O2 Loaded Germanosilicate Optical Fibers: Experimental In Situ Investigation and Ab Initio Simulation Study of GLPC Evolution under Irradiation
Abstract
:1. Introduction
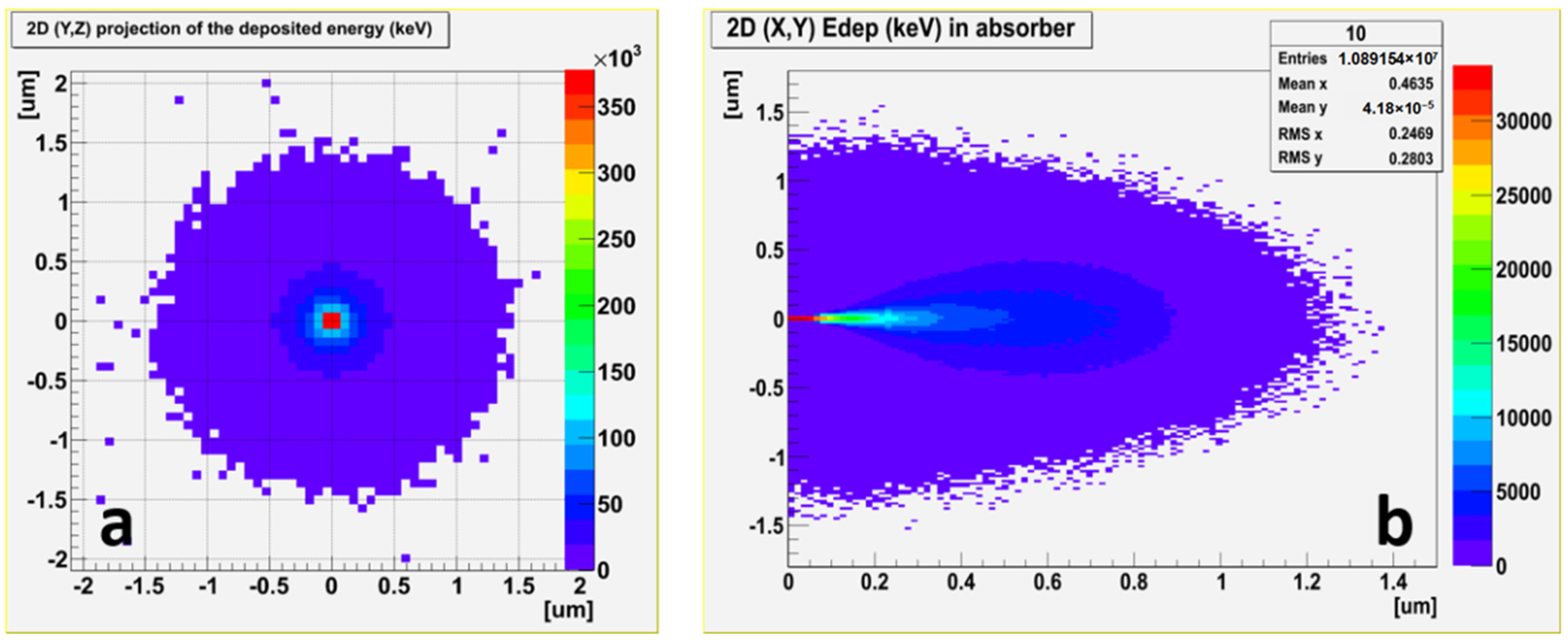
2. Materials and Methods
3. Results
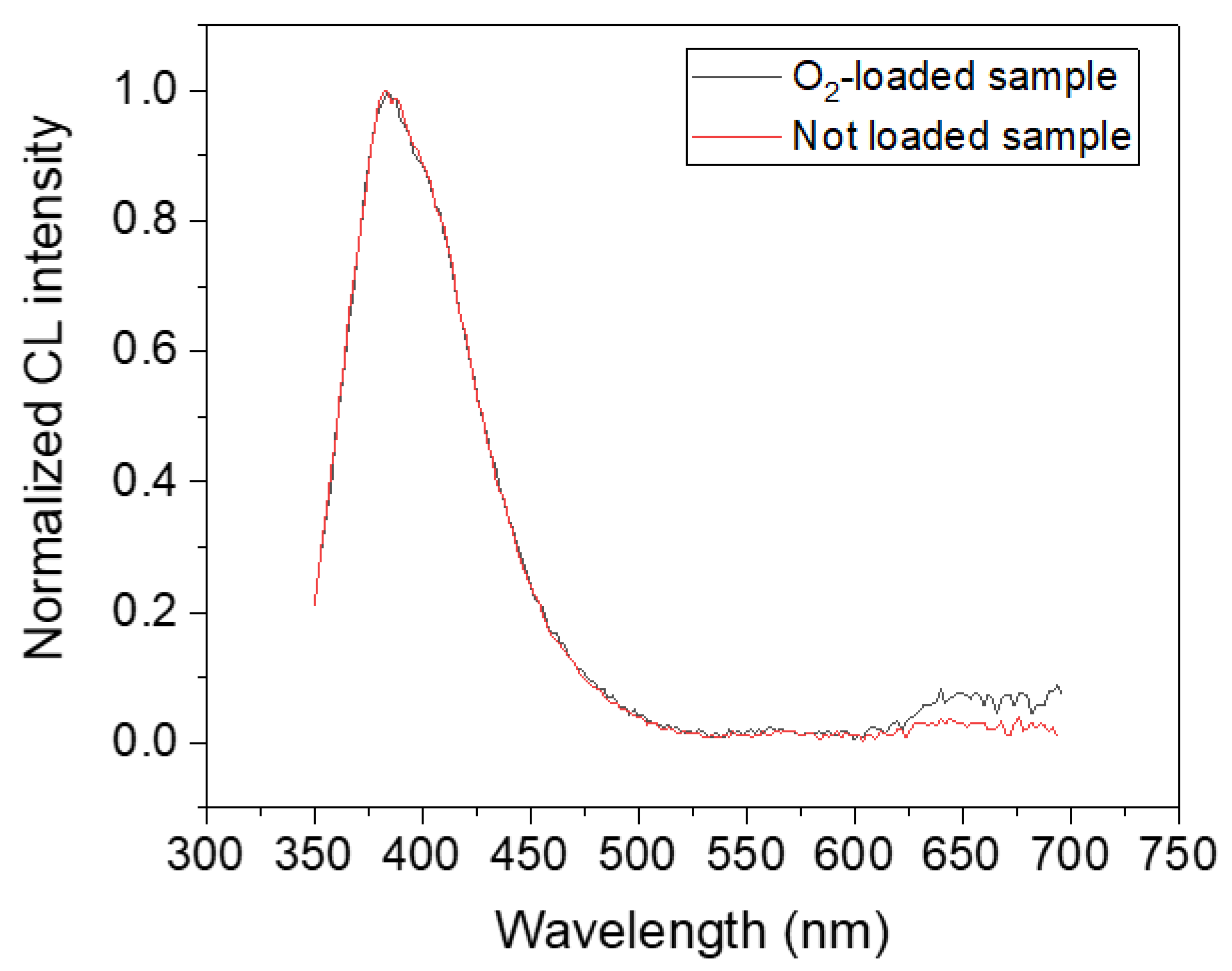
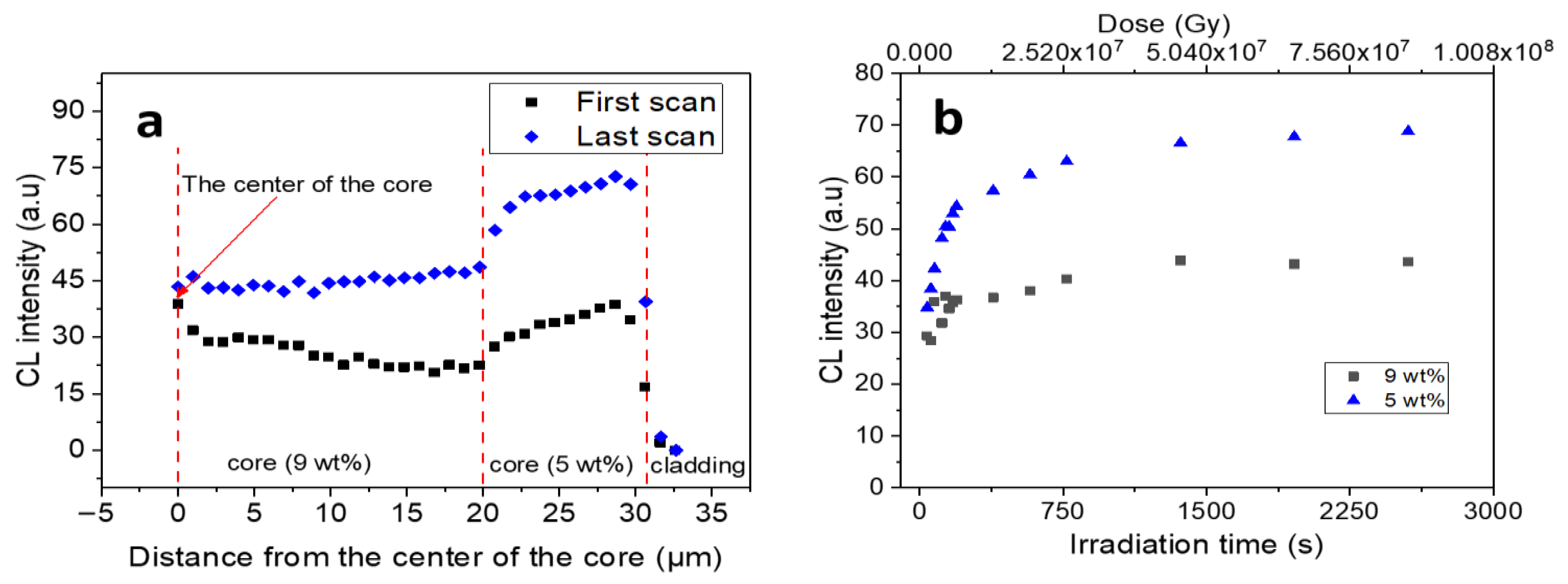
3.1. In Situ CL Investigation of GLPC Kinetics in the O2-Loaded Sample
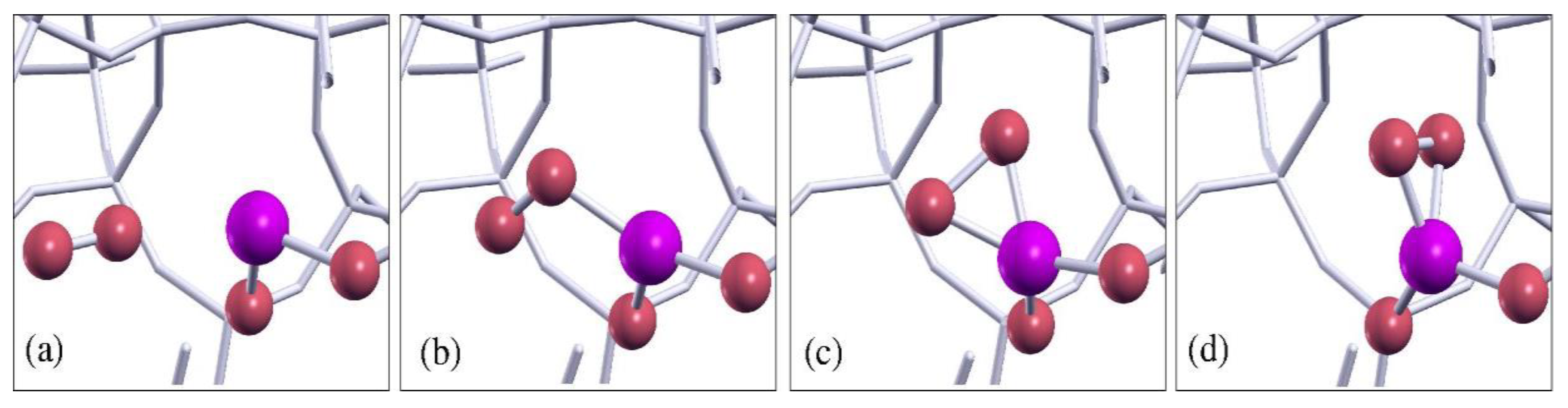
3.2. Ab Initio Calculations of Bulk Dioxagermirane Defects
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skuja, L.; Hirano, M.; Hosono, H.; Kajihara, K. Defects in oxide glasses. Phys. Stat. Sol. C 2005, 2, 15–24. [Google Scholar] [CrossRef]
- Girard, S.; Kuhnhenn, J.; Gusarov, A.; Brichard, B.; Van Uffelen, M.; Ouerdane, Y.; Boukenter, A.; Marcandella, C. Radiation Effects on Silica-Based Optical Fibers: Recent Advances and Future Challenges. IEEE Trans. Nucl. Sci. 2013, 60, 2015–2036. [Google Scholar] [CrossRef]
- Pacchioni, G.; Skuja, L.; Griscom, D.L. Defects in SiO2 and Related Dielectrics: Science and Technology; NATO Science Series II: Mathematics, Physics and Chemistry; Kluwer Academic: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Chiodini, N.; Meinardi, F.; Morazzoni, F.; Paleari, A.; Scotti, R. Optical transitions of paramagnetic Ge sites created by X-ray irradiation of oxygen-defect-free Ge-doped SiO2 by the sol-gel method. Phys. Rev. B 1999, 60, 2429–2435. [Google Scholar] [CrossRef]
- Kristensen, M. Ultraviolet-light-induced processes in germanium-doped silica. Phys. Rev. B 2001, 64, 144201. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Uchino, T.; Yoko, T. Correlation between Macro- and Microstructural Changes in Ge:SiO2 and SiO2 Glasses under Intense Ultraviolet Irradiation. J. Am. Ceram. Soc. 2002, 85, 1089–1092. [Google Scholar] [CrossRef]
- Buscarino, G.; Agnello, S.; Gelardi, F.M.; Boscaino, R. Polyamorphic transformation induced by electron irradiation in a-SiO2 glass. Phys. Rev. B 2009, 80, 94202. [Google Scholar] [CrossRef]
- Alessi, A.; Agnello, S.; Gelardi, F.M. Properties and Generation by Irradiation of Germanium Point Defects in Ge-Doped Silica. In Germanium: Properties, Production and Applications; Germanno, R.V., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 75–150. [Google Scholar]
- Griscom, D.L. Trapped-electron centers in pure and doped glassy silica: A review and synthesis. J. Non-Cryst. Solids 2011, 357, 1945–1962. [Google Scholar] [CrossRef]
- Di Francesca, D.; Agnello, S.; Girard, S.; Alessi, A.; Marcandella, C.; Paillet, P.; Boukenter, A.; Gelardi, F.M.; Ouerdane, Y. O2-Loading Treatment of Ge-Doped Silica Fibers: A Radiation Hardening Process. J. Lightw. Technol. 2016, 34, 2311–2316. [Google Scholar] [CrossRef]
- Griscom, D.L. On the natures of radiation-induced point defects in GeO2-SiO2 glasses: Reevaluation of a 26-year-old ESR and optical data set. Opt. Mat. Express 2011, 1, 400–412. [Google Scholar] [CrossRef]
- Griscom, D.L. Fractal kinetics of radiation-induced point-defect formation and decay in amorphous insulators: Application to color centers in silica-based optical fibers. Phys. Rev. B 2001, 64, 174201. [Google Scholar] [CrossRef]
- Kashaykin, P.F.; Tomashuk, A.L.; Khopin, V.F.; Firstov, S.V.; Guryanov, A.N.; Dianov, E.M. Observation of radiation-induced absorption of self-trapped holes in Ge-doped silica fiber in near infrared range at reduced temperature. J. Non-Cryst. Solids 2018, 496, 24–28. [Google Scholar] [CrossRef]
- Alessi, A.; Agnello, S.; Sporea, D.G.; Oproiu, C.; Brichard, B.; Gelardi, F.M. Formation of optically active oxygen deficient centers in Ge-doped SiO2 by γ-and β-ray irradiation. J. Non-Cryst. Solids 2010, 356, 275–280. [Google Scholar] [CrossRef]
- Giacomazzi, L.; Martin-Samos, L.; Boukenter, A.; Ouerdane, Y.; Girard, S.; Alessi, A.; de Gironcoli, S.; Richard, N. Photoactivated processes in optical fibers: Generation and conversion mechanisms of twofold coordinated Si and Ge atoms. Nanotechnology 2017, 28, 195202. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, M.; Watanabe, T.; Katoh, T.; Kasahara, T.; Miyazaki, N.; Ohki, Y. Structures and generation mechanisms of paramagnetic centers and absorption bands responsible for Ge-doped SiO2 optical-fiber gratings. Phys. Rev. B 1998, 57, 3920–3926. [Google Scholar] [CrossRef]
- Tomashuk, A.L.; Salgansky, M.Y.; Kashaykin, P.F.; Khopin, V.F.; Sultangulova, A.I.; Nishchev, K.N.; Borisovsky, S.E.; Guryanov, A.N.; Dianov, E.M. Enhanced Radiation Resistance of Silica Optical Fibers Fabricated in High O2 Excess Conditions. J. Lightw. Technol. 2014, 32, 213–219. [Google Scholar] [CrossRef]
- Zotov, K.V.; Likhachev, M.E.; Tomashuk, A.L.; Bubnov, M.M.; Yashkov, M.V.; Guryanov, A.N.; Klyamkin, S.N. Radiation-Resistant Erbium-Doped Fiber for Spacecraft Applications. IEEE Trans. Nucl. Sci. 2008, 55, 2213–2215. [Google Scholar] [CrossRef]
- Awazu, K.; Kawazoe, H.; Yamane, M. Simultaneous generation of optical absorption bands 5.14 and 0.452 eV in 9 SiO2:GeO2 glasses heated under H2 atmosphere. J. Appl. Phys. 1990, 68, 2713–2718. [Google Scholar] [CrossRef]
- Bradley, D.A.; Khandaker, M.U.; Alanazi, A. Irradiated glass and thermoluminescence yield: Dosimetric utility reviewed. Radiat. Phys. Chem. 2020, 170, 108680. [Google Scholar] [CrossRef]
- Campanella, C.; Gutilla, A.; Morana, A.; De Michele, V.; Muller, C.; Aubry, M.; Mady, F.; Marin, E.; Ouerdane, Y.; Boukenter, A.; et al. Photobleaching Effect on Infrared Radiation-Induced Attenuation of Germanosilicate Optical Fibers at MGy Dose Levels. IEEE Trans. Nucl. Sci. 2021, 68, 1688–1693. [Google Scholar] [CrossRef]
- Alessi, A.; Girard, S.; Reghioua, I.; Fanetti, M.; Di Francesca, D.; Agnello, S.; Cannas, M.; Marcandella, C.; Martin-Samos, L.; Richard, N.; et al. Gamma and X-ray irradiation effects on different Ge and Ge/F doped optical fibers. J. Appl. Phys. 2015, 118, 85901. [Google Scholar] [CrossRef]
- Skuja, L. Isoelectronic series of twofold coordinated Si, Ge, and Sn atoms in glassy SiO2: A luminescence study. J. Non-Cryst. Solids 1992, 149, 77–95. [Google Scholar] [CrossRef]
- Reghioua, I.; Girard, S.; Alessi, A.; DiFrancesca, D.; Martin-Samos, L.; Fanetti, M.; Richard, N.; Raine, M.; Valant, M.; Boukenter, A.; et al. Cathodoluminescence investigation of Ge-point defects in silica-based optical fibers. J. Lumin. 2016, 179, 1–7. [Google Scholar] [CrossRef]
- Simmons, H.W.; Nockolds, C.E.; Atkins, G.R.; Poole, S.B.; Sceats, M.G. Cathodoluminescence of defects in optical fibers and optical fiber preforms. Electron. Lett. 1992, 27, 1432–1433. [Google Scholar]
- Alessi, A.; Girard, S.; Cannas, M.; Agnello, S.; Boukenter, A.; Ouerdane, Y. Evolution of Photo-induced defects in Ge-doped fiber/preform: Influence of the drawing. Opt. Express 2011, 19, 11680–11690. [Google Scholar] [CrossRef] [Green Version]
- Raghavachari, K.; Pacchioni, G. Photoabsorption of dioxasilyrane and silanone groups at the surface of silica. J. Chem. Phys. 2001, 114, 4657–4662. [Google Scholar] [CrossRef]
- Radzig, V.A. Reactive silica—A new concept of the structure of active sites. Colloids Surf. A Physicochem. 1993, 74, 91–100. [Google Scholar] [CrossRef]
- Annenkov, V.V.; Danilovtseva, E.N.; Pal’shin, V.A.; Zelinskiy, S.N.; Chebykin, E.P.; Gak, V.Y.; Shendrik, R.Y. Luminescent siliceous materials based on sodium silicate, organic polymers and silicon analogs. Mater. Chem. Phys. 2017, 185, 65–72. [Google Scholar] [CrossRef]
- Yoo, J.; Han, S.; Park, W.; Lee, T.; Park, Y.; Chang, H.; Kwang-Hahn, S.; Kwon, W. Defect-Induced Fluorescence of Silica Nanoparticles for Bioimaging Applications. ACS Appl. Mater. Interfaces 2018, 10, 44247–44256. [Google Scholar] [CrossRef]
- Zyubin, A.S.; Mebel, A.M.; Lin, S.H. Photoluminescence of oxygen-containing surface defects in germanium oxides: A theoretical study. J. Chem. Phys. 2005, 123, 44701. [Google Scholar] [CrossRef]
- Li, L.; Fukawa, T.; Matsuo, T.; Hashizume, D.; Fueno, H.; Tanaka, K.; Tamao, K. A stable germanone as the first isolated heavy ketone with a terminal oxygen atom. Nat. Chem. 2012, 4, 361–365. [Google Scholar] [CrossRef]
- Kajihara, K.; Miura, T.; Kamioka, H.; Hirano, M.; Skuja, L.; Hosono, H. Surface Dissolution and Diffusion of Oxygen Molecules in SiO2 Glass. J. Ceram. Soc. Jpn. 2004, 112, 559–562. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, T.; Hata, M.; Neya, S.; Nishioka, Y.; Watanabe, T.; Tatsumura, K.; Ohdomari, I. Diffusion of Molecular and Atomic Oxygen in Silicon Oxide. Jpn. J. Appl. Phys. 2003, 42, 3560–3565. [Google Scholar] [CrossRef]
- Ixblue–Advanced Solutions for Navigation, Photonics and Maritime Autonomy. Available online: https://www.ixblue.com/ (accessed on 10 April 2022).
- Di Francesca, D.; Agnello, S.; Girard, S.; Marcandella, C.; Paillet, P.; Boukenter, A.; Ouerdane, Y.; Gelardi, F.M. Influence of O2-Loading Pretreatment on the Radiation Response of Pure and Fluorine-Doped Silica-Based Optical Fibers. IEEE Trans. Nucl. Sci. 2014, 61, 3302–3308. [Google Scholar] [CrossRef]
- Di Francesca, D.; Girard, S.; Agnello, S.; Marcandella, C.; Paillet, P.; Boukenter, A.; Gelardi, F.M.; Ouerdane, Y. Near infrared radio-luminescence of O2 loaded Rad-Hard silica optical fibers: A candidate dosimeter for harsh environments. Appl. Phys. Lett. 2014, 105, 183508. [Google Scholar] [CrossRef]
- Raine, M.; Gaillardin, M.; Paillet, P. Geant4 physics processes for silicon microdosimetry simulation: Improvements and extension of the energy-range validity up to 10 GeV/nucleon. Nucl. Instrum. Methods Phys. Res. B 2014, 325, 97–100. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Reghioua, I.; Girard, S.; Raine, M.; Alessi, A.; DiFrancesca, D.; Fanetti, M.; Martin-Samos, L.; Richard, N.; Valant, M.; Boukenter, A.; et al. Cathodoluminescence Characterization of Point Defects in Optical Fibers. IEEE Trans. Nucl. Sci. 2017, 64, 2318–2324. [Google Scholar] [CrossRef]
- Reghioua, I.; Girard, S.; Alessi, A.; DiFrancesca, D.; Marin, E.; Morana, A.; Fanetti, M.; Martin-Samos, L.; Richard, N.; Raine, M.; et al. Study of point defects in as-drown and irradiated Ge-doped optical fibers using cathodoluminescence. IOP Conf. Ser. Mater. Sci. Eng. 2017, 169, 12006. [Google Scholar] [CrossRef] [Green Version]
- Giacomazzi, L.; Umari, P.; Pasquarello, A. Vibrational spectra of vitreous germania from first-principles. Phys. Rev. B 2006, 74, 155208. [Google Scholar] [CrossRef]
- Hamann, D.R. Diffusion of atomic oxygen in SiO2. Phys. Rev. Lett. 1998, 81, 3447. [Google Scholar] [CrossRef]
- Bongiorno, A.; Pasquarello, A. Multiscale modeling of oxygen diffusion through the oxide during silicon oxidation. Phys. Rev. B 2004, 70, 195312. [Google Scholar] [CrossRef]
- Winkler, B.; Martin-Samos, L.; Richard, N.; Giacomazzi, L.; Alessi, A.; Girard, S.; Boukenter, A.; Ouerdane, Y.; Valant, M. Correlations between Structural and Optical Properties of Peroxy Bridges from First Principles. J. Phys. Chem. C 2017, 121, 4002–4010. [Google Scholar] [CrossRef]
- Krasheninnikov, A.V.; Nordlund, K. Ion and Electron irradiation-induced effects in nanostructured materials. J. Appl. Phys. 2010, 107, 71301. [Google Scholar] [CrossRef]
- Takeguchi, M.; Furuya, K.; Yoshihara, K. Electron energy loss spectroscopy study of the formation process of Si nanocrystals in SiO2 due to electron stimulated desorption-decomposition. Micron 1999, 30, 147–150. [Google Scholar] [CrossRef]
- Yakabe, T.; Fujita, D.; Yoshihara, K. Electron irradiation effect on depth profiling of a SiO2/Si (1 0 0) surface by Auger electron spectroscopy. Appl. Surf. Sci. 2005, 241, 127–130. [Google Scholar] [CrossRef]
- Kajihara, K.; Skuja, L.; Hosono, H. Diffusion and Reactions of Photoinduced Interstitial Oxygen Atoms in Amorphous SiO2 Impregnated with 18O-Labeled Interstitial Oxygen Molecules. J. Phys. Chem. C 2014, 118, 4282–4286. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reghioua, I.; Giacomazzi, L.; Alessi, A.; Winkler, B.; Martin-Samos, L.; Girard, S.; Di Francesca, D.; Fanetti, M.; Richard, N.; Paillet, P.; et al. O2 Loaded Germanosilicate Optical Fibers: Experimental In Situ Investigation and Ab Initio Simulation Study of GLPC Evolution under Irradiation. Appl. Sci. 2022, 12, 3916. https://doi.org/10.3390/app12083916
Reghioua I, Giacomazzi L, Alessi A, Winkler B, Martin-Samos L, Girard S, Di Francesca D, Fanetti M, Richard N, Paillet P, et al. O2 Loaded Germanosilicate Optical Fibers: Experimental In Situ Investigation and Ab Initio Simulation Study of GLPC Evolution under Irradiation. Applied Sciences. 2022; 12(8):3916. https://doi.org/10.3390/app12083916
Chicago/Turabian StyleReghioua, Imene, Luigi Giacomazzi, Antonino Alessi, Blaz Winkler, Layla Martin-Samos, Sylvain Girard, Diego Di Francesca, Mattia Fanetti, Nicolas Richard, Philippe Paillet, and et al. 2022. "O2 Loaded Germanosilicate Optical Fibers: Experimental In Situ Investigation and Ab Initio Simulation Study of GLPC Evolution under Irradiation" Applied Sciences 12, no. 8: 3916. https://doi.org/10.3390/app12083916






