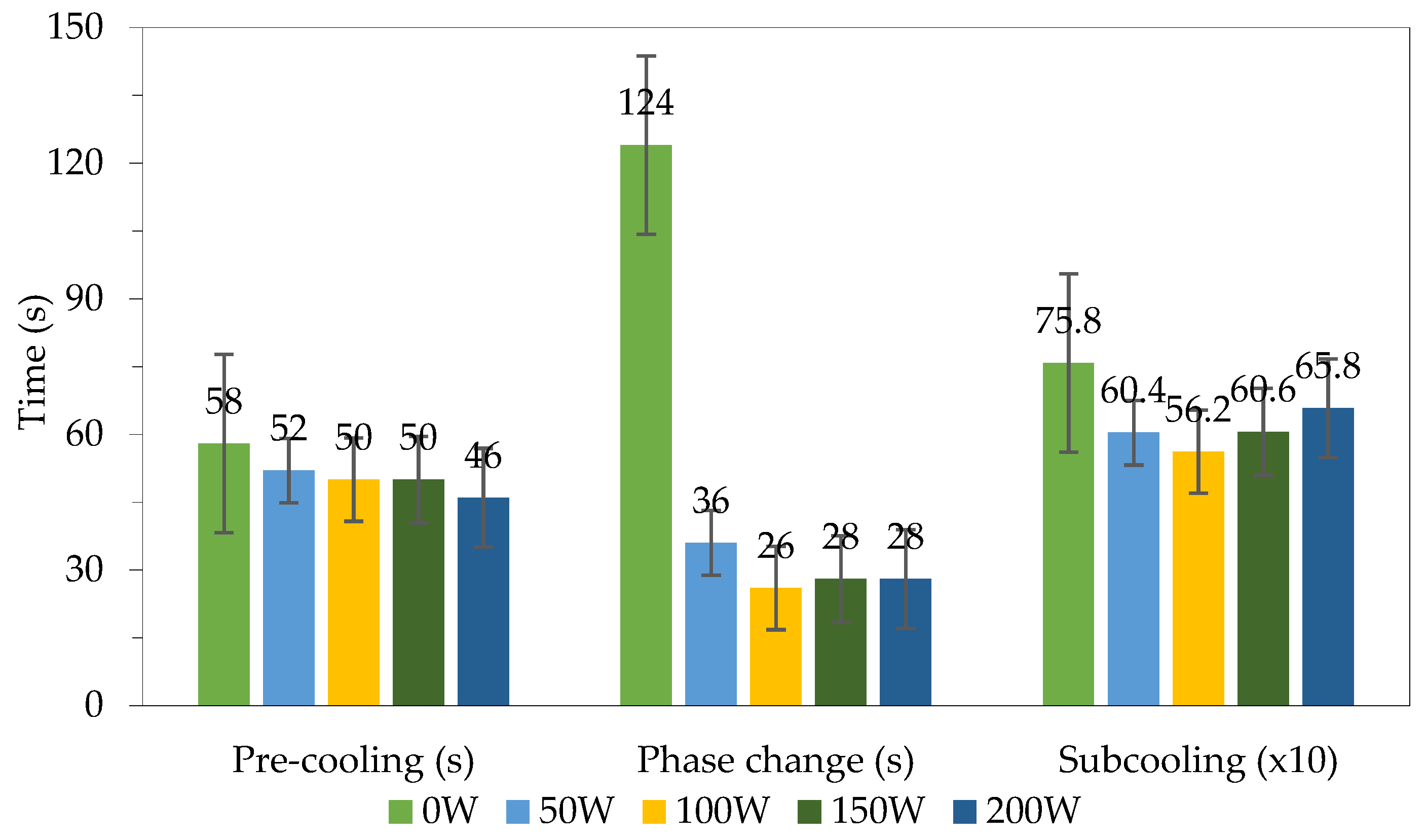Abstract
The main purpose of this study was to select the appropriate ultrasound parameters that support the freezing process of Bo Chinh ginseng. This process involves placing Bo Chinh ginseng in an open-air environment and ensuring that the transducer does not come into contact with the material. The research results show that the ultrasound power, ultrasound irradiation temperature and intermittency ratio all affect the freezing time, nucleation temperature, color and microstructure of the materials. When the ultrasound continuously operated during the freezing process, at a frequency of 20 kHz, there was a 29.1% reduction in the freezing time at a power level of 100 W as compared to freezing without the help of ultrasound irradiation. The irradiation temperature and nucleation temperature have a linear relationship () which can help to control the nucleation temperature, thereby changing the ice crystal size. Ultrasound-assisted freezing at a 0.6 intermittency ratio had the shortest freezing time. The microstructure of the material changed after ultrasound-assisted freezing and many microchannels and holes were generated. When frozen at a wave rate of 0.4, the microchannels that were created in the material effectively supported the process of water drainage in the drying sublimation stage. At the same time, ultrasound irradiation did not affect the color of the post-freezing material when compared to the color of the frozen material that did not undergo ultrasound irradiation.
1. Introduction
Bo Chinh ginseng (Abelmoschus sagittifolius (Kurz) Merr) is one of the precious ginseng species grown in Vietnam. Bo Chinh ginseng has important medicinal ingredients, including triterpene saponins, which are considered to be a group of compounds that determine the typical pharmacological characteristics of the medicinal plants in the ginseng family. In addition, Bo Chinh ginseng also contains other important components, such as mucilage, starch, phytosterols, coumarin, fatty acids, organic acids, reducing sugar and ionic compounds, lipids, proteins and protids. Hence, the process of preservation and processing is very important in order to keep the medicinal ingredients in ginseng intact. Currently, ginseng is dried after it is harvested and before it is stored. The medicinal ingredients in ginseng are easily lost when they are dried at high temperatures. Therefore, freeze-drying is a promising method for preserving high levels of the valuable properties of ginseng. In particular, the first stage of freeze-drying involves freezing the material in order to completely crystallize the water in the material, which is also an important stage in determining the quality of the material after drying [1]. This study aimed to investigate the effects of ultrasound parameters on the freezing process of Bo Chinh ginseng. In addition, in order to reduce the drying times in the freeze-drying process and enhance the quality of the dried material, having more control over the freezing stage is important. This is because freezing phenomena, such as nucleation and ice crystal growth, are related to ice crystal morphology parameters, and these parameters directly affect drying rates.
In recent years, ultrasound irradiation has been widely studied and applied in the field of food preservation and processing, including research into ultrasound irradiation methods in order to assist in the freezing process of materials. Ultrasound irradiation effectively assists in the phase transition to nucleate and promote ice crystal growth, and to reduce the size of ice crystals. This is very important in ensuring the quality of the material after freezing; smaller ice crystals will help to maintain the protein structure, and the biochemical properties of the material will be preserved [2]. Xuan Ma et al. [3] indicated that ultrasound irradiation accelerates heat and water transfer because ultrasound irradiation creates microchannels by disrupting the bubbles generated during nucleation. In addition, some studies show that ultrasound irradiation increases the nucleation temperature of materials compared to samples of materials frozen without ultrasound support, thereby accelerating the crystallization process, reducing the crystal size and shortening the freezing time. Some other studies have indicated that the nucleation step can be divided into primary nucleation and secondary nucleation stages when assisted by ultrasound [4,5,6,7,8,9,10,11]. In particular, at the primary nucleation stage, ultrasound irradiation breaks the bubbles to create cavitation bubbles, which increases the nucleation temperature. In the secondary nucleation stage, ultrasound irradiation helps to promote heat and mass transfer. At the stage of crystal growth, the crystal size is reduced and uniform crystals are formed because ultrasound irradiation creates microchannels that increase the rate of heat transfer, which shortens the crystallization time.
However, not all intensities and durations of ultrasound irradiation produce positive effects in the freezing process. Ultrasound irradiation helps to increase the nucleation temperature during freezing compared to freezing without ultrasound support. However, if the ultrasound intensity is too large and the transmitting time is long, it will have an adverse effect on the freezing process. This is due to the ice crystals crystallizing again after the thermal effect melts them, which increases the freezing time [12]. Different materials have different effects on the intensity and duration of ultrasound transmission. Therefore, a separate study is required for each type of material.
In most of the studies on ultrasound-assisted freezing, the material is immersed in a refrigerant. Kiani et al. [13] experimented with freezing Agar gel dipped in an ethylene glycol solution. They found that ultrasound irradiation supports the freezing process at a frequency of 25 kHz with an intensity of 0.07, 0.14, 0.25, 0.35 and 0.42 W/cm2 when the temperature starts the transmission process at −2, −3, −4 and −5 °C, respectively. These research results showed that the nucleation temperature of the agar gel formed in response to the super-cooling temperature was different and corresponded to the intensity and time of transmission. Xu et al. [12] studied the freezing of radish cylinders dipped in a CaCl2 solution with the help of ultrasound irradiation at 25 kHz with wave intensities of 0.09, 0.17, 0.26 and 0.37 W/cm2. Transmittance started at temperatures of −0.5, −1, −1.5 and −2 °C, and there was a transmitting delay of 0, 3, 7, 10 and 15 s. A temperature of −0.5 °C and a 7 s delay corresponding to a transmitting intensity of 0.26 W/cm2 was the optimal condition for the nucleation of water in the material. Shi et al. [14] studied the freezing of grass carp by applying a 40 kHz ultrasound with wave intensities of 0.3, 0.38, 0.48 and 0.6 W/cm2 and an intermittent transmitting rate of 8 s on/2 s off. Their experiments showed that ultrasound irradiation at an intensity of 0.38 W/cm2 helped to reduce the freezing time, as well as shorten the duration of the cooling phase and the transition phase. Xin et al. [15,16] studied the effect of ultrasound irradiation on the freezing process of broccoli at 20 kHz and 30 kHz with a power of 0, 125, 150, 175 and 195 W and an intermittency ratio of 60 s on/60 s off. Their research results showed that ultrasound irradiation significantly shortened the cooling time and supercooling time, especially at 150 W (30 kHz) and 175 W (30 kHz). Sun et al. [17] studied the freezing of common carp with the help of ultrasound irradiation at 30 kHz with a power of 175 W and a transmitting rate 30 s on 30 s off; ultrasound irradiation helped to shorten the freezing time and reduce water crystallization in the material. Zhang et al. [9] performed the ultrasound-assisted freezing of pork (porcine longissimus muscle) at a frequency of 30 kHz, with capacities of 120, 180, 240 and 300 W and the proportional intermittent ratio of 30 s on/30 s off. Experimental results showed that ultrasound irradiation helped to reduce freezing time, and at the 180 W power level, provided the most effective support with the shortest freezing time. Islam et al. [6] conducted the direct freezing of mushrooms without dipping them in the refrigerant, and thus, the material was not contaminated. The form of ultrasound irradiation that supports the freezing process has a frequency of 20 kHz and a transmitting intensity of 0, 0.13, 0.27 and 0.39 W/cm2. The results showed that at an intensity of 0.39 W/cm2, the fungus kept its original hardness. However, the transmitting efficiency was reduced due to transmission in a cold-air environment. There are many studies on the ultrasound-assisted freezing process for many different materials, but no article has researched the application of ultrasound irradiation to support, in general, the freezing process of ginseng materials and, in particular, that of Bo Chinh ginseng.
Therefore, this research will study the appropriate ultrasound parameters that support the freezing process of Bo Chinh ginseng, without it coming into direct contact with the transducer, in order to analyze the effects of the ultrasound power, irradiation temperature and intermittency ratio on the freezing time, nucleation temperature, color and microstructure of the materials.
2. Materials and Methods
2.1. Experimental Material
The fresh Bo Chinh ginseng root used in the experiment was one-year-old ginseng with an average diameter of 25 mm, which was grown in Quang Binh Province. The purchased ginseng was stored at a temperature of 6 ÷ 8 °C to ensure freshness during the experiment. The ginseng root was sliced to be 7 mm thick by using a specialized Ritter slicer (model: RITTER E16 515000; manufacturer: Ritter; made in Gröbenzell, Germany), and the mass of ginseng for each frozen batch was 200 g. The temperature of the ginseng before freezing was close to the ambient temperature.
2.2. Experimental Equipment
The freezer used in the experiment was from AOBEISI (made in Suzhou, China):
- Freezing-room dimensions: 450 × 450 × 500 (mm).
- Cascade refrigeration system:
- + Compressors of the 1st and 2nd stage (model: CAJ2464Z; manufacturer: Tecumseh; made in Selangor, Malaysia).
+ Condenser unit with a fan (model: A069842 manufacturer: Weiguang; made in Hangzhou, China).
+ Evaporator: v = 2.3 m/s.
- 4.
- Ultrasound frequency generator (model: CDEXIM0620; frequency: 20 kHz; manufacturer: Conprofe Technology Group Co., Ltd.; made in Guangdong, China).
2.3. Measurement Equipment
2.3.1. Temperature Measurement
The temperature of the center of the material was measured and recorded automatically by the FX1000 Yokogawa temperature logger (model: FX1000; manufacturer: Yokogawa; made in Tokyo, Japan).
Temperature probes (type-T thermocouples) were attached to the machine (range temperature: −200 °C to +260 °C; nominal size: 1.7 × 3.0 mm; length: 4 m)
This temperature logger has a 2 s interval between recordings, and an accuracy of ±0.05%.
2.3.2. Color Measurement
The color measuring procedure is presented as follows:
Step 1: after the freezing process is complete, the frozen sample is taken out of the freeze chamber and the sample is attached to the holder.
Step 2: the instrument is placed on the target while making sure no light will enter into the instrument (the measurement port of the instrument should be in close contact with the sample); then, “test” (name of button) is pressed to begin the measurements.
Step 3: after hearing the sound of a beep, the values of a*, b* and L* are recorded on the screen of the instrument (repeat 3 times).
L*a*b is the color space introduced by the CIE (English name: International Commission on Illumination). The value of L* represents white or black (L* > 0 represents too much white and L* < 0 represents too much black), a* represents red or green (a* > 0 represents too much red and a* < 0 represents too much green) and b* represents yellow or blue (b* > 0 represents too much yellow and b* < 0 represents too much blue).
The characteristic parameters of L* (light/dark), a* (red/green) and b* (yellow/blue) were measured with a CHN SPEC CS-10 colorimeter (model: CS-10; manufacturer: CHN SPEC; made in Hangzhou, China). The color index, WI (white index), was calculated according to the above three parameters by using the following formula [18]:
2.3.3. Microscopic Capture of Material Surface Structure via SEM (Scanning Electron Microscope)
The microstructure of the material was captured using a JSM-IT100 scanning electron microscope from JEOL, which was made in Tokyo, Japan. Surface microstructure images of the frozen material samples were obtained with a scanning electron microscope following the procedure below:
Step 1: after the freezing process is complete, the material samples are stored in a refrigerator at a temperature of (−22) ± 0.5 °C.
Step 2: before scanning a sample by using scanning electron microscopy (SEM), the sample is cut into a maximum size of 2 cm × 2 cm to fit the holder (the ginseng samples were not coated with a thin layer of gold to maintain the temperature of the frozen sample).
Step 3: the sample is attached to the holder with conductive carbon tape.
Step 4: the holder is placed into the vacuum chamber of the machine.
Step 5: scanning electron microscopy is conducted with a JSM-IT100 microscope (JEOL, Tokyo, Japan) at a voltage of 5 kV.
2.4. Experimental Procedure
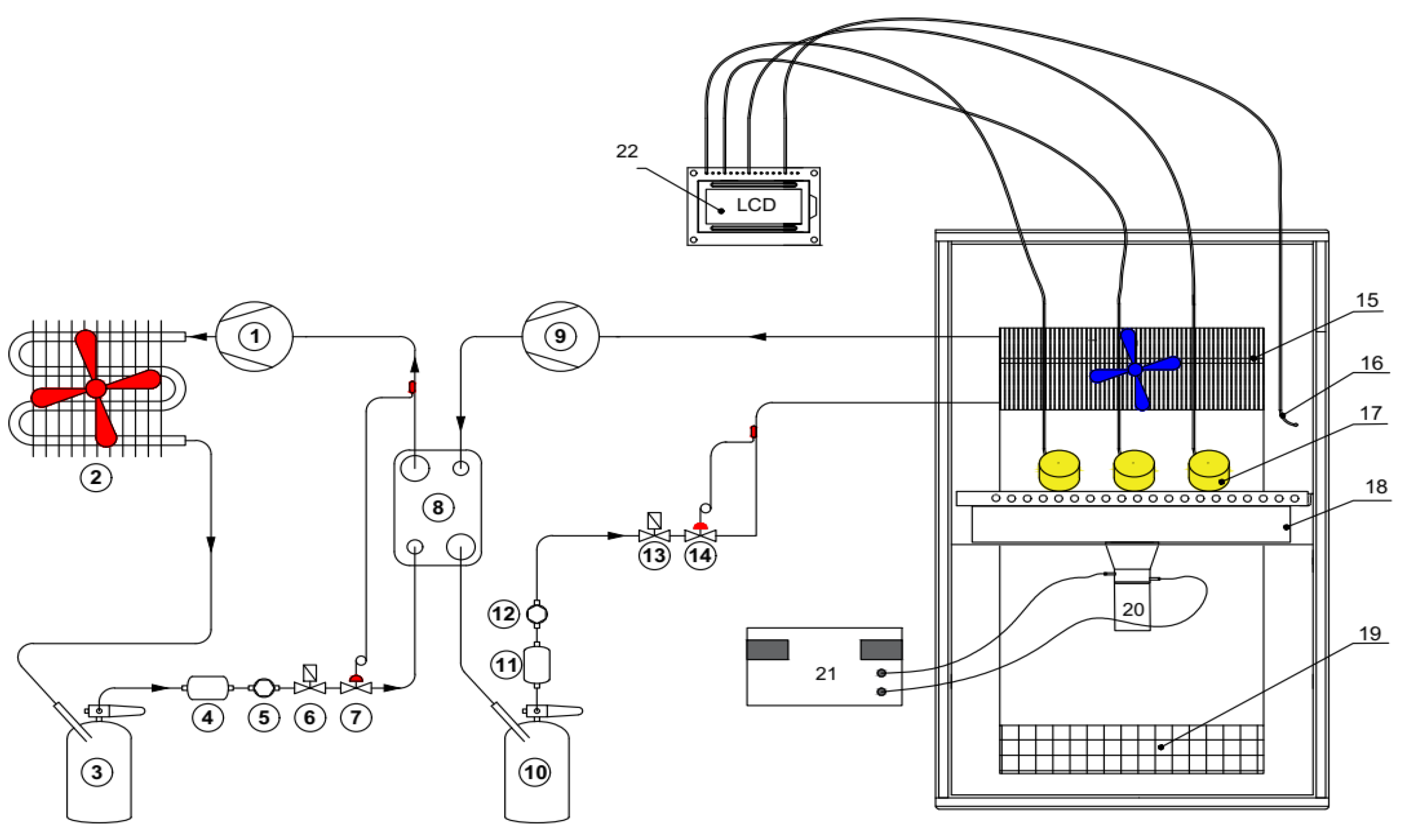
The required temperature of the freezer chamber was set at −40 ± 0.5 °C and recorded on the temperature recorder (Figure 1). The machine was operated until the required temperature was reached. Ginseng roots were sliced to be 7 ± 0.5 mm thick and placed on a tray in contact with the ultrasound generator, and then the temperature recorder probe was placed in the center of the ginseng slice. When the chamber temperature reached −40 °C, the material tray was put into the freezing chamber. The freezing process ended when the material’s center temperature reached −35 °C to ensure that all the water within the finished material was completely frozen.

Figure 1.
Schematic diagram of an ultrasound-assisted freezer: 1—compressor of the 1st stage; 2—condenser; 3—high-pressure receiver of the 1st stage; 4—filter-drier of the 1st stage; 5—sight glass of the 1st stage; 6—solenoid valve of the 1st stage; 7—expansion valve of the 1st stage; 8—heat exchanger; 9—compressor of the 2nd stage; 10—high-pressure receiver of the 2nd stage; 11—filter-drier of the 2nd stage; 12—sight glass of the 2nd stage; 13—solenoid valve of the 2nd stage; 14—expansion valve of the 2nd stage; 15—evaporator; 16—temperature probe; 17—freezing material; 18—emission plate; 19—return air grille; 20—transducer; 21—ultrasound generator; 22—temperature recorder.
2.5. Experimental Method
The single-factor test method was used to determine the effects of ultrasound irradiation on the freezing process of materials:
- Experiment 1: experiment on how ultrasound irradiation with a power of 0, 50, 100, 150 and 200 W at a 20 kHz frequency influences the material freezing time and nucleation temperature of water in the material.
- Experiment 2: experiment on how the irradiation temperature affects the nucleation temperature of the material.
- Experiment 3: experiment on how the rate of transmitting intermittency affects the freezing time.
Finally, the effects of ultrasound irradiation on nucleation temperature, freezing time, color and the microstructure of the materials after freezing were determined.
3. Results and Discussions
3.1. Experimental Effect of Ultrasound Power on Freezing Time of Bo Chinh Ginseng
The experimental freezing of Bo Chinh ginseng was supported by ultrasound irradiation at a 20 kHz frequency with different ultrasound powers of 0 W, 50 W, 100 W, 150 W and 200 W.
The test results are shown in the figure below:
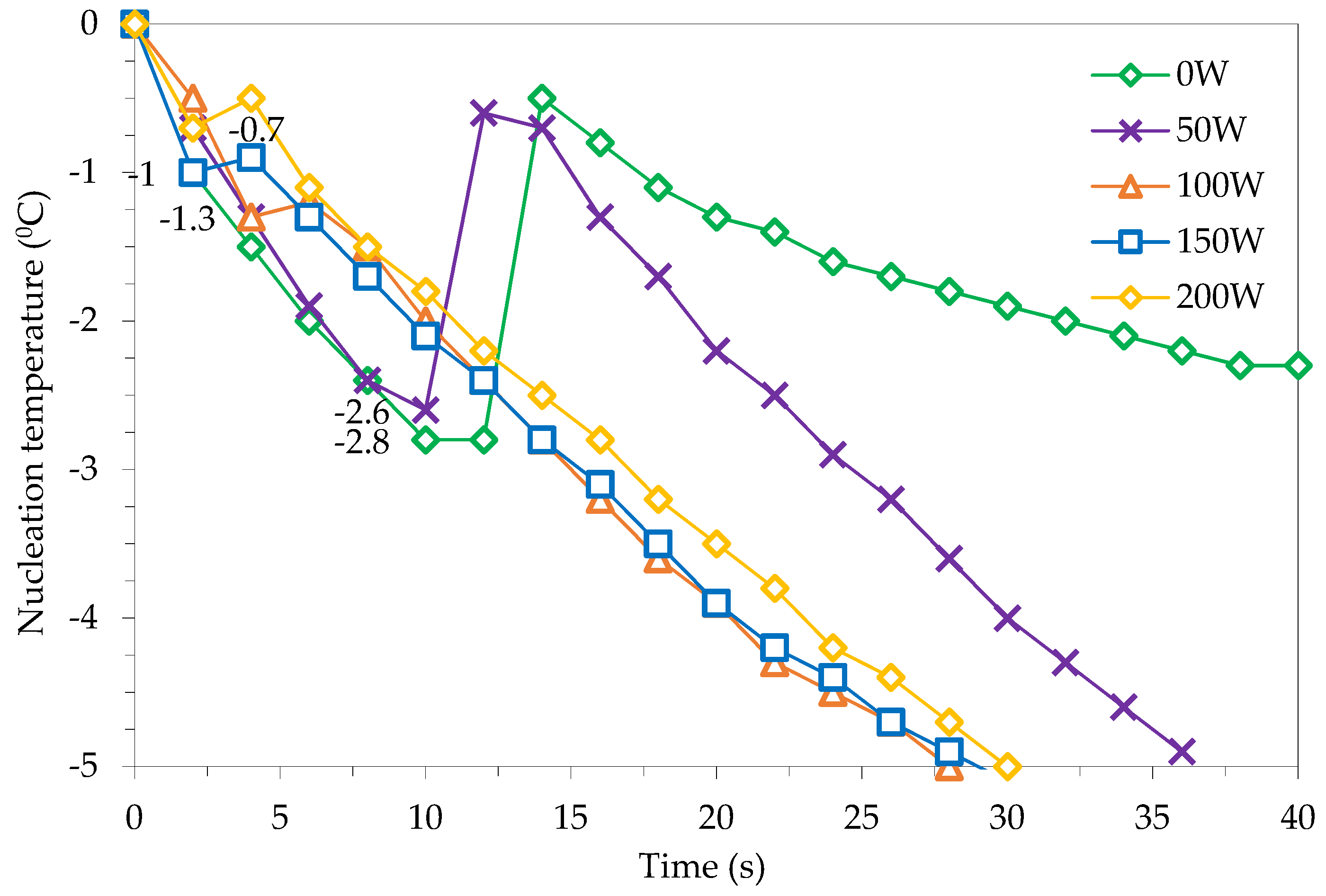
Under the same conditions of ambient temperature, initial material temperature, freezing temperature and air velocity in the freezing chamber, the experimental results show that the ultrasound-assisted freezing mode at a 20 kHz frequency with power levels of 50 W, 100 W, 150 W and 200 W all had a shorter freezing time than the method of freezing without ultrasound support. Previous studies have also shown that ultrasound irradiation traveling through a solid medium causes the solid’s molecules to vibrate, causing compression and expansion along with pressure changes, and, ultimately, creating a “bubble effect” which creates microchannels that promote heat and mass transfer and shorten the freezing time [19].
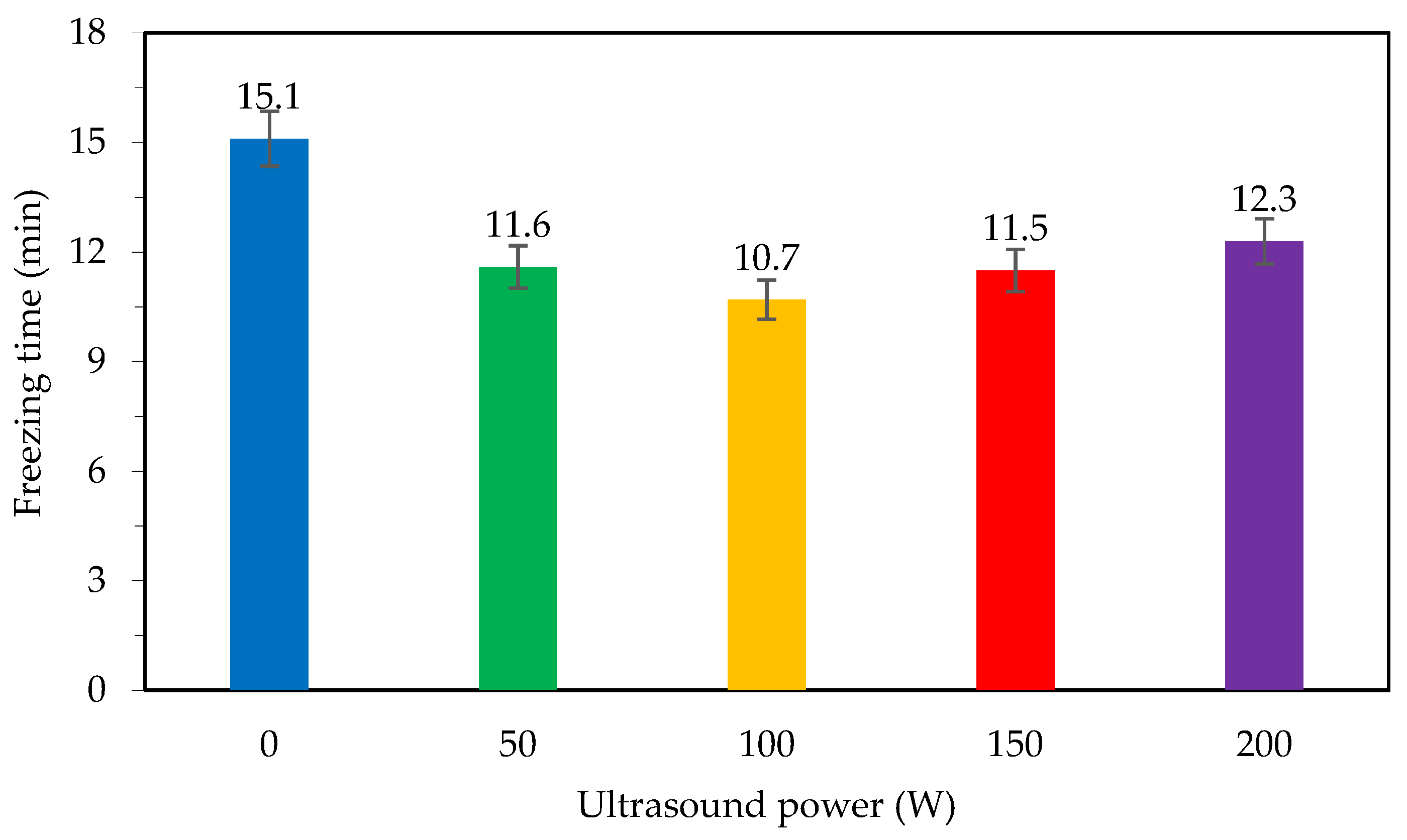
As shown in Figure 2, when the ultrasound power level was increased from 0 to 50 W, the freezing time decreased from 15.1 to 11.6 min (23.2%). Increasing the ultrasound power to 100 W only reduced the freezing time to 0.9 min, which was 7.8% better as compared to the freezing time at 50 W. However, if the ultrasound power continues to increase to 150 W and 200 W, the freezing time tends to increase again as compared to the minimum freezing time corresponding to the 100 W ultrasound power. Specifically, at the power level of 150 W, the freezing time increases by 7.5% as compared to the freezing time at 100 W, and at the ultrasound power of 200 W, the increase is 15% compared to the freezing time at 100 W, which is only a decrease of 18% compared to freezing without ultrasound. The time needed to freeze materials with the help of ultrasound irradiation was lowest at the power level of 100 W Freezing with the help of ultrasound irradiation with a capacity of 100 W saves 29.1% of the freezing time as compared to freezing without ultrasound. However, when the transmitting power is increased, the freezing time also increases due to the thermal effect generated by the ultrasound irradiation [12].

Figure 2.
Freezing time with different ultrasound power levels.
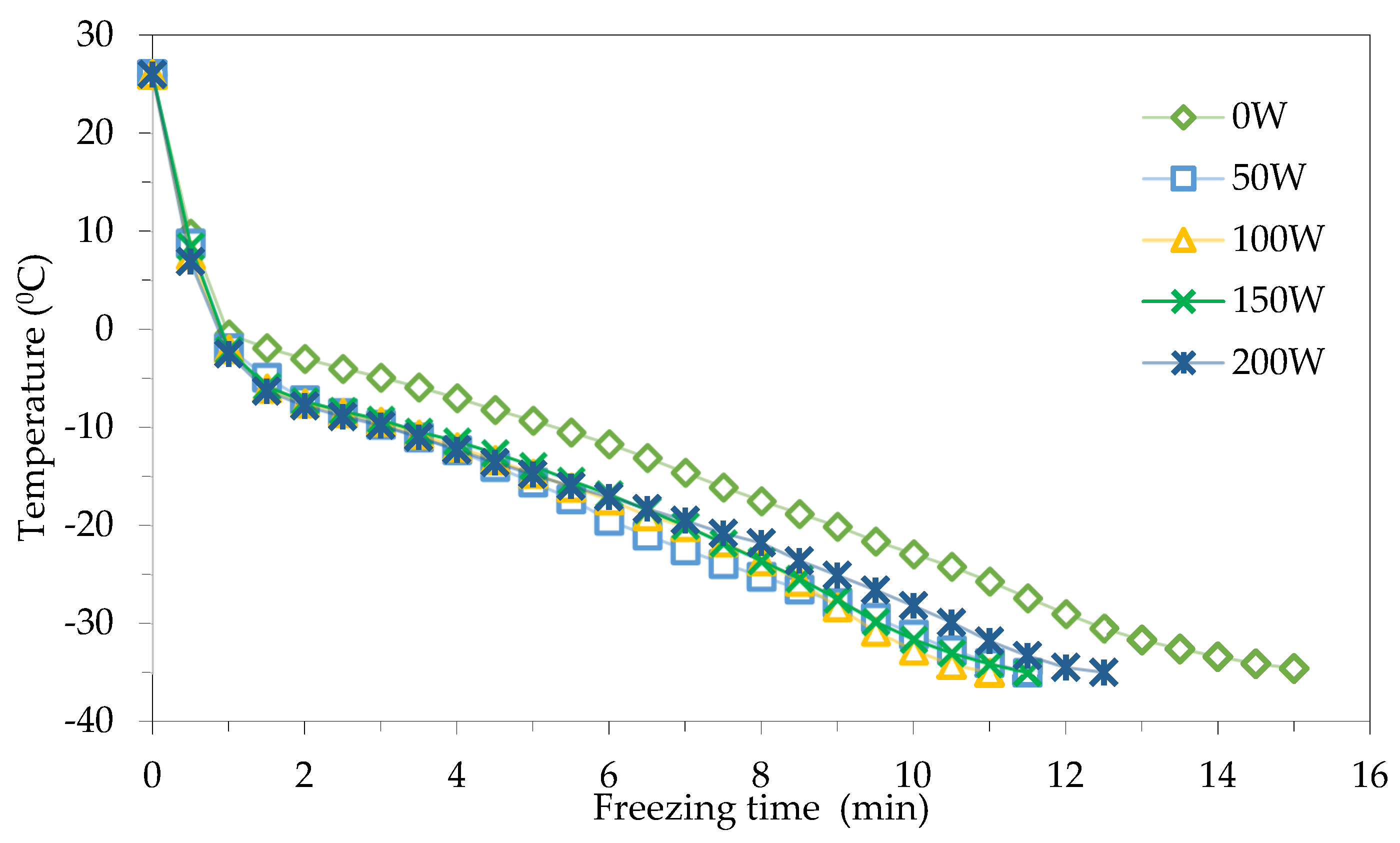
In fact, the total freezing time does not accurately reflect how ultrasound irradiation affects the freezing process of Bo Chinh ginseng. The freezing time is often calculated by obtaining the sum of the time of the three stages: cooling, phase transition and supercooling. Figure 3 shows the freezing curves for the freezing modes with and without the help of ultrasound irradiation, which can be divided into three stages: the cooling phase (from 26 °C to 0 °C), phase transition (0 ÷ −5 °C) and supercooling period (−5 ÷ −35 °C). From Figure 3, it can be seen that the cooling and phase transition times are faster than the time required for the supercooling stage in all different freezing modes.

Figure 3.
Freezing curves with different ultrasound power levels.
Figure 4 shows the time at each stage, in which the cooling and phase transition stages in the freezing mode without ultrasound support have shorter times than the other modes. Considering the phase transition, it can be clearly seen that for ultrasound-assisted freezing, the time is much shorter than that of the unassisted freezing mode. Specifically, the phase transition time only takes 26 and 28 s at 100 W, 150 W and 200 W, respectively. Meanwhile, the phase transition time in the freezing mode without the help of ultrasound irradiation is up to 124 s. In the subcooling stage, ultrasound irradiation has a noticeable effect, helping to shorten the time of subcooling compared to the time required for the freezing mode without the help of ultrasound irradiation. The freezing mode that uses ultrasound irradiation with a capacity of 100 W had the shortest time. Through the above experiments, it can be concluded that the continuous transmitting mode with a power level of 100 W is the most effective at shortening the freezing time.

Figure 4.
Time of each freezing stage with different ultrasound power levels.
3.2. Experimental Effect of Ultrasound Power on Nucleation Temperature during Freezing of Bo Chinh Ginseng
The nucleation temperature is the lowest supercooling temperature in the cooling phase and is lower than 0 °C. At this temperature, the water in the material has not yet crystallized, but remains in the liquid phase. The nucleation temperature of water in the materials cannot be predicted, as it depends on the different structure and water bonding in each material. The nucleation temperatures of different materials were different because the structures of the materials were complex and contained different components that probably act as ice nuclei, resulting in the different nucleation temperatures [12,20]. The nucleation temperature affects the morphology, size and distribution of ice crystals and it is considered a critical factor for the optimization of freezing processes. Previous studies have shown that using ultrasound can control crystallization and has a positive effect on the nucleation rate and crystal growth rate [3,5,12,20].
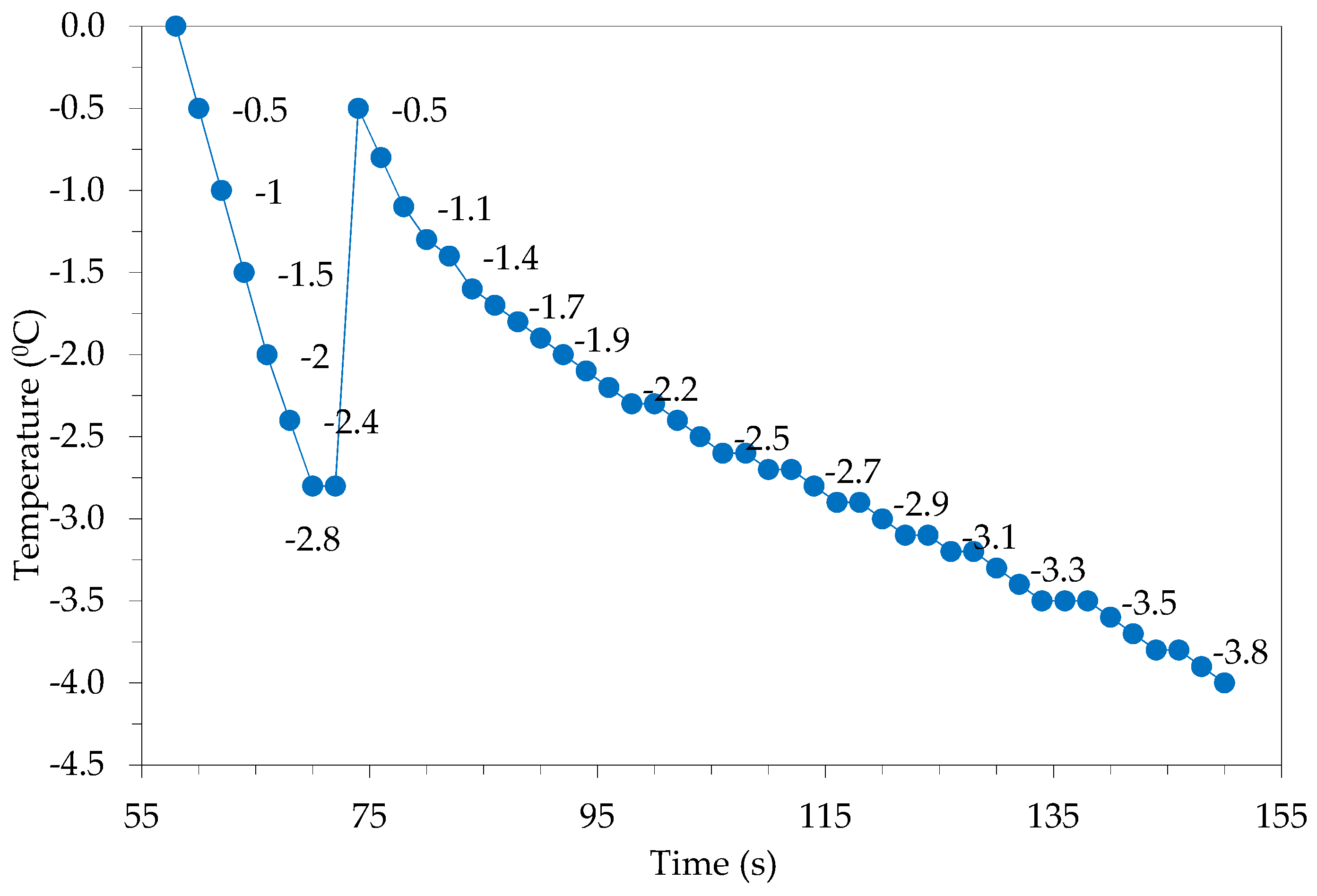
Figure 5 shows the nucleation temperature of water in the material during the ultrasound-unassisted freezing process (−2.8 °C).

Figure 5.
The nucleation temperature in freezing mode without ultrasound irradiation.
Figure 6 shows the effect of ultrasound power on the nucleation temperature of water in the material during freezing. Ultrasound irradiation at higher power levels increases the nucleation temperature of water in the material compared to the ultrasound-unassisted freezing mode [13]. The lowest nucleation temperature (−2.8 °C) corresponds to the freezing mode without ultrasound support and the highest (−0.7 °C) corresponds to the freezing process with an ultrasound power level of 200 W. All freezing modes corresponding to different levels of ultrasound power had an initial crystallization temperature of −0.5 °C or less.

Figure 6.
Ginseng nucleation temperature with different ultrasound power levels.
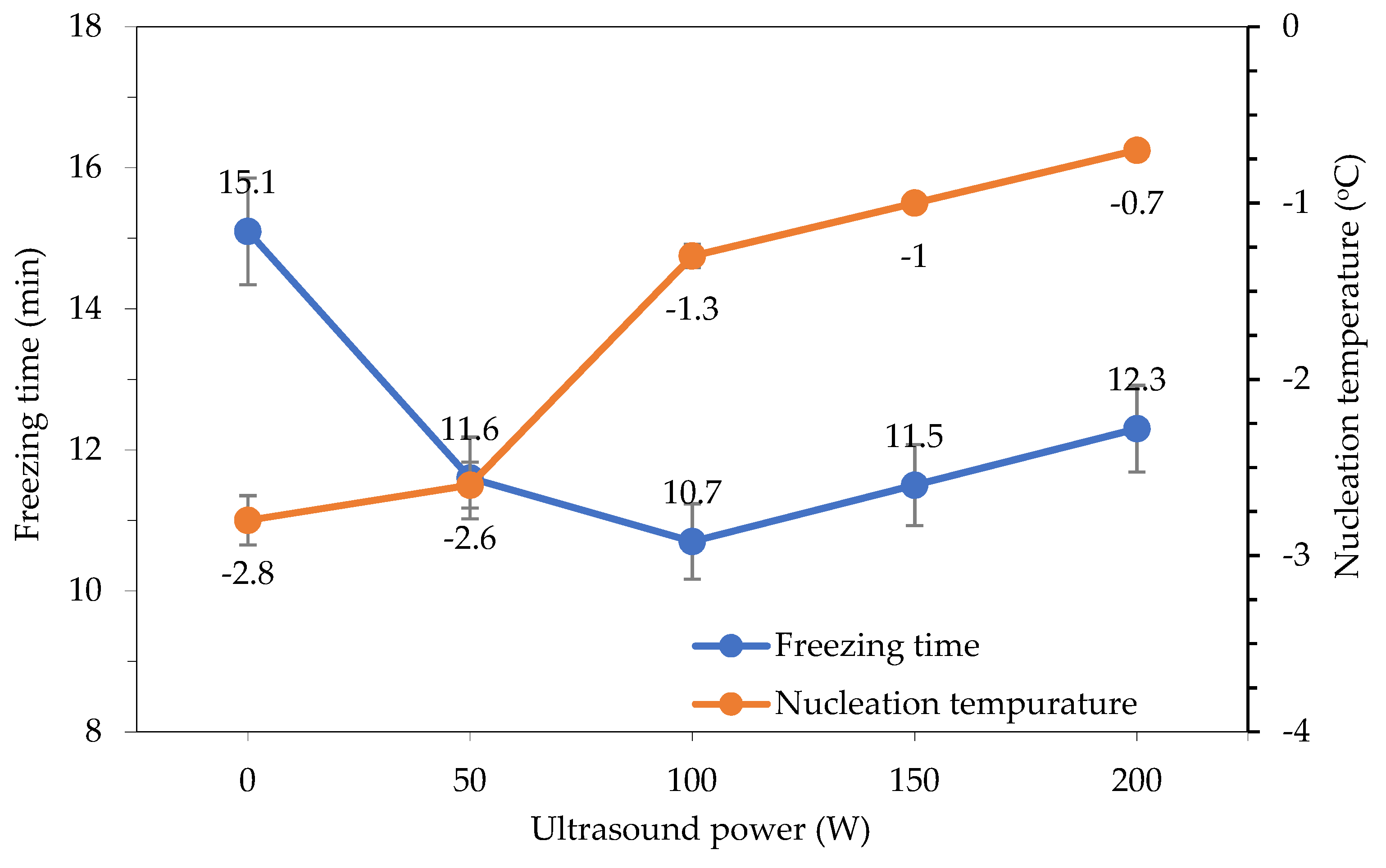
The results of the comparison between nucleation temperature and freezing time for different freezing modes are shown in Figure 7. As the ultrasound power increased, the nucleation temperature increased accordingly. However, at the 150 W and 200 W power levels, the nucleation temperature increased by −1 °C and −0.7 °C, respectively, and the freezing time also increased. It can be said that the higher the ultrasound power, the higher the nucleation temperature, but the longer the broadcasting time, the more likely it is that the ultrasound irradiation will create a thermal effect that increases the freezing time in the third stage, which is the subcooling stage. Ultrasound irradiation at a power level of 100 W undergoes continuous transmission to support the freezing process of the materials. The effects of ultrasound application on the freezing time and the nucleation temperature of the material are summarized in Table 1.

Figure 7.
Effect of ultrasound power on nucleation temperature and freezing time.

Table 1.
Parameters for ultrasound-assisted freezing of ginseng material.
3.3. Effect of Ultrasound Initiation Temperature on Nucleation Temperature during Freezing of Materials
Previous studies have also shown that ultrasound irradiation is instrumental in controlling the nucleation temperature. Baogou Xu et al. [12] studied the effect of the broadcast initiation temperature on the nucleation temperature of radish cylinders. The temperature at the start of ultrasound irradiation in this study was located in the temperature range lower than 0 °C and higher than the nucleation temperature of the material when undergoing unsupported ultrasound freezing. The experimental results show that the higher the temperature during the application of the ultrasound, the sooner the nucleation takes place, i.e., the higher the nucleation temperature. In addition, some studies have shown that ultrasound-assisted frozen samples have higher nucleation temperatures than those frozen without ultrasound assistance. According to the studies of Kiani et al. [21] that used agar gel and Nakagawa et al. [20] that used mannitol and mannitol samples of bovine serum albumin (BSA), the lower the nucleation temperature of the ultrasound-assisted frozen samples, the smaller the ice crystal size.
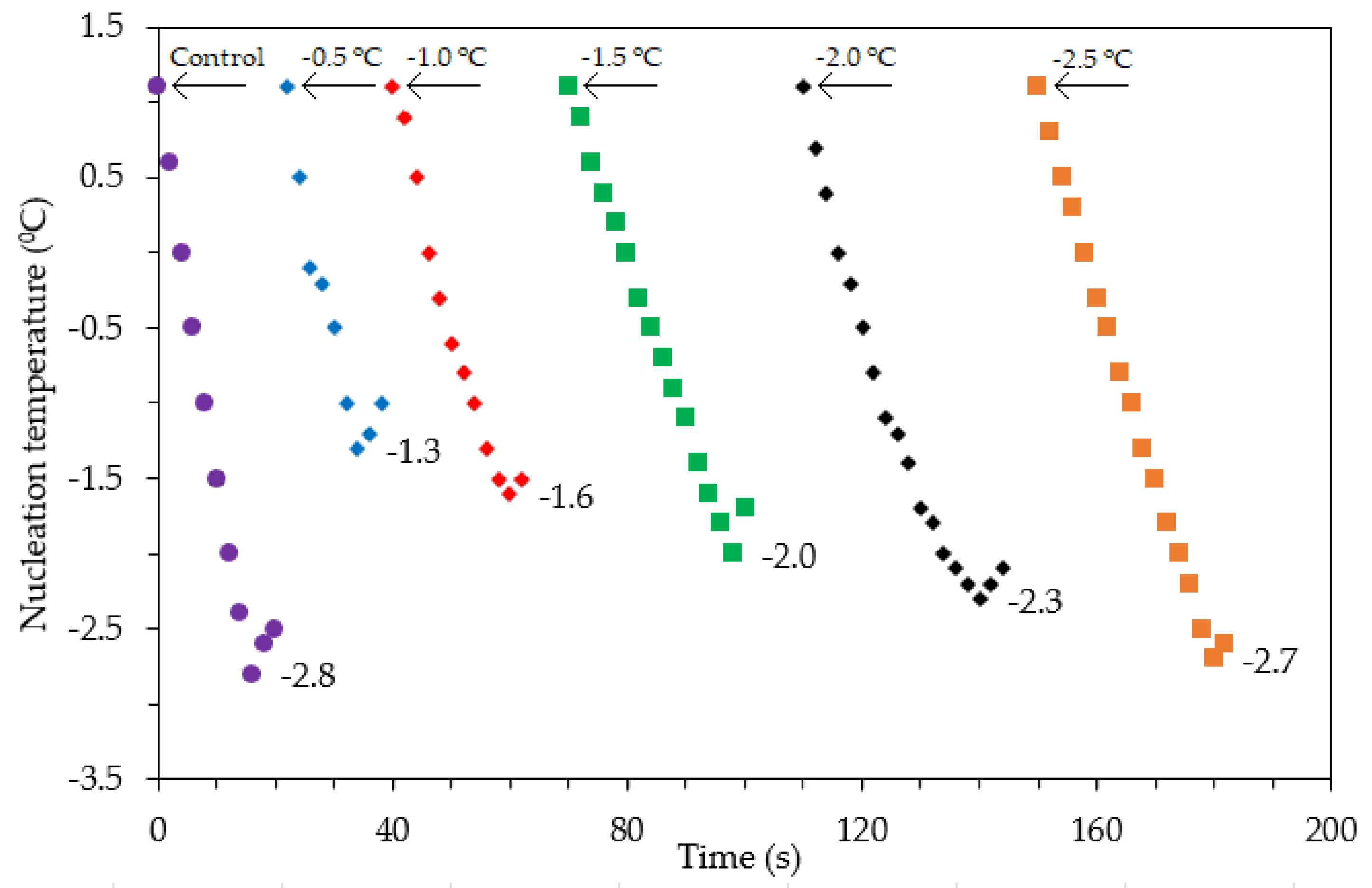
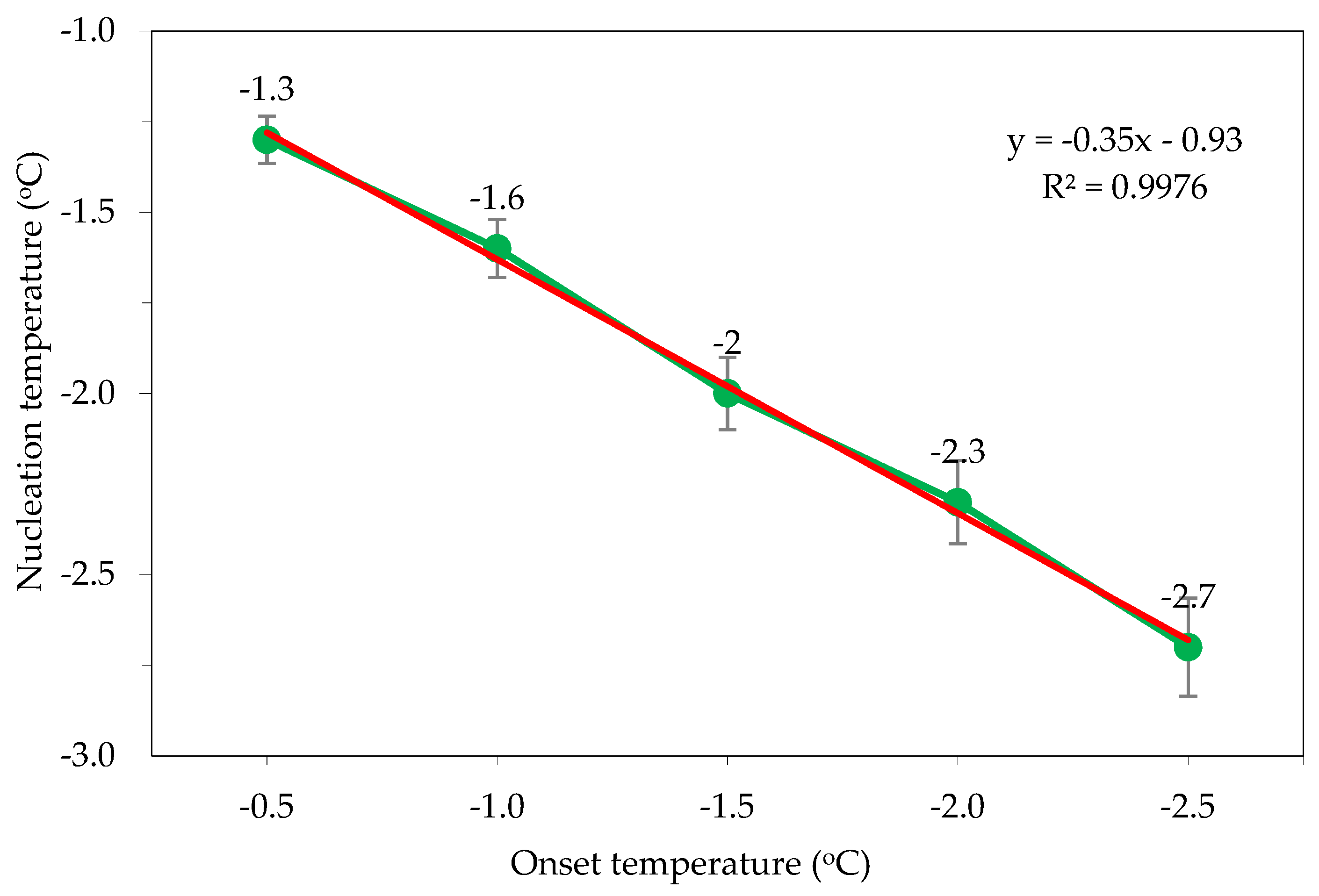
Figure 8 shows the effect of different irradiation initiation temperatures on the nucleation temperature of the materials. Corresponding to different transmitting initiation temperatures, the nucleation temperatures of the materials during freezing are also different. The lowest nucleation temperature (−2.8 °C) corresponds to the freezing process without ultrasound support (control). The nucleation temperature further decreases as the irradiation initiation temperature decreases. The highest nucleation temperature of −1.3 °C corresponds to the transmitting initiation temperature of −0.5 °C, and the lowest nucleation temperature of −2.7 °C corresponds to the lowest transmitting temperature of −2.5 °C.

Figure 8.
Effect of ultrasound irradiation initiation temperature at 100 W (ultrasound power) on the nucleation temperature: control—frozen sample obtained without ultrasound-assisted freezing. Arrows indicate the ultrasound-application-triggered temperature points.
Figure 9 shows a linear relationship between the irradiation temperature and nucleation temperature (y = −0.35x − 0.93, R2 = 0.9976). The results show that ultrasound irradiation can control the nucleation of materials. This is very important for the research and application of ultrasound irradiation to support the freezing process of ginseng, as it helps to ensure product quality and freezing time, especially in the sublimation drying of materials that require freezing before drying.

Figure 9.
Relationship between irradiation and nucleation temperatures.
3.4. Experimental Effect of Interruption Rate on Freezing Time of Bo Chinh Ginseng
Materials exposed for a long time to ultrasound are at risk of experiencing thermal effects. Kiani et al. [21] studied how the duration of ultrasound irradiation affects the freezing of agar gel. The study showed that higher duty cycles resulted in larger crystals as they reduced the freezing rate, and the elongation of the duration of ultrasound produced heat that melted the ice crystals. The other study also showed that a long duration of ultrasound irradiation can result in heat generation in the material, which is not good for the nucleation process [22]. When the time of ultrasound irradiation is prolonged and the ultrasonic thermal effect is enhanced, the heat can hinder the formation of ice crystals and slow down the freezing rate [3]. Therefore, the effect of the intermittency ratio of ultrasound irradiation was studied.
In this experiment, freezing occurred at a power level of 100 W (irradiation temperature of −0.5 °C) with an intermittency radio of 0, 0.2, 0.4, 0.6 and 0.8. The intermittency ratio is calculated as [23]:
in which: Ton—freezing time with ultrasound assistance;
Toff—freezing time without ultrasound assistance;
A = 0: continuous ultrasound transmitting;
A = 0.2: 60 s on 15 s off;
A = 0.4: 60 s on 40 s off;
A = 0.6: 60 s on 90 s off;
A = 0.8: 60 s on 240 s off;
A = 1: no ultrasound.
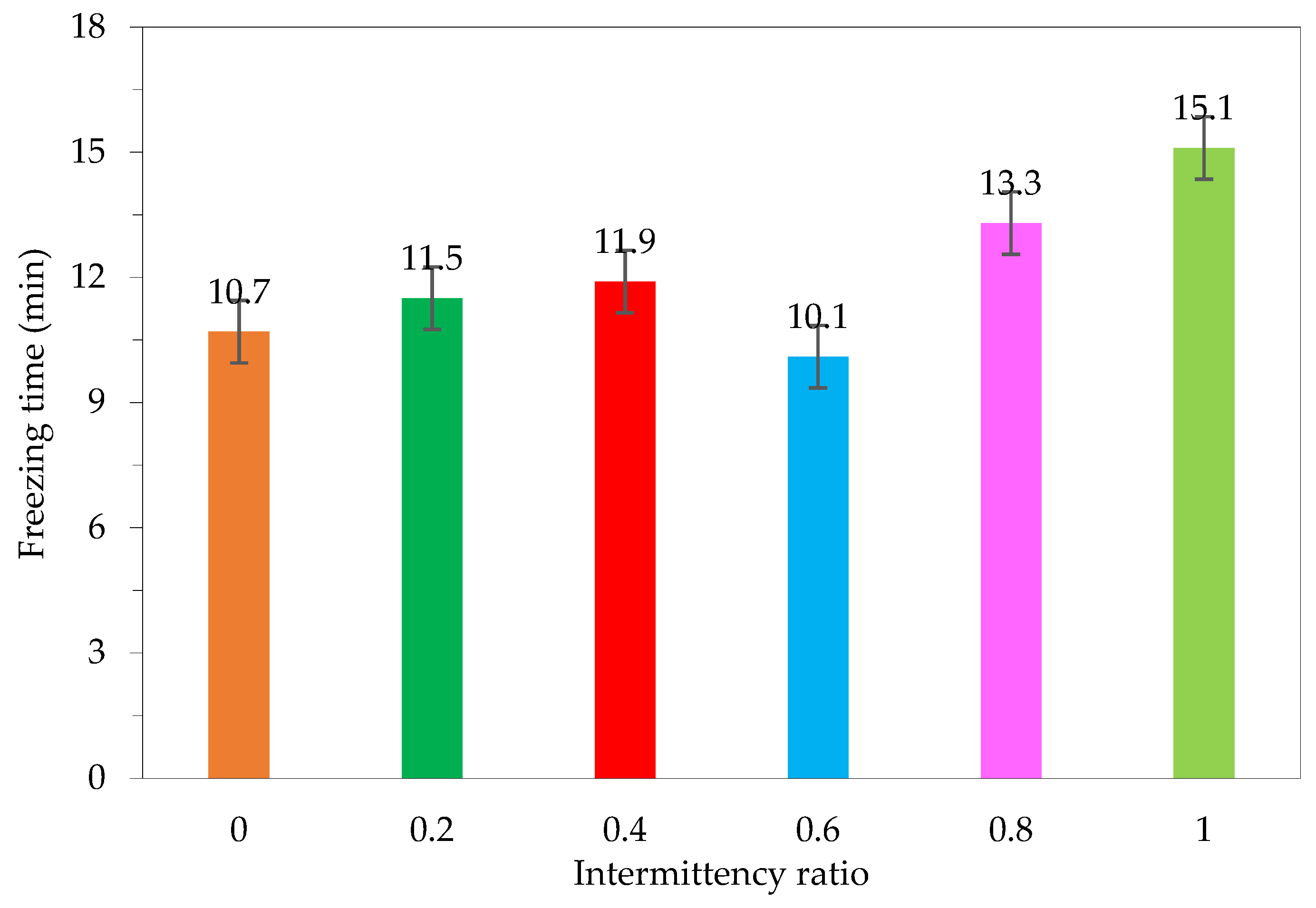
Previous studies have shown that continuous transmitting affects freezing time. Extending the airing time will generate heat, which increases the freezing time. As a result, the freezing time is not as reduced as that of the non-ultrasound freezing regime [8]. Some studies have examined how interrupting the transmitting rate affects freezing time [5,15,17,24,25]. These research results also show that the rate of the transmitting intermittency affects the freezing time. Figure 10 indicates that at an intermittency ratio of 0.2, 0.4 and 0.8, the freezing time is lower than that of the unsupported mode of ultrasound irradiation, but higher than that of the continuous broadcasting mode. At the rate of 0.6, the freezing time is the lowest among the freezing modes. This shows that choosing the appropriate ratio helps to shorten the freezing time, reduce the air time and ensure product quality.

Figure 10.
Effect of intermittent transmitting rate on material freezing time.
3.5. Effect of Ultrasound Irradiation on the Color of Materials after Freezing
Color is an important indicator to assess the sensory quality of materials. The indices of L*, a* and b* are used to evaluate the color change of product samples.
In Table 2, it can be seen that the values of L*, which were obtained using the WI of frozen material samples corresponding to different modes, are higher than that of fresh material samples. This is because when freezing, water moves from the inside of the material to the surface and crystallizes. As a result, frozen samples will have a lighter color than fresh samples. The frozen samples do not have much of a difference between the L* and WI values, which shows that using ultrasound irradiation to support the freezing process does not affect the color of materials as compared to non-ultrasound freezing. A similar phenomenon was reported by Tu et al. [25], as their work showed that freezing lotus root by using ultrasound did not cause a significant change in its brightness in comparison to freezing without ultrasound, but both samples were brighter compared to the raw material. However, the values of the a* and b* color parameters of the frozen samples changed under different freezing conditions. The values of the a* of the control sample increased significantly, whereas those of the ultrasound-treated sample slightly went up. Ultrasonic treatment samples decreased in yellowness compared with the raw and control samples.

Table 2.
Color index of material samples.
3.6. Experimental Effect of Ultrasound Irradiation on the Microstructure of Bo Chinh Ginseng
The microstructure plays an important role in assessing the quality of materials during food processing.
Previous studies have shown that ultrasound irradiation aids in freezing, and besides promoting early nucleation and reducing the size of ice crystals, it also creates microchannels that enhance transmission heat and mass transfer [3]. Sun et al. [17] presented their findings showing that an appropriate ultrasonic power applied to the assisted freezing process can decrease the ice crystal size. Zhang et al. [9] showed that the crystal size of frozen samples treated by ultrasound was smaller and the distribution of ice crystals was uniform.
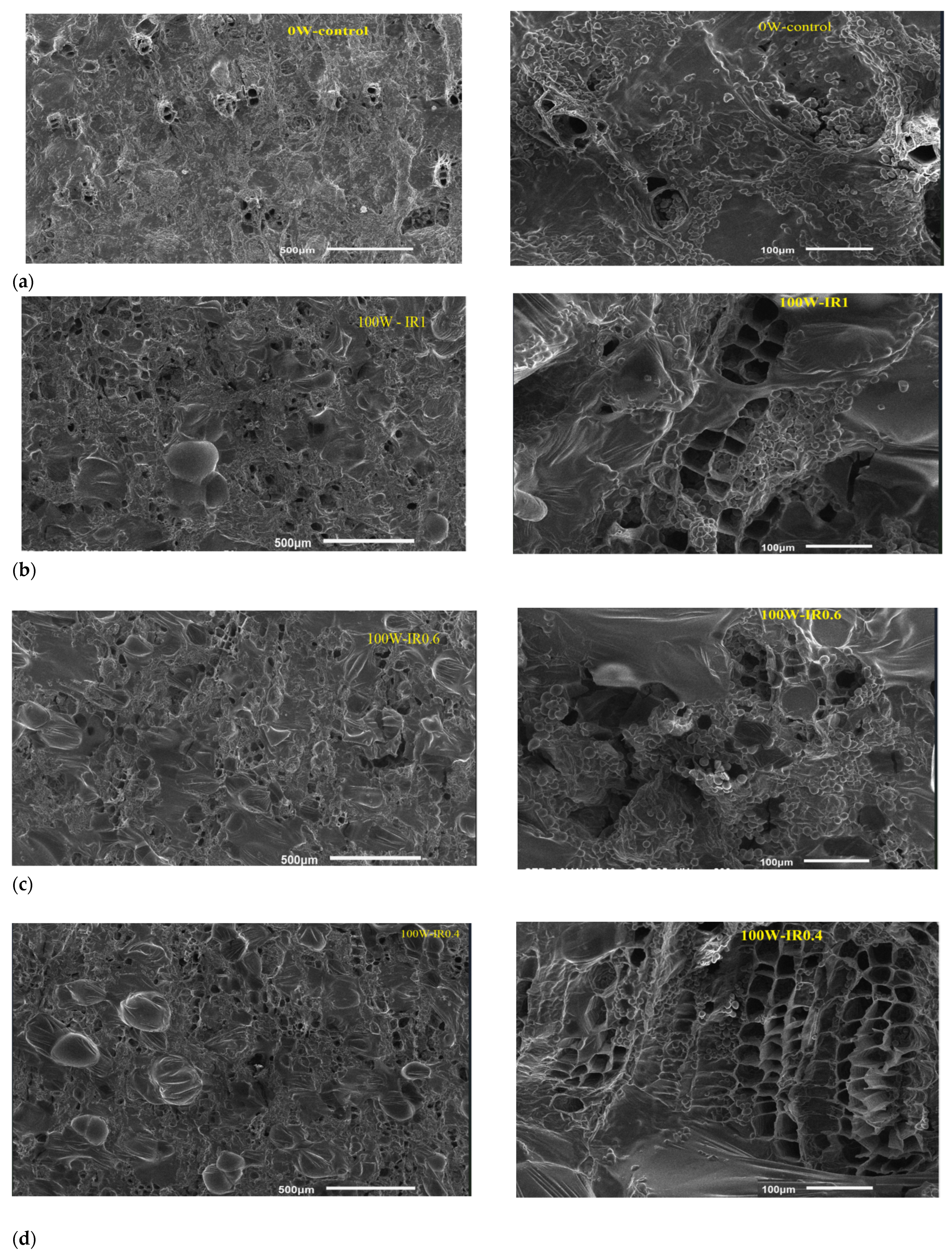
The SEM images of the materials are shown in Figure 11 with two different magnification ratios (50 and 200 times). Through the image, it can be seen that the surface of the material sample frozen without the support of ultrasound irradiation (0 W-control) has very few holes. Meanwhile, in the ultrasound-assisted freezing mode, a number of holes appeared on the surface of the dense material. This proves that super waves greatly influence the creation of the microchannels, which are shown through holes on the surface of the material. The fact that holes appear on the surface of the material proves that this experimental study produced results consistent with previous studies. In addition, the creation of microchannels in the material after freezing is of great significance for the sublimation drying phase, as the microchannels promote the heat transfer process, helping to shorten the drying time and improve the quality of the material.

Figure 11.
SEM images of ginseng microstructures under different freezing conditions: (a) 0W-control sample at ×50 and ×200 magnifications; (b) 100W-IR1—continuous ultrasound-assisted freezing at ×50 and ×200 magnifications; (c) 100W-IR 0.6—intermittent ultrasound-assisted freezing at ×50 and ×200 magnifications; (d) 100W-IR0.4—intermittent ultrasound-assisted freezing at ×50 and ×200 magnifications.
The surface of the continuously frozen ultrasound-assisted (100 W-IR1) sample was much more porous than that of the non-ultrasound (0 W-control) sample. This may be attributed to the cavitation bubbles produced by ultrasound-induced nucleation, and to microstreaming that may have fragmented pre-existing ice crystals into smaller nuclei, promoting secondary nucleation and resulting in more ice nuclei and a shorter freezing time [26]. However, compared with the 100 W-IR1 sample, the intermittently frozen ultrasound-assisted (100 W-IR0.4 and 100 W-IR0.6) samples had more pores on their surfaces at the 500 μm magnification. In the corresponding image at a 100 μm magnification, it is easy to see that the pores appear evenly and more frequently in the material sample with an intermittency ratio of 0.4 compared to that with a ratio of 0.6.
4. Conclusions
The research results show that ultrasound irradiation has a positive effect on the freezing process of ginseng materials. Factors such as broadcast power level (at the same 20 kHz frequency), irradiation temperature and intermittency ratio all affect the freezing process. It can be concluded that it is very important to choose the appropriate ultrasound parameters and conditions to support the material freezing process before sublimation drying. In comparing ultrasound-assisted frozen samples with ultrasound-unassisted frozen samples, the freezing time, nucleation temperature, microstructure and color all have better results at a 100 W (20 kHz) power and ratio interruption rate of 0.6.
When a higher ultrasound power is continuously transmitted to the material, it does not shorten the freezing time; instead, the larger the power capacity, the less effective it is in helping to shorten the freezing time, and at the same time, it increases the nucleation temperature. The results also show that the nucleation temperature is a function of the irradiation temperature in the studied temperature range from −0.5 to −2.5 °C. In addition, the intermittency ratio also has a positive effect on shortening the freezing time, as it helps to reduce the heat generation effect of ultrasound irradiation. Ultrasound irradiation also causes many microchannels and holes on the surface of the material, enhancing the process of heat and substance transfer. Moreover, ultrasound irradiation do not change the material color.
The results show that selecting the appropriate parameters during the ultrasound-assisted freezing process of ginseng root is crucial. Although this study only shows quantitative research results on the influence that ultrasound application has on the freezing process, the results provide useful information for the future application of ultrasound-assisted food freezing.
Author Contributions
Investigation, experimenting and writing, M.-H.N.; editing and approval, H.N. and T.-B.N.; experimenting, T.-D.L.; providing and setting up the experiments, Q.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kapoor, N.; Mohite, A.M.; Sharma, N.; Sharma, D. Comparative analysis of freeze-dried and spray dried beet-root powder according to physico-chemical, functional and color properties. Bull. Transilv. Univ. Brasov. Ser. II For. Wood Ind. Agric. Food Eng. 2021, 14, 153–162. [Google Scholar] [CrossRef]
- Heldman, D.R.; Daryl, B.L. Handbook of Food Engineering, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Ma, X.; Mei, J.; Xie, J. Mechanism of ultrasound assisted nucleation during freezing and its application in food freezing process. Int. J. Food Prop. 2021, 24, 68–88. [Google Scholar] [CrossRef]
- Chow, R.; Blindt, R.; Chivers, R.; Povey, M. A study on the primary and secondary nucleation of ice by power ultrasound. Ultrasonics 2005, 43, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.E.; Zheng, L.; Sun, D.W. Influence of ultrasound on freezing rate of immersion-frozen apples. Food Bioprocess Technol. 2009, 2, 263–270. [Google Scholar] [CrossRef]
- Islam, M.N.; Zhang, M.; Adhikari, B.; Cheng, X.; Xu, B.G. The effect of ultrasound-assisted immersion freezing on selected physicochemical properties of mushrooms. Int. J. Refrig. 2014, 42, 121–133. [Google Scholar] [CrossRef]
- Islam, M.N.; Zhang, M.; Fang, Z.; Sun, J. Direct contact ultrasound assisted freezing of mushroom (Agaricus bisporus): Growth and size distribution of ice crystals. Int. J. Refrig. 2015, 57, 46–53. [Google Scholar] [CrossRef]
- Mortazavi, S.; Tabatabaei, F. Study of ice cream freezing process after treatment with ultrasound. World Appl. Sci. J. 2008, 4, 188–190. [Google Scholar]
- Zhang, M.; Haili, N.; Chen, Q.; Xia, X.; Kong, B. Influence of ultrasound-assisted immersion freezing on the freezing rate and quality of porcine longissimus muscles. Meat Sci. 2018, 136, 1–8. [Google Scholar] [CrossRef]
- Zhang, M.; Xia, X.; Liu, Q.; Chen, Q.; Kong, B. Changes in microstructure, quality and water distribution of porcine longissimus muscles subjected to ultrasound-assisted immersion freezing during frozen storage. Meat Sci. 2019, 151, 24–32. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, P.; Sun, D.W. Effects of multi-frequency ultrasound on freezing rates and quality attributes of potatoes. Ultrason. Sonochem. 2020, 60, 104733. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, M.; Bhandari, B.; Cheng, X. Influence of power ultrasound on ice nucleation of radish cylinders during ultrasound-assisted immersion freezing. Int. J. Refrig. 2014, 46, 1–8. [Google Scholar] [CrossRef]
- Kiani, H.; Sun, D.W.; Delgado, A.; Zhang, Z. Investigation of the effect of power ultrasound on the nucleation of water during freezing of agar gel samples in tubing vials. Ultrason. Sonochem. 2012, 19, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhong, S.; Yan, W.; Liu, M.; Yang, Z.; Qiao, X. The effects of ultrasonic treatment on the freezing rate, physicochemical quality, and microstructure of the back muscle of grass carp (Ctenopharyngodon idella). LWT Food Sci. Technol. 2019, 111, 301–308. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. The effects of ultrasound-assisted freezing on the freezing time and quality of broccoli (Brassica oleracea L. var. botrytis L.) during immersion freezing. Int. J. Refrig. 2014, 41, 82–91. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. Ultrasound assisted immersion freezing of broccoli (Brassica oleracea L. var. botrytis L.). Ultrason. Sonochem. 2014, 21, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhao, X.; Zhang, C.; Xia, X.; Sun, F.; Kong, B. Ultrasound-assisted immersion freezing accelerates the freezing process and improves the quality of common carp (Cyprinus carpio) at different power levels. LWT Food Sci. Technol. 2019, 108, 106–112. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Wang, H.; Xia, X.; Kong, B. Ultrasound-assisted Immersion Freezing Reduces the Structure and Gel Property Deterioration of Myofibrillar Protein from Chicken Breast. Ultrason. Sonochem. 2020, 67, 105137. [Google Scholar] [CrossRef]
- Kentish, S.; Ashokkumar, M. The physical and chemical effect of ultrasound. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Canovas, G.V., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 1–12. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hottot, A.; Vessot, S.; Andrieu, J. Influence of Controlled Nucleation by Ultrasounds on Ice Morphology of Frozen Formulations for Pharmaceutical Proteins Freeze-drying. Chem. Eng. Process. Process Intensif. 2006, 45, 783–791. [Google Scholar] [CrossRef]
- Kiani, H.; Zhang, Z.; Sun, D.W. Effect of ultrasound irradiation on ice crystal size distribution in frozen agar gel samples. Innov. Food Sci. Emerg. Technol. 2013, 18, 126–131. [Google Scholar] [CrossRef]
- Kiani, H.; Zhang, Z.; Delgado, A.; Sun, D.W. Ultrasound assisted nucleation of some liquid and solid model foods during freezing. Food Res. Int. 2011, 44, 2915–2921. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Z.; Yu, F.; Tao, Z. Ultrasound-assisted heat pump intermittent drying of adzuki bean seeds: Drying characteristics and parameter optimization. J. Food Process Eng. 2020, 43, e13501. [Google Scholar] [CrossRef]
- Hu, S.Q.; Liu, G.; Li, L.; Li, Z.X.; Hou, Y. An improvement in the immersion freezing process for frozen dough via ultrasound irradiation. J. Food Eng. 2013, 114, 22–28. [Google Scholar] [CrossRef]
- Tu, J.; Zhang, M.; Xu, B.; Liu, H. Effects of different freezing methods on the quality and microstructure of lotus (Nelumbo nucifera) root. Int. J. Refrig. 2015, 52, 59–65. [Google Scholar] [CrossRef]
- Saclier, M.; Peczalski, R.; Andrieu, J. Effect of ultrasonically induced nucleation on ice crystals’ size and shape during freezing in vials. Chem. Eng. Sci. 2010, 65, 3064–3071. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).