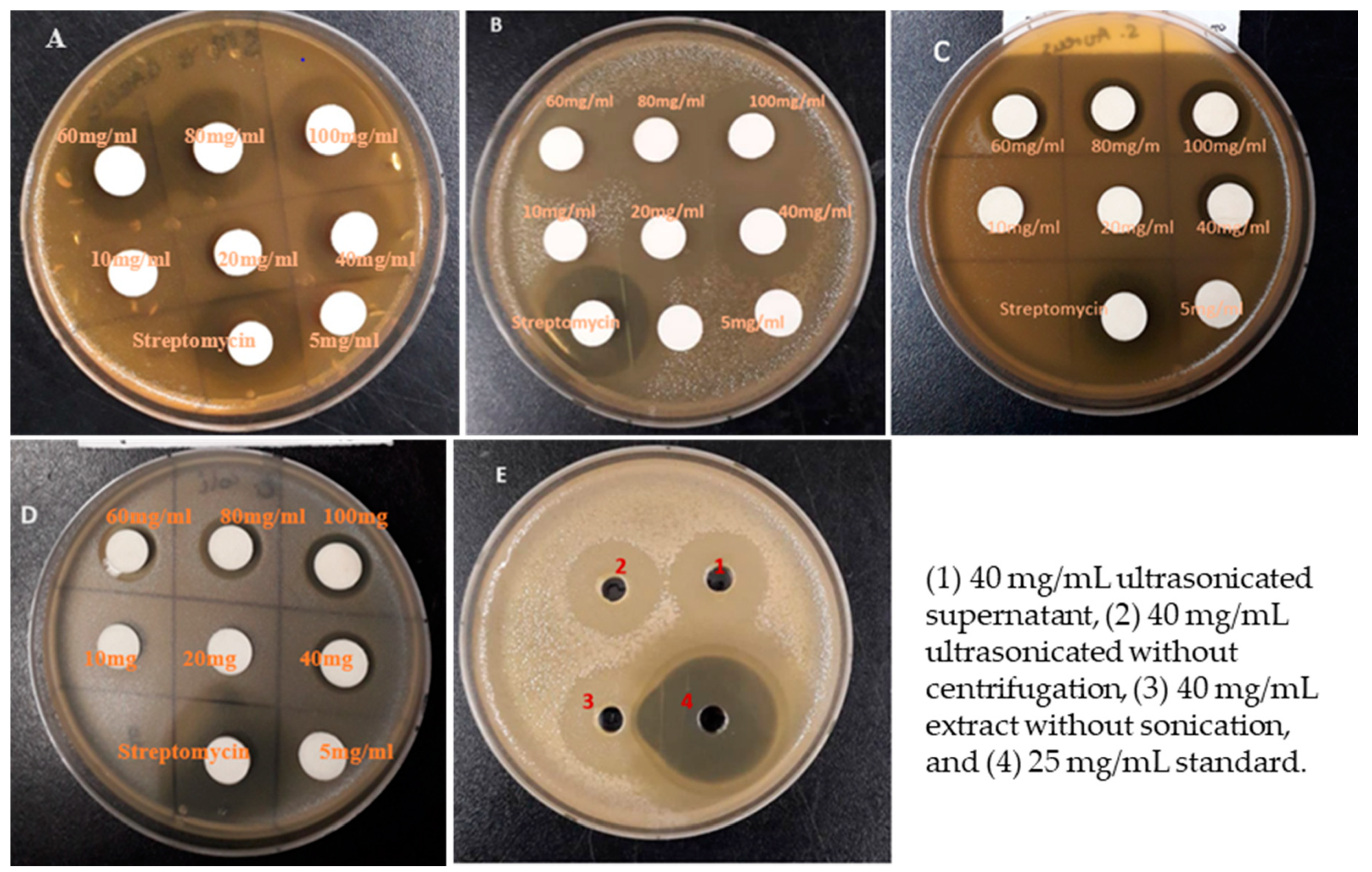





| Garlic Extract (mg/mL) | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| S. mutans | S. aureus sub. aureus | E. coli | P. gingivalis | |
| 5 | 0.0 ± 0.0 g | 0.0 ± 0.0 d | 0.0 ± 0.0 f | 0.0 ± 0.0 g |
| 10 | 0.0 ± 0.0 g | 0.0 ± 0.0 d | 0.0 ± 0.0 f | 0.0 ± 0.0 g |
| 20 | 12.1 ± 0.7 f | 0.0 ± 0.0 d | 0.0 ± 0.0 f | 11.2 ± f |
| 40 | 20.2 ± 0.6 e | 15.7 ± 0.6 c | 14.2 ± 0.8 e | 19.3 ± e |
| 60 | 22.2 ± 1.0 d | 16.0 ± 1.0 c | 15.3 ± 0.6 d | 21.5 ± d |
| 80 | 24.2 ± 0.7 c | 18.1 ± 0.9 b | 16.4 ± 0.5 c | 23.2 ± c |
| 100 | 26.2 ± 0.8 b | 19.1 ± 1.0 b | 17.4 ± 0.5 b | 25.3 ± b |
| Standard | 33.0 ± 0.0 a | 26.3 ± 0.0 a | 24.1 ± 0.0 a | 31.9 ± 0.0 a |
| R-Square | 0.9982 | 0.9922 | 0.9985 | 0.9989 |
| Coeff Var | 3.41 | 8.84 | 3.91 | 2.71 |
| Root MSE | 0.59 | 1.05 | 0.43 | 0.45 |
| Garlic Extract (mg/mL) | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| S. mutans | S. aureus sub. aureus | E. coli | P. gingivalis | |
| 5 | 0.0 ± 0.0 g | 0.0 ± 0.0 e | 0.0 ± 0.0 e | 0.0 ± 0.0 g |
| 10 | 0.0 ± 0.0 g | 0.0 ± 0.0 e | 0.0 ± 0.0 e | 0.0 ± 0.0 g |
| 20 | 15.6 ± 0.6 f | 0.0 ± 0.0 e | 0.0 ± 0.0 e | 14.6 ± 0.7 f |
| 40 | 22.2 ± 0.4 e | 17.3 ± 0.8 d | 16.2 ± 0.7 d | 21.1 ± 0.4 e |
| 60 | 24.5 ± 0.9 d | 18.9 ± 1.5 c | 17.4 ± 0.4 c | 23.6 ± 0.5 d |
| 80 | 26.8 ± 0.6 c | 20.0 ± 1.1 c | 18.1 ± 0.4 c | 25.2 ± 0.9 c |
| 100 | 28.7 ± 0.9 b | 21.4 ± 0.5 b | 19.3 ± 0.6 b | 27.1 ± 0.7 b |
| Standard | 34.1 ± 0.0 a | 27.2 ± 0.0 a | 25.5 ± 0.0 a | 35.4 ± 0.0 a |
| R-Square | 0.9985 | 0.9967 | 0.9990 | 0.9987 |
| Coeff Var | 2.94 | 5.60 | 3.05 | 2.82 |
| Root MSE | 0.56 | 0.73 | 0.37 | 0.52 |
| Garlic Extract (40 mg/mL) | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| S. mutans | S. aureus sub. aureus | E. coli | P. gingivalis | |
| Extract without sonication | 16.4 ± 0.5 d | 12.7 ± 0.3 d | 11.7 ± 0.4 d | 15.1 ± 0.3 d |
| Sonicated without centrifugation | 18.6 ± 0.5 c | 14.7 ± 0.4 c | 13.5 ± 0.3 c | 17.5 ± 0.6 c |
| Sonicated supernatant | 19.5 ± 0.5 b | 15.5 ± 0.4 b | 14.5 ± 0.5 b | 18.7 ± 0.9 b |
| Standard | 33.0 ± 0.0 a | 26.3 ± 0.0 a | 24.1 ± 0.0 a | 34.2 ± 0.0 a |
| R-Square | 0.9973 | 0.9976 | 0.9967 | 0.9960 |
| Coeff Var | 1.88 | 1.84 | 2.12 | 2.73 |
| Root MSE | 0.41 | 0.32 | 0.34 | 0.59 |
| Garlic Extract (40 mg/mL) | Inhibition Zone (mm) | |||
|---|---|---|---|---|
| S. mutans | S. aureus sub. aureus | E. coli | P. gingivalis | |
| Extract without sonication | 18.3 ± 0.3 d | 14.3 ± 0.5 d | 13.2 ± 0.3 d | 17.6 ± 0.5 d |
| Sonicated without centrifugation | 20.2 ± 0.5 c | 16.2 ± 0.3 c | 14.3 ± 0.4 c | 19.3 ± 0.6 c |
| Sonicated supernatant | 21.9 ± 0.3 b | 17.2 ± 0.4 b | 16.4 ± 0.6 b | 20.4 ± 0.7 b |
| Standard | 34.1 ± 0.0 a | 27.2 ± 0.0 a | 25.5 ± 0.0 a | 35.4 ± 0.0 a |
| R-Square | 0.9983 | 0.9971 | 0.9958 | 0.9961 |
| Coeff Var | 1.32 | 1.75 | 2.21 | 2.36 |
| Root MSE | 0.31 | 0.32 | 0.38 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabriel, T.; Vestine, A.; Kim, K.D.; Kwon, S.J.; Sivanesan, I.; Chun, S.C. Antibacterial Activity of Nanoparticles of Garlic (Allium sativum) Extract against Different Bacteria Such as Streptococcus mutans and Poryphormonas gingivalis. Appl. Sci. 2022, 12, 3491. https://doi.org/10.3390/app12073491
Gabriel T, Vestine A, Kim KD, Kwon SJ, Sivanesan I, Chun SC. Antibacterial Activity of Nanoparticles of Garlic (Allium sativum) Extract against Different Bacteria Such as Streptococcus mutans and Poryphormonas gingivalis. Applied Sciences. 2022; 12(7):3491. https://doi.org/10.3390/app12073491
Chicago/Turabian StyleGabriel, Tuyishime, Abimana Vestine, Ki Deok Kim, Seong Jung Kwon, Iyyakkannu Sivanesan, and Se Chul Chun. 2022. "Antibacterial Activity of Nanoparticles of Garlic (Allium sativum) Extract against Different Bacteria Such as Streptococcus mutans and Poryphormonas gingivalis" Applied Sciences 12, no. 7: 3491. https://doi.org/10.3390/app12073491
APA StyleGabriel, T., Vestine, A., Kim, K. D., Kwon, S. J., Sivanesan, I., & Chun, S. C. (2022). Antibacterial Activity of Nanoparticles of Garlic (Allium sativum) Extract against Different Bacteria Such as Streptococcus mutans and Poryphormonas gingivalis. Applied Sciences, 12(7), 3491. https://doi.org/10.3390/app12073491








