Surfactant-Modified Construction Waste Bricks for the Removal of Diclofenac from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Adsorbents
2.3. Characterization
2.4. The Adsorption Experiments
3. Results and Discussion
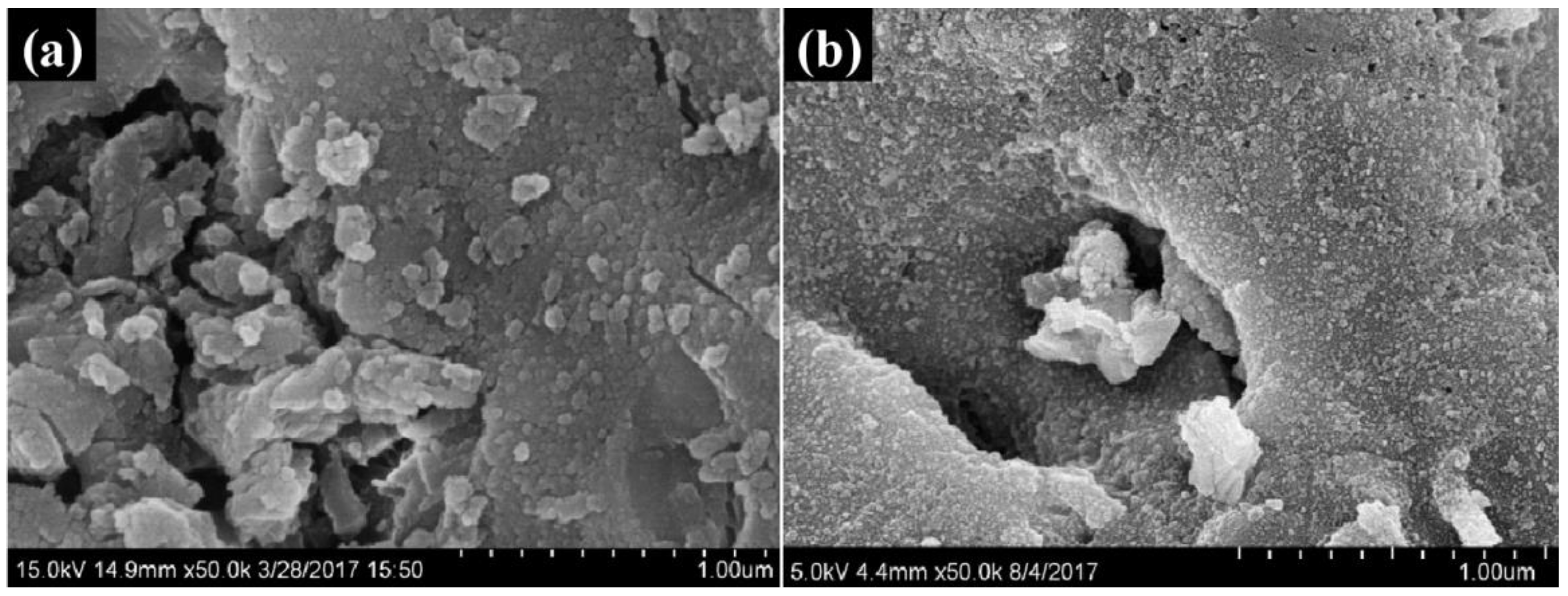
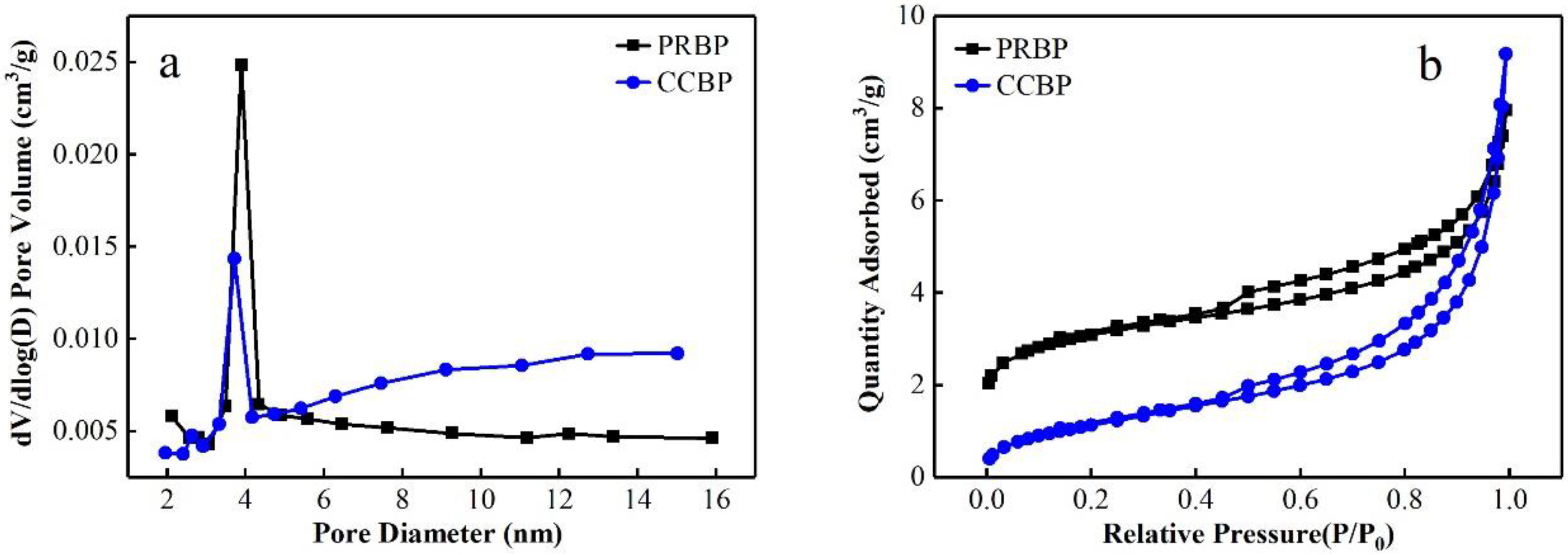
3.1. Characteristics of Waste Bricks
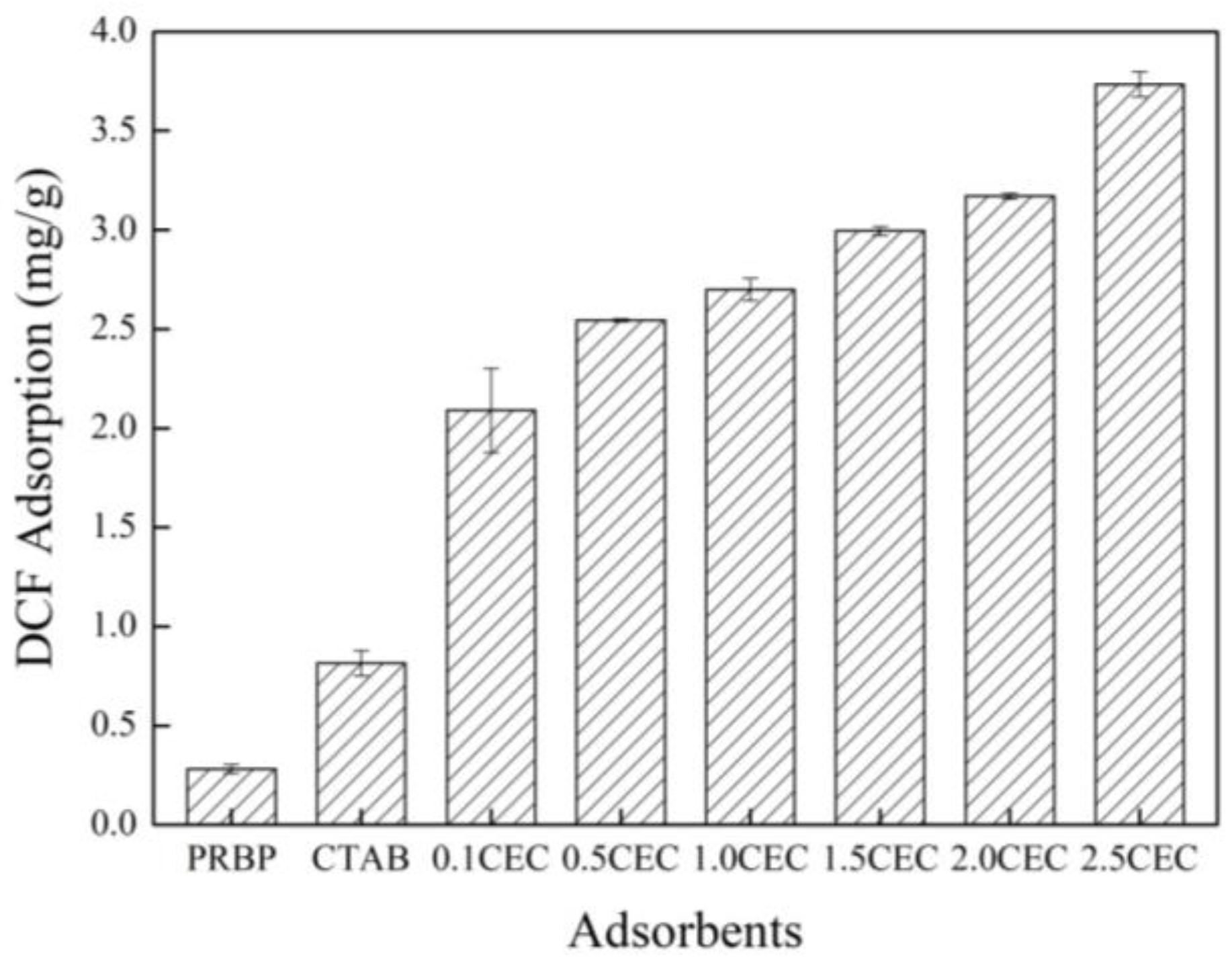
3.2. Adsorption Performance of Adsorbents
3.3. Effect of pH on the Adsorption of DCF
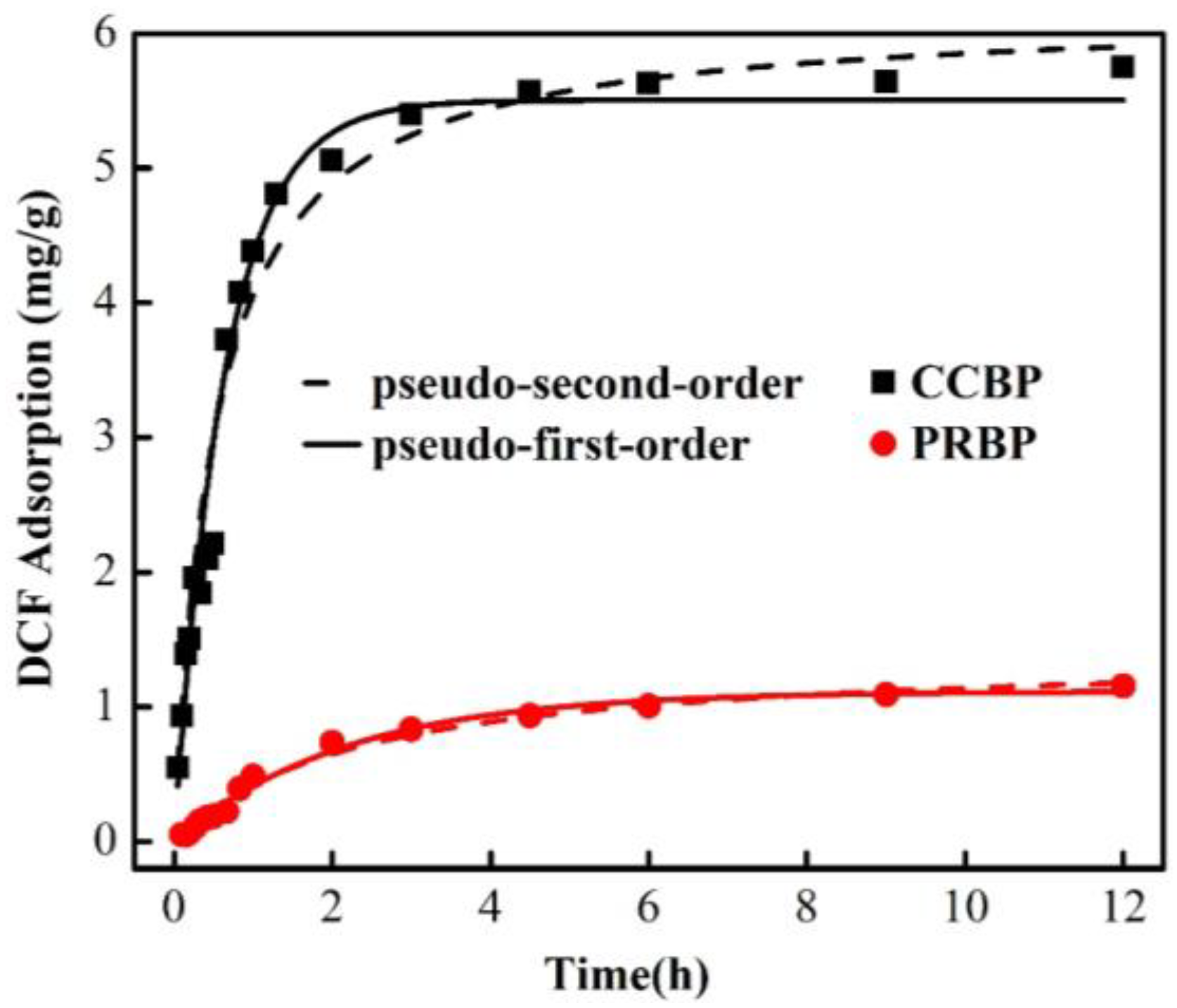
3.4. Adsorption Kinetics
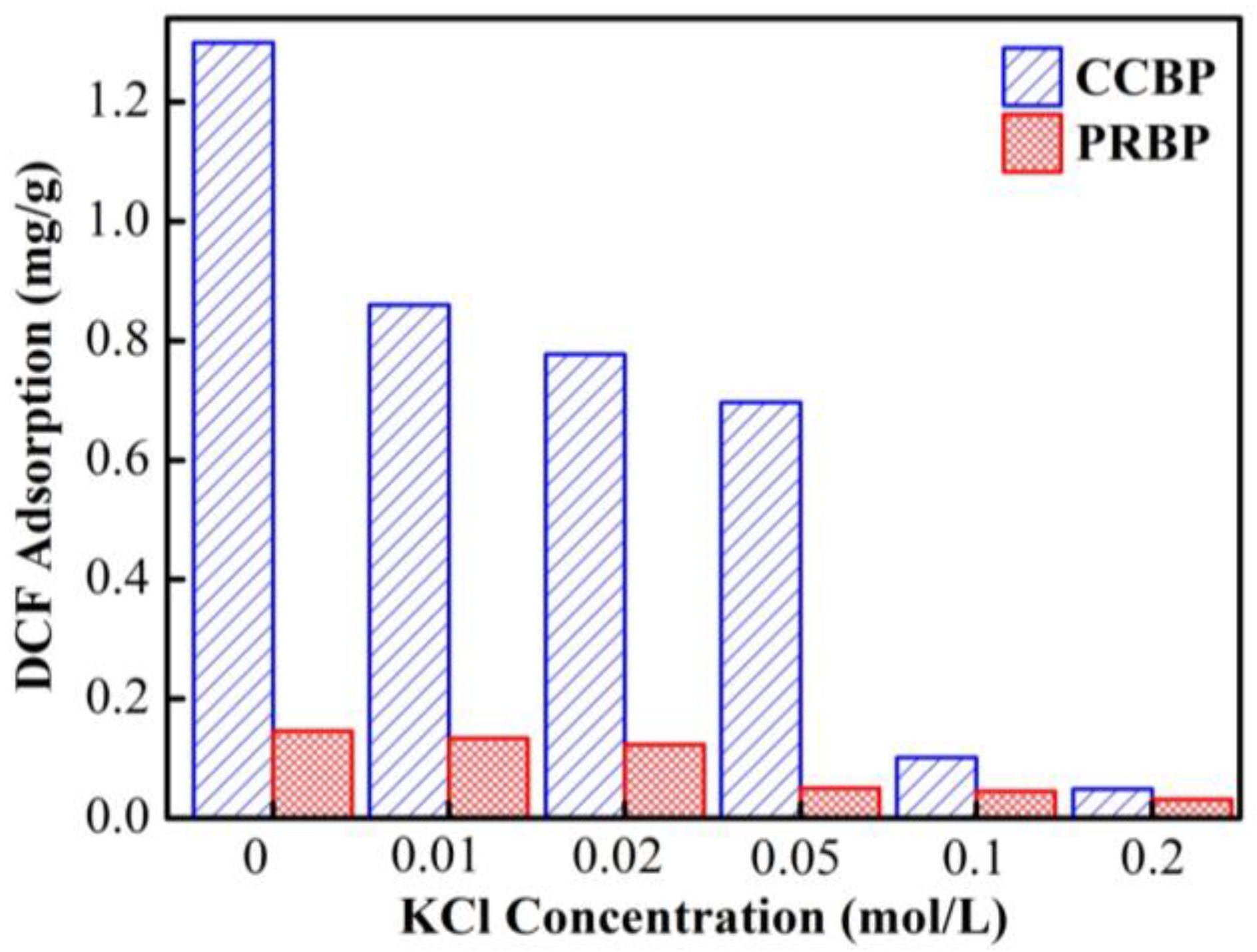
3.5. Effect of Ionic Strength
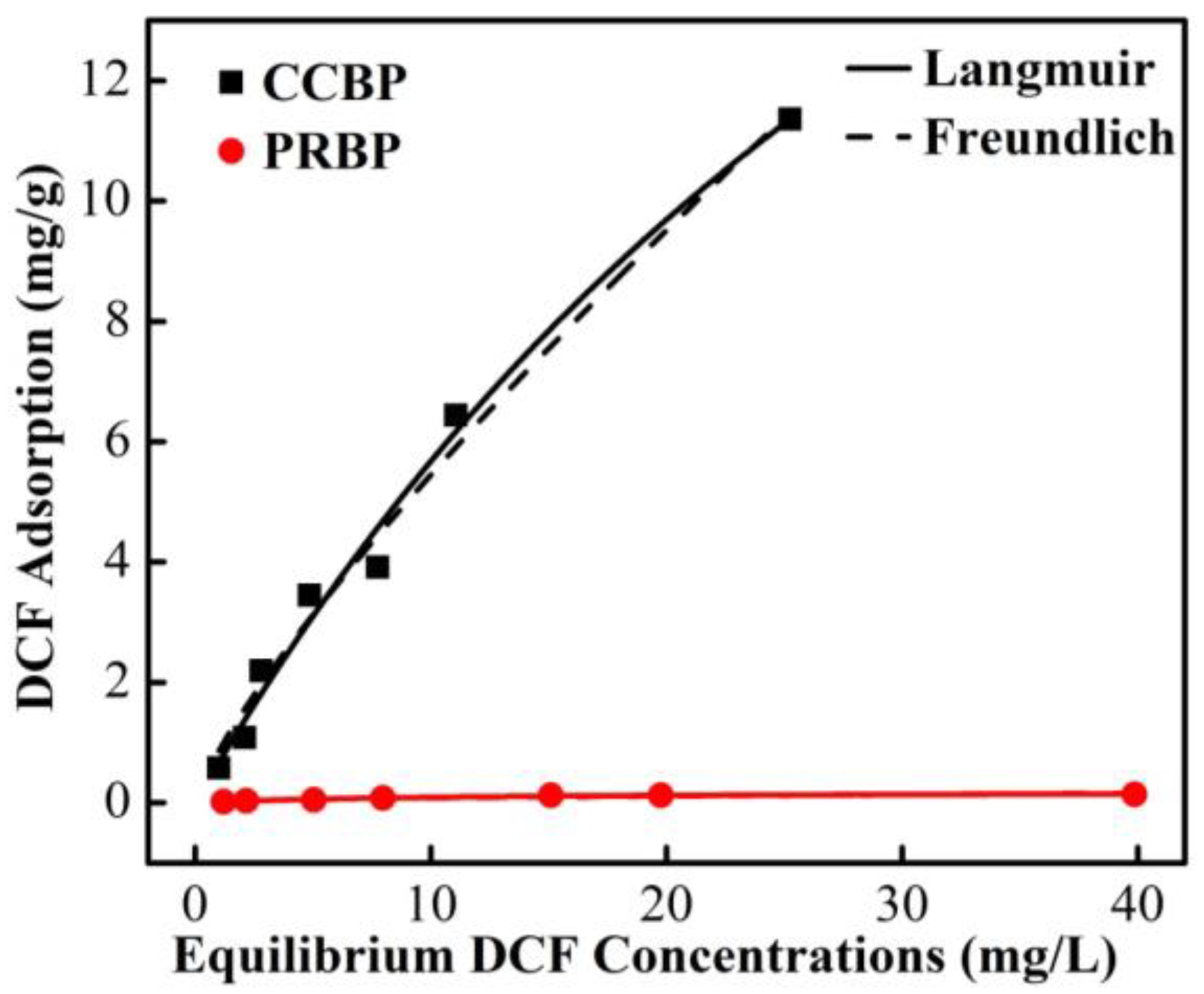
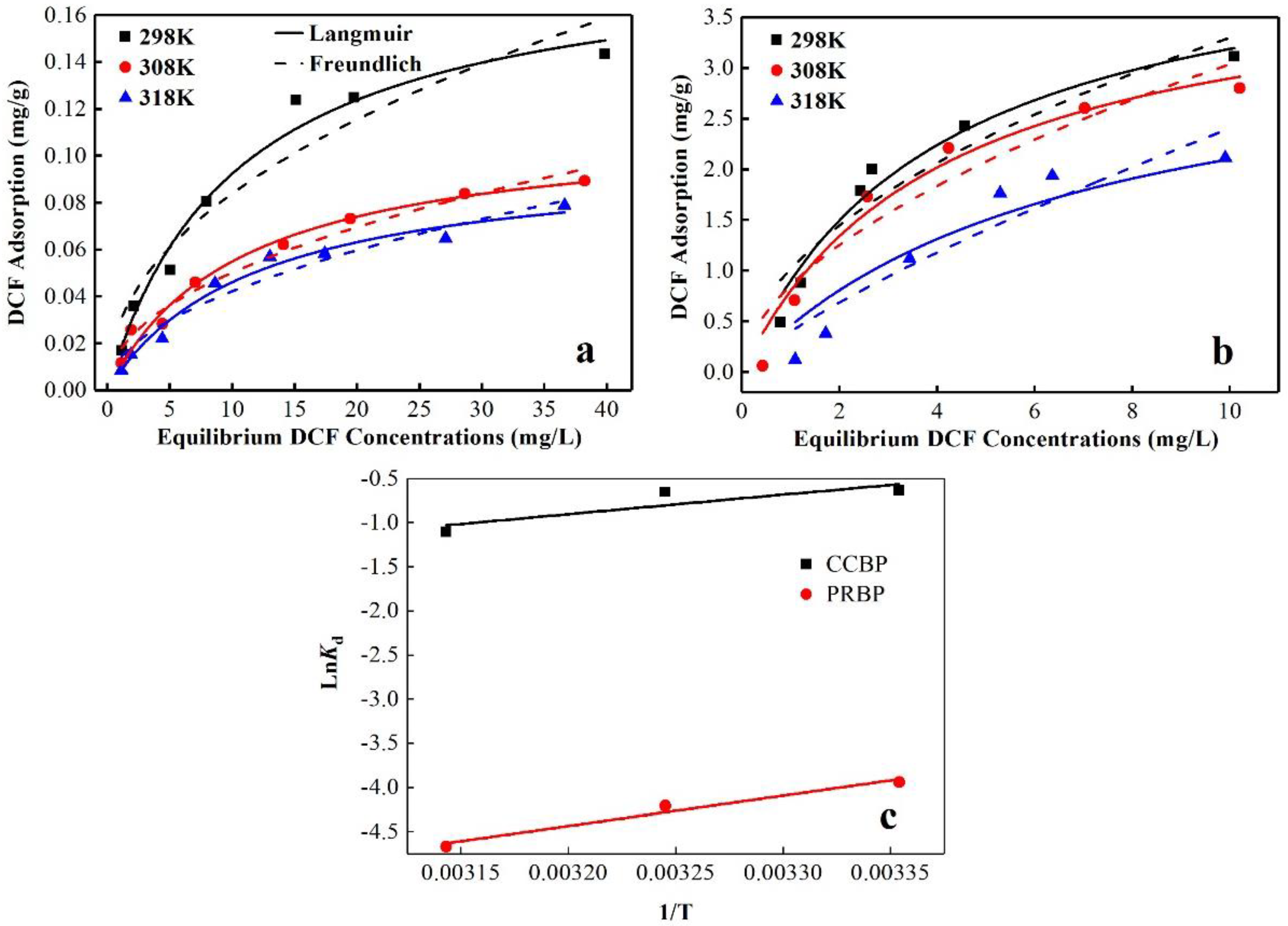
3.6. Adsorption Isotherms
3.7. Adsorption Thermodynamics
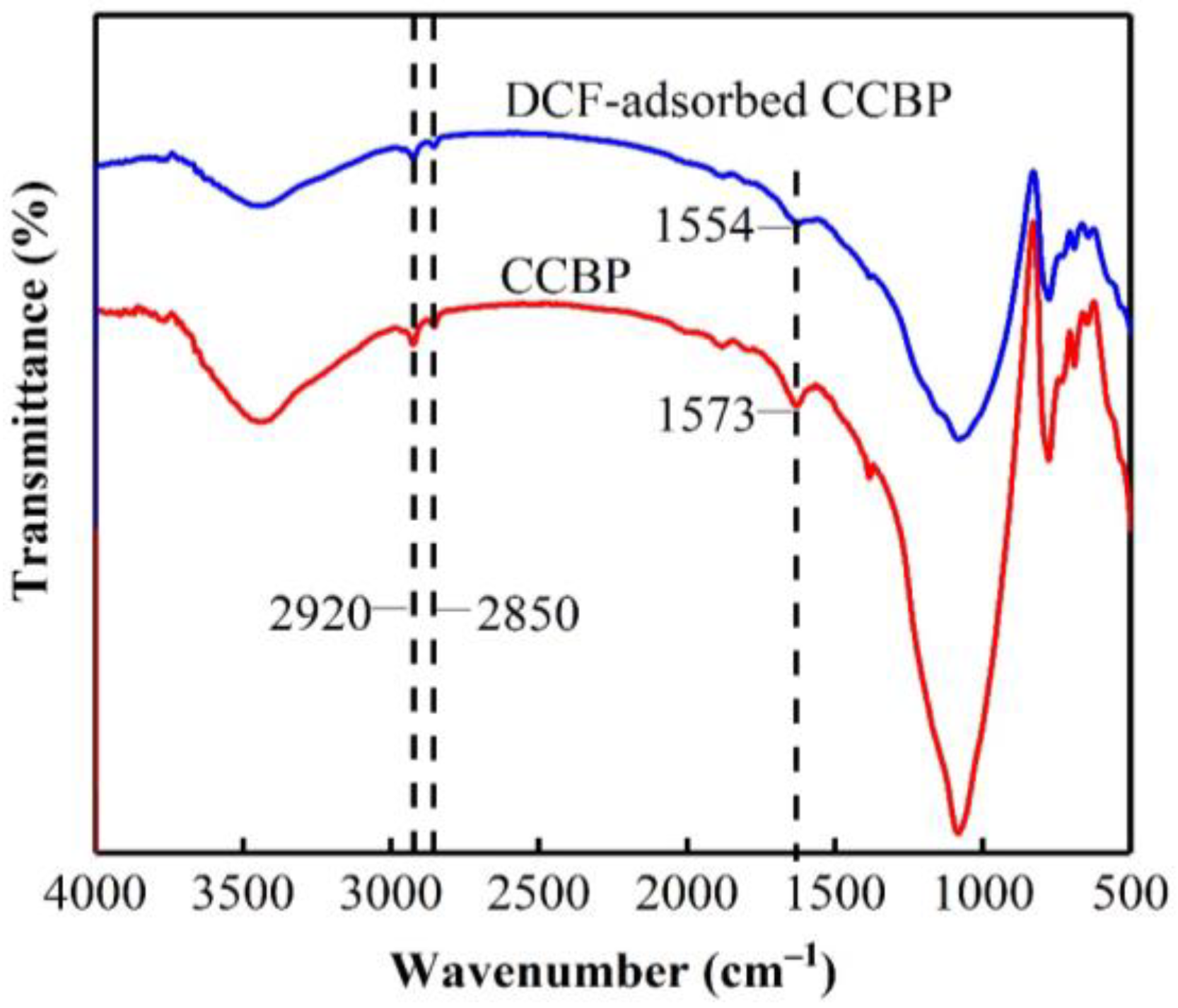
3.8. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, H.J.; Bhadra, B.N.; Khan, N.A.; Jhung, S.H. Adsorptive removal of wide range of pharmaceutical and personal care products from water by using metal azolate framework-6-derived porous carbon. Chem. Eng. J. 2018, 343, 447–454. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Spaniol, O.; Bergheim, M.; Dawick, J.; Kötter, D.; McDonough, K.; Schowanek, D.; Stanton, K.; Wheeler, J.; Willing, A. Comparing the European Union System for the Evaluation of Substances (EUSES) environmental exposure calculations with monitoring data for alkyl sulphate surfactants. Environ. Sci. Eur. 2021, 33, 3. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Yuan, S.; Sun, J.; Zheng, S. Effect of resin charged functional group, porosity, and chemical matrix on the long-term pharmaceutical removal mechanism by conventional ion exchange resins. Chemosphere 2016, 160, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.A.; Sorkin, E.M. Diclofenac sodium: A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1988, 35, 244–285. [Google Scholar] [CrossRef]
- Bariguian Revel, F.; Fayet, M.; Hagen, M. Topical diclofenac, an efficacious treatment for osteoarthritis: A narrative review. Rheumatol. Ther. 2020, 7, 217–236. [Google Scholar] [CrossRef]
- Bickley, L.K.; van Aerle, R.; Brown, A.R.; Hargreaves, A.; Huby, R.; Cammack, V.; Jackson, R.; Santos, E.M.; Tyler, C.R. Bioavailability and kidney responses to diclofenac in the fathead minnow (Pimephales promelas). Environ. Sci. Technol. 2017, 51, 1764–1774. [Google Scholar] [CrossRef]
- Donati, M.; Conforti, A.; Lenti, M.C.; Capuano, A.; Bortolami, O.; Motola, D.; Moretti, U.; Vannacci, A.; Rafaniello, C.; Vaccheri, A. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: Data from drug-induced liver injury case–control study in Italy. Br. J. Clin. Pharmacol. 2016, 82, 238–248. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.; Ahmed, S.; Iqbal Chaudhry, M.J.; Arshad, M. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Hena, S.; Gutierrez, L.; Croué, J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef] [PubMed]
- Schröder, P.; Helmreich, B.; Škrbić, B.; Carballa, M.; Papa, M.; Pastore, C.; Emre, Z.; Oehmen, A.; Langenhoff, A.; Molinos, M. Status of hormones and painkillers in wastewater effluents across several European states—Considerations for the EU watch list concerning estradiols and diclofenac. Environ. Sci. Pollut. Res. 2016, 23, 12835–12866. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Guo, H.; Li, D.; Li, Z.; Lin, S.; Wang, Y.; Pan, S.; Han, J. Promoted elimination of antibiotic sulfamethoxazole in water using sodium percarbonate activated by ozone: Mechanism, degradation pathway and toxicity assessment. Sep. Purif. Technol. 2021, 266, 118543. [Google Scholar] [CrossRef]
- Ali, M.; Song, X.; Ding, D.; Wang, Q.; Zhang, Z.; Tang, Z. Bioremediation of PAHs and heavy metals co-contaminated soils: Challenges and enhancement strategies. Environ. Pollut. 2022, 295, 118686. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Varsha, M.; Kumar, P.S.; Rathi, B.S. A review on recent trends in the removal of emerging contaminants from aquatic environment using low-cost adsorbents. Chemosphere 2022, 287, 132270. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Lu, J.; Zhou, Y.; Wang, Z.; Zhou, Y. Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review. Chin. Chem. Lett. 2022, 33, 3585–3593. [Google Scholar] [CrossRef]
- Guo, D.; You, S.; Li, F.; Liu, Y. Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation: A review. Chin. Chem. Lett. 2022, 33, 1–10. [Google Scholar] [CrossRef]
- Diotti, A.; Perèz Galvin, A.; Piccinali, A.; Plizzari, G.; Sorlini, S. Chemical and leaching behavior of construction and demolition wastes and recycled aggregates. Sustainability 2020, 12, 10326. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Yang, L.; Huang, T. Adsorption characteristics of construction waste for heavy metals from urban stormwater runoff. Chin. J. Chem. Eng. 2015, 23, 1542–1550. [Google Scholar] [CrossRef]
- Gorsky, A.; Racanelli, G.; Belvin, A.; Chambers, R. Greenhouse gas flux from stormwater ponds in southeastern Virginia (USA). Anthropocene 2019, 28, 100218. [Google Scholar] [CrossRef]
- Shamsudin, M.S.; Azha, S.F.; Ismail, S. A review of diclofenac occurrences, toxicology, and potential adsorption of clay-based materials with surfactant modifier. J. Environ. Chem. Eng. 2022, 10, 107541. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Q.; Zhu, J.; Xi, Y.; He, H.; Zhu, R.; Tao, Q.; Ayoko, G.A. Adsorption of phenol and Cu (II) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems. Chem. Eng. J. 2016, 283, 880–888. [Google Scholar] [CrossRef]
- Sun, K.; Shi, Y.; Wang, X.; Li, Z. Sorption and retention of diclofenac on zeolite in the presence of cationic surfactant. J. Hazard. Mater. 2017, 323, 584–592. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Bilal, M.; Hussain, T.; Sher, F.; Rizwan, K. Surfactants-based remediation as an effective approach for removal of environmental pollutants—A review. J. Mol. Liq. 2020, 318, 113960. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Mittal, A.; Usman, M.; Mittal, J.; Yu, G.; Núñez-Delgado, A.; Kornaros, M. A review on halloysite-based adsorbents to remove pollutants in water and wastewater. J. Mol. Liq. 2018, 269, 855–868. [Google Scholar] [CrossRef]
- Tabrizi, S.H.; Tanhaei, B.; Ayati, A.; Ranjbari, S. Substantial improvement in the adsorption behavior of montmorillonite toward Tartrazine through hexadecylamine impregnation. Environ. Res. 2022, 204, 111965. [Google Scholar] [CrossRef]
- Wang, L.-C.; Ni, X.-j.; Cao, Y.-H.; Cao, G.-q. Adsorption behavior of bisphenol A on CTAB-modified graphite. Appl. Surf. Sci. 2018, 428, 165–170. [Google Scholar] [CrossRef]
- Zawrah, M.; Khattab, R.; Saad, E.; Gado, R. Effect of surfactant types and their concentration on the structural characteristics of nanoclay. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Yuan, B.; Shen, C.; Fu, M.; Cui, H.; Sun, W. Large scale preparation of Cu-doped α-FeOOH nanoflowers and their photo-Fenton-like catalytic degradation of diclofenac sodium. Chem. Eng. J. 2016, 291, 174–183. [Google Scholar] [CrossRef]
- Jia, J.; Qin, F.; Guo, J.; Liu, Z.; Zhao, Q.; Guan, W. FeOOH coupled Bi2WO6 for efficient photocatalysis-Fenton synergistic degradation of organic pollutants. Mater. Lett. 2023, 336, 133862. [Google Scholar] [CrossRef]
- Labidi, N. Removal of mercury from aqueous solutions by waste brick. Int. J. Environ. Res. 2008, 2, 275–278. [Google Scholar]
- Zheng, H.; Gao, Y.; Zhu, K.; Wang, Q.; Wakeel, M.; Wahid, A.; Alharbi, N.S.; Chen, C. Investigation of the adsorption mechanisms of Pb (II) and 1-naphthol by β-cyclodextrin modified graphene oxide nanosheets from aqueous solution. J. Colloid Interface Sci. 2018, 530, 154–162. [Google Scholar] [CrossRef]
- Oki, J.; Watanabe, D.; Uekusa, T.; Sugano, K. Mechanism of supersaturation suppression in dissolution process of acidic drug salt. Mol. Pharm. 2019, 16, 1669–1677. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wei, J. Fabrication of poly (β-cyclodextrin)-conjugated magnetic graphene oxide by surface-initiated RAFT polymerization for synergetic adsorption of heavy metal ions and organic pollutants. J. Mater. Chem. A 2019, 7, 2055–2065. [Google Scholar] [CrossRef]
- Jeirani, Z.; Niu, C.H.; Soltan, J. Adsorption of emerging pollutants on activated carbon. Rev. Chem. Eng. 2017, 33, 491–522. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, L.; Zhuang, S. Sorption of polycyclic aromatic hydrocarbons to soils enhanced by heavy metals: Perspective of molecular interactions. J. Soils Sediments 2016, 16, 1509–1518. [Google Scholar] [CrossRef]
- Bernardo, M.; Rodrigues, S.; Lapa, N.; Matos, I.; Lemos, F.; Batista, M.; Carvalho, A.; Fonseca, I. High efficacy on diclofenac removal by activated carbon produced from potato peel waste. Int. J. Environ. Sci. Technol. 2016, 13, 1989–2000. [Google Scholar] [CrossRef]
- Shu, J.; Wang, Z.; Huang, Y.; Huang, N.; Ren, C.; Zhang, W. Adsorption removal of Congo red from aqueous solution by polyhedral Cu2O nanoparticles: Kinetics, isotherms, thermodynamics and mechanism analysis. J. Alloys Compd. 2015, 633, 338–346. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Xu, Z.; Zhang, L.; Cheng, B.; Ho, W. Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr (VI) ions. Appl. Surf. Sci. 2019, 478, 981–990. [Google Scholar] [CrossRef]
- Kaur, H.; Bansiwal, A.; Hippargi, G.; Pophali, G.R. Effect of hydrophobicity of pharmaceuticals and personal care products for adsorption on activated carbon: Adsorption isotherms, kinetics and mechanism. Environ. Sci. Pollut. Res. 2018, 25, 20473–20485. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xie, J.; Zhang, M.; Zhou, Q.; Liu, F. Insight into the adsorption of PPCPs by porous adsorbents: Effect of the properties of adsorbents and adsorbates. Environ. Pollut. 2016, 214, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhai, Y.; Chen, T.; Yu, L.; Wang, H. Synergistic and antagonistic effects on removal of diclofenac and cadmium onto hydrous manganese dioxide. Nanosci. Nanotechnol. Lett. 2016, 8, 985–992. [Google Scholar] [CrossRef]




| Name | Molecular Mass (g/mol) | Water Solubility (mg/L) | Structure |
|---|---|---|---|
| CTAB | 364.45 | 13 (293 K) |  |
| DCF | 296.15 | 50 (293 K) |  |
| Adsorbents | BET Surface Area (m²/g) | BJH Pore Volume (cm³/g) | BJH Pore Size (nm) |
|---|---|---|---|
| PRBP | 10.42 | 0.0059 | 4.51 |
| CCBP | 4.33 | 0.0068 | 5.16 |
| Adsorbents | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| K1 (mg/h) | qe (mg/g) | R2 | K2 (mg/h) | qe (mg/g) | R2 | |
| PRBP | 0.46 | 1.12 | 0.99 | 0.30 | 1.40 | 0.99 |
| CCBP | 1.56 | 5.51 | 0.93 | 0.31 | 6.12 | 0.96 |
| Adsorbents | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| B (L/mg) | qmax (mg/g) | R2 | Kf (mg/g) | n | R2 | |
| PRBP | 0.096 | 0.19 | 0.97 | 0.029 | 2.17 | 0.90 |
| CCBP | 0.021 | 33.0 | 0.99 | 0.86 | 1.25 | 0.98 |
| Adsorbents | T (K) | Kd (L/mol) | ΔH (kJ/mol) | ΔS (J/mol·K) | ΔG (kJ/mol) |
|---|---|---|---|---|---|
| PRBPs | 298 | 0.019 | −28.64 | 112.37 | −4.86 |
| 308 | 0.015 | −4.67 | |||
| 318 | 0.0094 | −3.41 | |||
| CCBPs | 298 | 0.53 | −18.49 | 80.06 | −5.38 |
| 308 | 0.52 | −5.46 | |||
| 318 | 0.33 | −5.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Jin, X.; Chen, H.; Zhang, X.; Tan, C.; Bai, X.; Gong, Y. Surfactant-Modified Construction Waste Bricks for the Removal of Diclofenac from Aqueous Solutions. Appl. Sci. 2023, 13, 6306. https://doi.org/10.3390/app13106306
Zhang Z, Jin X, Chen H, Zhang X, Tan C, Bai X, Gong Y. Surfactant-Modified Construction Waste Bricks for the Removal of Diclofenac from Aqueous Solutions. Applied Sciences. 2023; 13(10):6306. https://doi.org/10.3390/app13106306
Chicago/Turabian StyleZhang, Ziyang, Xiao Jin, Hongrui Chen, Xiaoran Zhang, Chaohong Tan, Xiaojuan Bai, and Yongwei Gong. 2023. "Surfactant-Modified Construction Waste Bricks for the Removal of Diclofenac from Aqueous Solutions" Applied Sciences 13, no. 10: 6306. https://doi.org/10.3390/app13106306





