Amino-Acid-Derived Oxazolidin-5-Ones as Chemical Markers for Schiff Base Formation in Glycation Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Spiking Experiments with Selected Strecker Aldehydes
2.4. ESI/qTOF/MS Analysis
2.5. Structural Elucidation and Isotope Labeling Studies
2.6. Criteria Applied for Tentative Identification of the Listed Ions
3. Results and Discussion
3.1. Detection of Schiff Bases of Alanine with Sugar-Derived Aldehydes through Isotope Labeling
3.2. Detection of Iron Complexes
3.3. Spiking Experiments with Simple Aldehydes
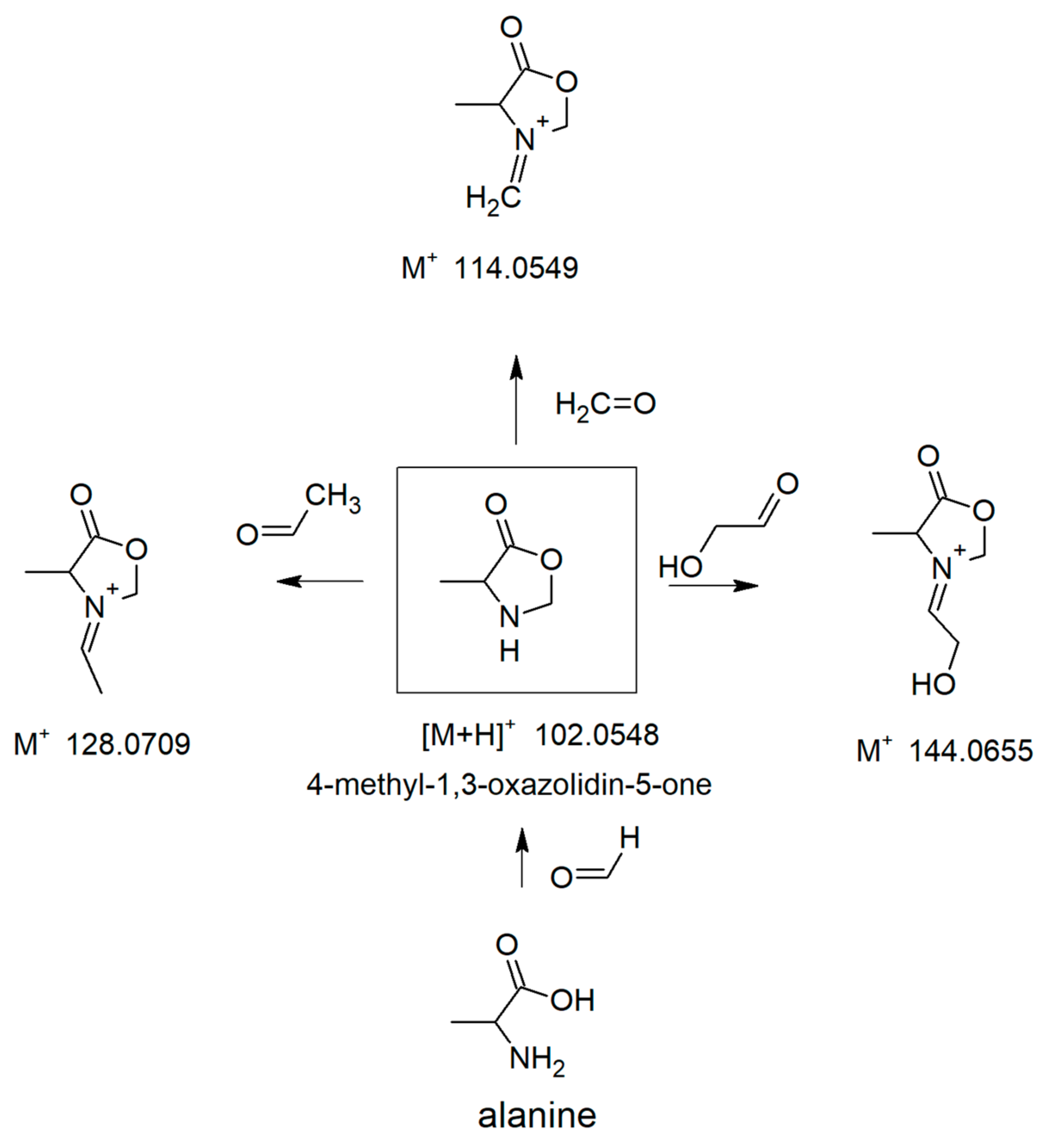
3.4. Further Reactions of Oxazolidin-5-Ones with Aldehydes
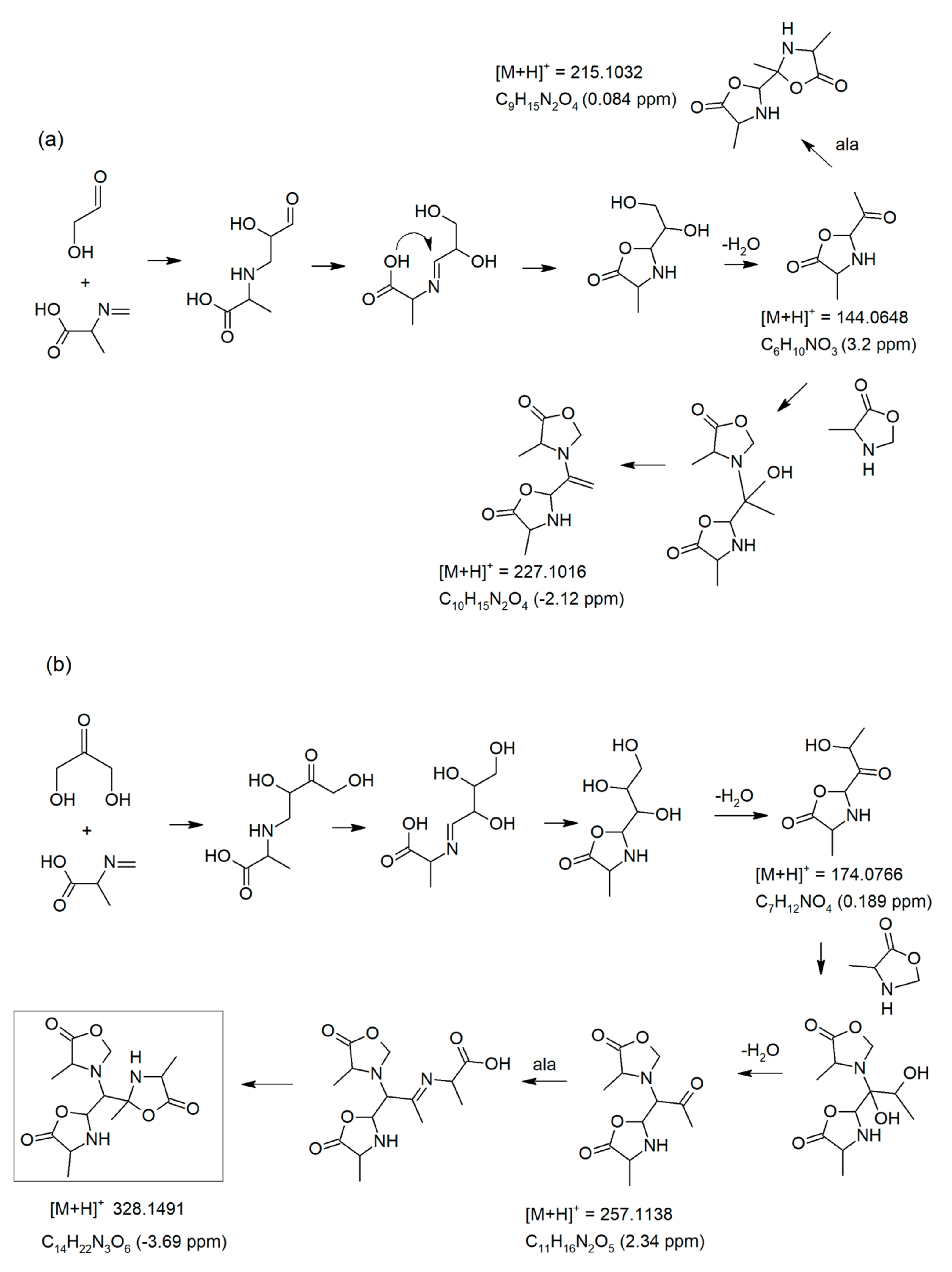
3.5. Formation of Oxazolidin-5-One Oligomers
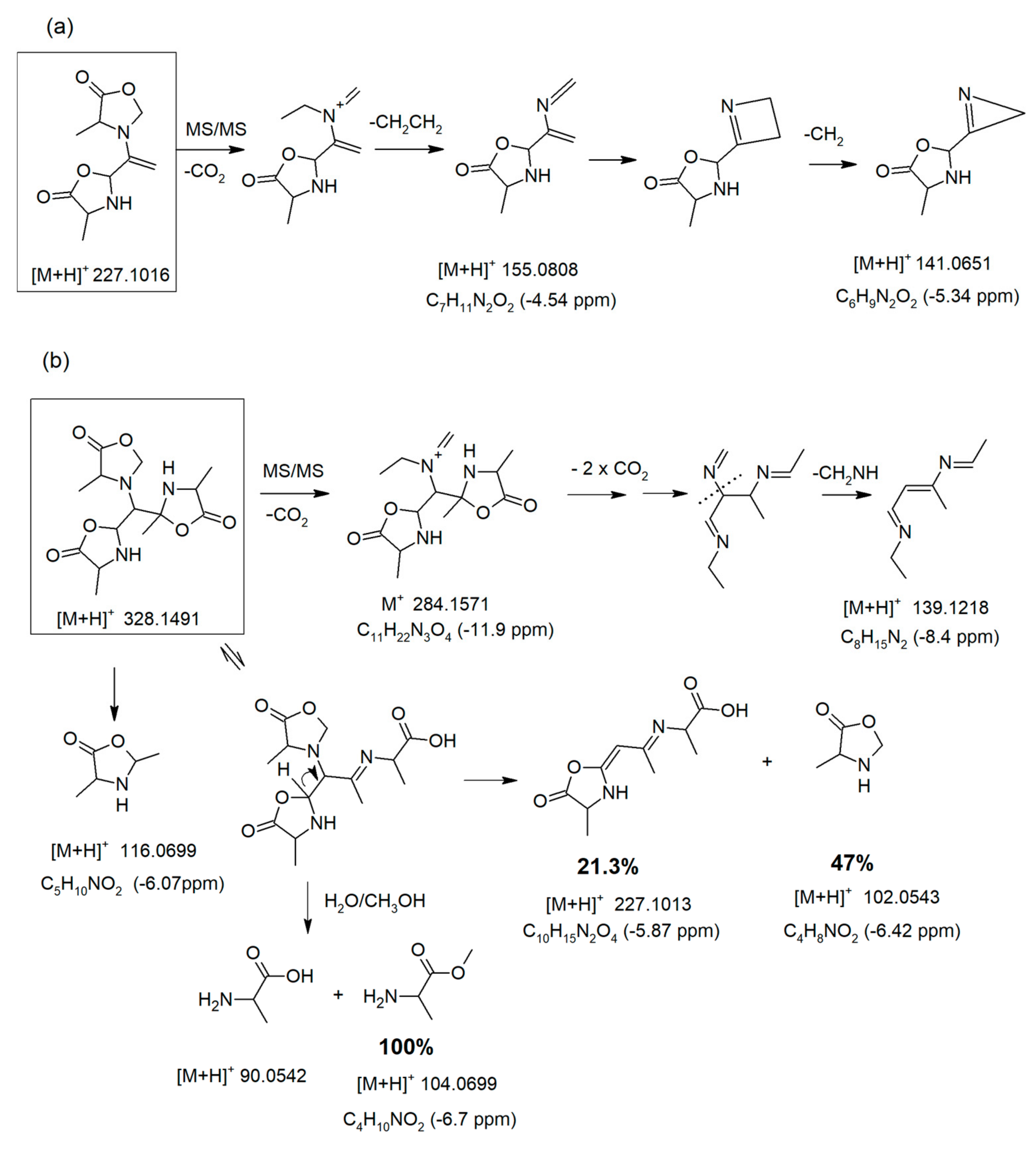
3.6. Generation of Dimeric and Trimeric Oxazolidin-5-Ones Observed at [M + H]+ 227.1016 and 328.1491
3.7. MS/MS Fragmentations of Ions at m/z 227 and 328 under 10eV Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hellwig, M.; Henle, T. Baking, Ageing, Diabetes: A Short History of the Maillard Reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef] [PubMed]
- Mortzfeld, F.B.; Hashem, C.; Vranková, K.; Winkler, M.; Rudroff, F. Pyrazines: Synthesis and Industrial Application of these Valuable Flavour and Frarance Compounds. Biotechnol. J. 2020, 15, 2000064. [Google Scholar] [CrossRef]
- Frank, O.; Hofmann, T. Characterization of Key Chromospheres Formed by Nonenzymatic Browning of Hexoses and L-Alanine by using the Colour Activity Concept. J. Agric. Food Chem. 2000, 48, 6303–6311. [Google Scholar] [CrossRef] [PubMed]
- Yaylayan, V. Acrylamide formation and its impact on the mechanism of the early Maillard reaction. J. Food Nutr. Res. 2009, 48, 1–7. [Google Scholar]
- Hofmann, T. Application of Site Specific 13C Enrichment and 13C NMR Spectroscopy for the Elucidation of the Formation Pathway Leading to a Red 1H-Pyrrol-3(2H)-one during the Maillard reaction of Furna-2-carboxaldehyde and L-Alanine. J. Agric. Food Chem. 1998, 46, 941–945. [Google Scholar] [CrossRef]
- Rizzi, G.P. Chemical structure of coloured Maillard reaction products. Food Rev. Int. 1997, 13, 1–28. [Google Scholar] [CrossRef]
- RajanBabu, T.V. Stereochemistry of intramolecular free-radical cyclization reaction. Accounts Chem. Res. 1991, 24, 139–145. [Google Scholar] [CrossRef]
- Chu, F.L.; Yaylayan, V.A. Post-schiff chemistry of the Maillard reaction. Ann. N. Y. Acad. Sci. 2008, 1126, 30–37. [Google Scholar] [CrossRef]
- Chu, F.L.; Yaylayan, V.A. FTIR monitoring of oxazolidinone-5-one formation and decomposition in a glycolaldehyde-phenylalanine model system by isotope labelling techniques. Carbohydr. Res. 2009, 344, 229–236. [Google Scholar] [CrossRef]
- Xing, H.; Yaylayan, V. Insight into the mechnochemistry of the Maillard reaction: Degradation of Schiff bases via 5-oxazolidinone intermediate. Eur. Food Res. Technol. 2021, 247, 1095–1106. [Google Scholar] [CrossRef]
- Manini, P.; D’Ischi, M.; Prota, G. An Unusual Decarboxylative Maillard Reaction between l-DOPA and d-Glucose under Biomimetic Conditions: Factors Governing Competition with Pictet−Spengler Condensation. J. Org. Chem. 2001, 66, 5048–5053. [Google Scholar] [CrossRef]
- Manini, P.; Napolitano, A.; D’ischia, M. Reactions of d-glucose with phenolic amino acids: Further insights into the competition between Maillard and Pictet–Spengler condensation pathways. Carbohydr. Res. 2005, 340, 2719–2727. [Google Scholar] [CrossRef]
- Shimasaki, C.; Hirata, F.; Ohta, H.; Tsukurimichi, E.; Yoshimura, T. Thermal behaior and mass spectrometry studies of 2-oxazolidinone derivatives. J. Anal. Appl. Pyrolysis 1993, 24, 291–300. [Google Scholar] [CrossRef]
- Kim, E.S.; Yaylayan, V. Identification of the Maillard reaction intermediates as divalent iron complexes in alanine/glucose/FeCl2 model system using ESI/qTOF/MS/MS and isotope labelling technique. Curr. Res. Food Sci. 2021, 4, 287–294. [Google Scholar] [CrossRef]
- Patiny, L.; Borel, A. ChemCalc: A Building Block for Tomorrow’s Chemical Infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef]
- Smuda, M.; Glomb, M.A. Fragmentation Pathways during Maillard-Induced Carbohydrate Degradation. J. Agric. Food Chem. 2013, 61, 10198–10208. [Google Scholar] [CrossRef]
- Davídek, T.; Robert, F.; Devaud, S.; Vera, F.A.; Blank, I. Sugar Fragmentation in the Maillard Reaction Cascade: Formation of Short-Chain Carboxylic Acids, by a New Oxidative α-Dicarbonyl Cleavage Pathway. J. Agric. Food Chem. 2006, 54, 6677–6684. [Google Scholar] [CrossRef]
- Davídek, T.; Robert, F.; Devaud, S.; Vera, F.A.; Blank, I. Sugar Fragmentation in the Maillard Reaction Cascade: Isotope Labeling Studies on the Formation of Acetic Acid by a Hydrolytic β-Dicarbonyl Cleavage Mechanism. J. Agric. Food Chem. 2006, 54, 6667–6676. [Google Scholar] [CrossRef]
- Thornalley, P.J. Dicarbonyl Intermediates in the Maillard Reaction. Ann. N. Y. Acad. Sci. 2002, 1043, 111–117. [Google Scholar] [CrossRef]
- Gensberger-Reigl, S.; Glomb, M.A.; Pischetsrieder, M. Analysis of Sugar Degradation Products with α-Dicarbonyl Structure in Carbonated Soft Drinks by UHPLC-DAD-MS/MS. J. Agric. Food Chem. 2013, 61, 10238–10245. [Google Scholar] [CrossRef]
- Breslow, R. On the mechanism of the formose reaction. Tetrahedron Lett. 1959, 1, 22–26. [Google Scholar] [CrossRef]
- Breslow, R.; Appayee, C. Deuterium Studies Reveal a New Mechanism for the Formose Reaction Involvoing Hydride Shifs. J. Am. Chem. Soc. 2014, 136, 3720–3723. [Google Scholar] [CrossRef]
- Usui, T.; Yanagisawa, S.; Ohguchi, M.; Yoshino, M.; Kawabata, R.; Kishimoto, J.; Arai, Y.; Watanabe, H.; Hayase, F. Identification and determination of α-Dicarbonyl Compounds Formed in the Degradation of Sugars. Biosci. Biotechnol. Biochem. 2007, 71, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, O.; Kanemasa, S.; Ohe, M.; Takenaka, S. Simple Generation of Nonstabilized Azomethine Ylides through Decarboxylative Condensation of α-Amino Acids with Carbonyl Compounds via 5-Oxazolidinone Intermediates. Bull. Chem. Soc. Jpn. 1987, 60, 4079–4089. [Google Scholar] [CrossRef]
- Choury, M.; Lopes, A.B.; Blond, G.; Gulea, M. Synthesis of Medium-Sized Heterocycles by Transition-Metal-Catalyzed Intramolecular Cyclization. Molecules 2020, 25, 3147. [Google Scholar] [CrossRef]
- Feather, M.S.; Mossine, V.V. Correlations between Structure and Reactivity of Amadori Compounds: The Reactivity of Acyclic Forms. In The Maillard Reaction in Foods and Medicine; Woodhead Publishing: Cambridge, UK, 2005; pp. 37–42. [Google Scholar] [CrossRef]
- Schmeyers, J.; Toda, F.; Boy, J.; Kaupp, G. Quantitative solid–solid synthesis of azomethines. J. Chem. Soc. Perkin Trans. 1998, 2, 989–994. [Google Scholar] [CrossRef]
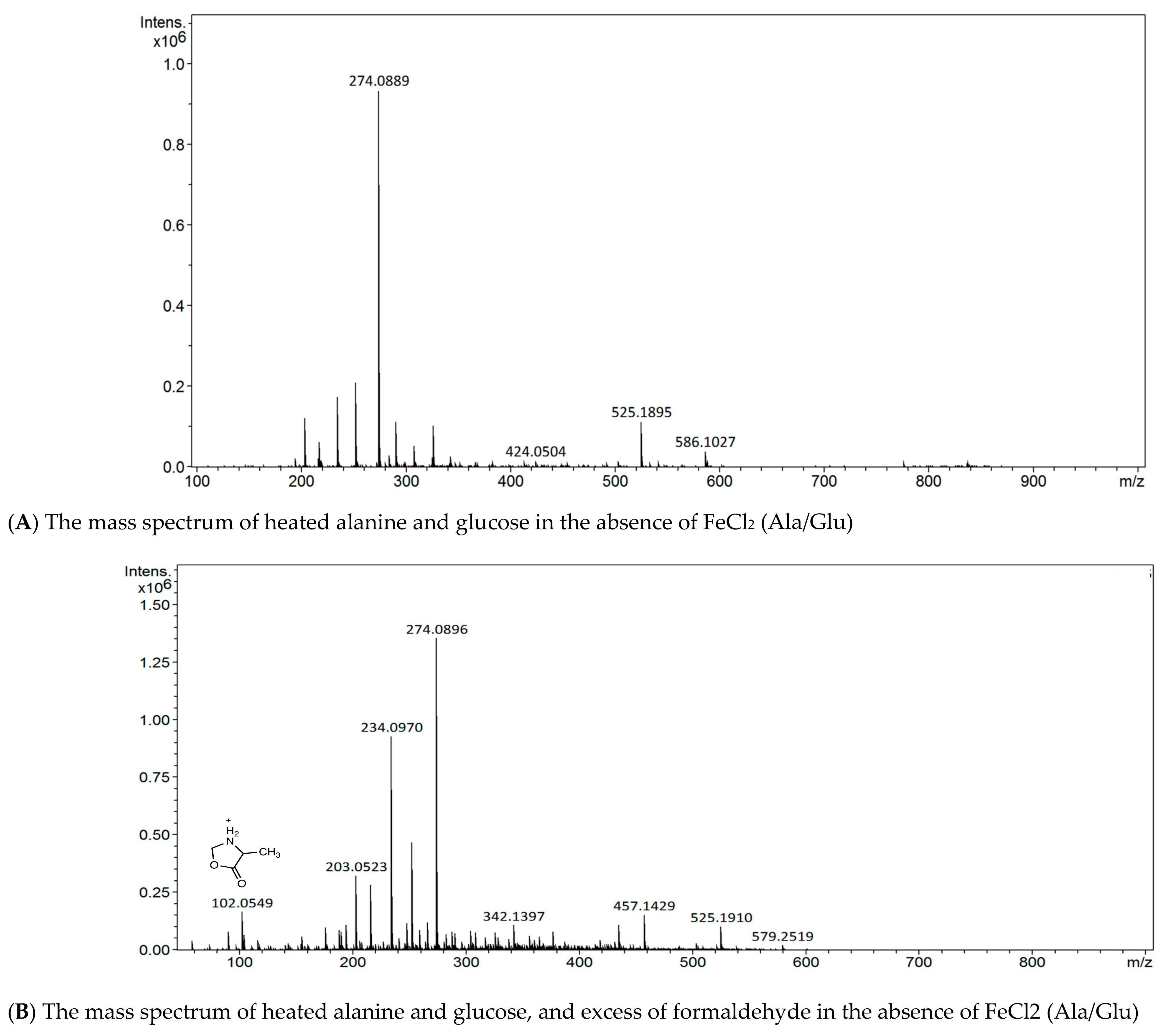
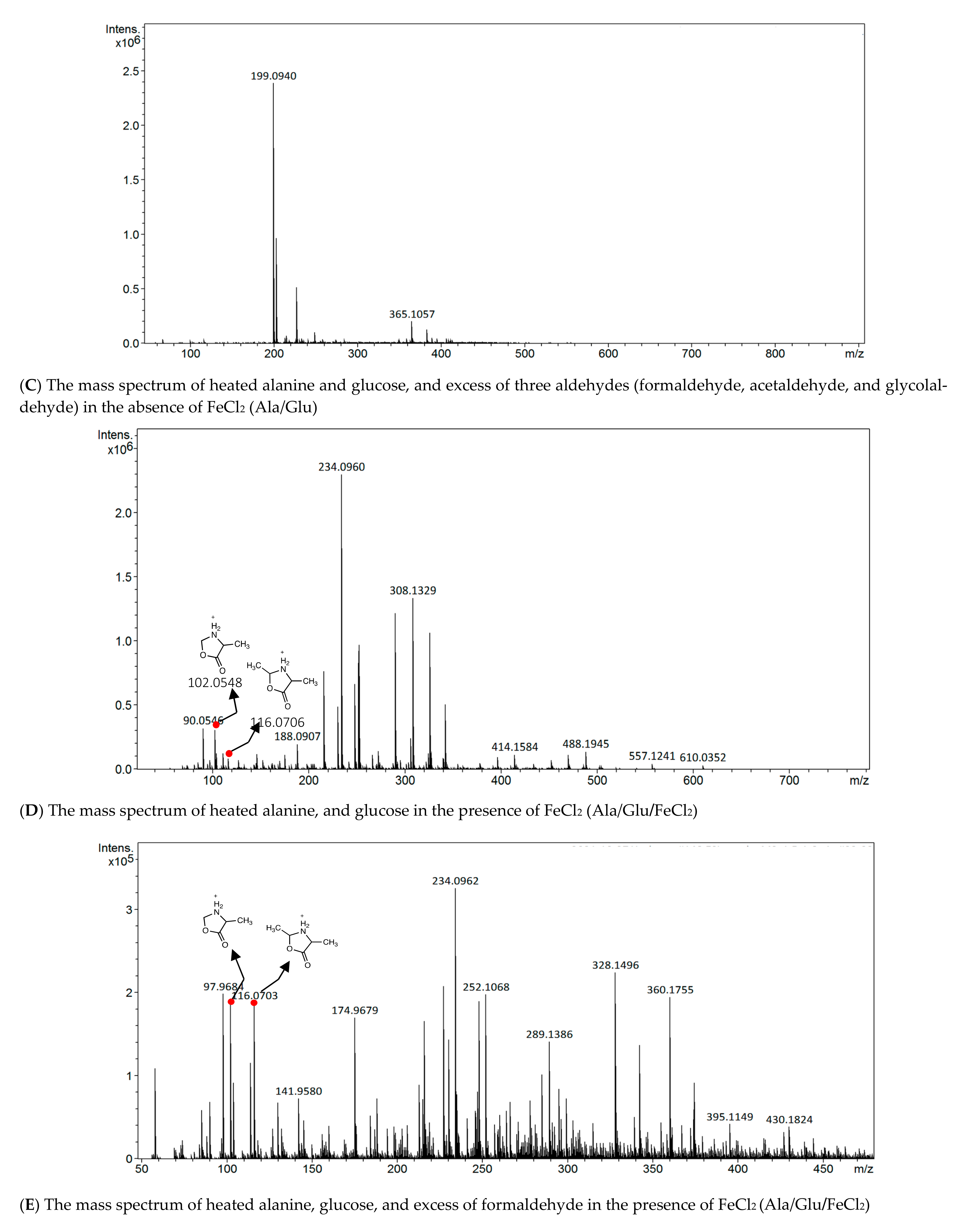


| Model System | |
|---|---|
| Control Model System | Control model systems contained no metal ions—Ala/Glu |
| Spiked Model System | Paraformaldehyde was added to an alanine and glucose and heated in the absence of metal ions—Ala/Glu Three reactive aldehydes (paraformaldehyde, acetaldehyde, and glycolaldehyde) were added to an alanine and glucose solution, followed by heating without FeCl2—Ala/Glu Reactive aldehyde (paraformaldehyde: acetaldehyde: B, and glycolaldehyde: C) were added to an alanine and glucose solution, followed by heating in the presence of FeCl2—Ala/Glu/FeCl2, Ala/Glu/FeCl2, and Ala/Glu/FeCl2 Three reactive aldehydes (paraformaldehyde, acetaldehyde, and glycolaldehyde) were added to an alanine and glucose solution, followed by heated in the presence of FeCl2 |
| Test Model System | Alanine was added to a glucose solution and heated in the presence of FeCl2—Ala/Glu/FeCl2 |
| Isotope-Labeled Model System | Alanine [13C-3] was added to a glucose solution and heated in the presence of FeCl2-[13C-3] Ala/Glu/FeCl2 |
| Alanine was added to a glucose [13C-U] solution and heated in the presence of FeCl2—Ala/[13C-U] Glu/FeCl2 | |
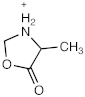 [M + H]+ = 102.0548 C4H8NO2 (−1.52 ppm) | 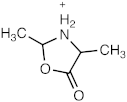 [M + H]+ = 116.0706 C5H10NO2 (−0.04 ppm) | 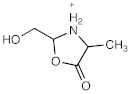 [M + H]+ = 132.0656 C5H10NO3 (0.61 ppm) | |
|---|---|---|---|
| Label incorporation data | |||
| Glu [13C-U]/Ala/FeCl2 | C3[13C]H8NO2 (−3.01 ppm) | C3[13C]2H10NO2 (−0.97 ppm) | C3[13C]2H10NO3 (0.96 ppm) |
| Ala [13C-3]/Glu/FeCl2 | C3[13C]H8NO2 (−2.04 ppm) | C4[13C]H10NO2 (−3.07ppm) | C4[13C]H9NaNO3 (−3.35 ppm) |
| % intensities of the detected ions in the presence FeCl2 and spiking with formaldehyde | |||
| Ala/Glu/MeOH | 0.6 | Not detected | Not detected |
| Ala/Glu/FeCl2/MeOH | 14.5 ± 0.98 | 6.43 ± 0.32 | 0.83 ± 0.12 |
| Ala/Glu/FeCl2/H2O | 0.0 | 0.0 | 0.0 |
| Ala/Glu/FeCl2/CH2O/ | 62.35 ± 7.42 | 62.1 ± 6.36 | 3.5 ± 0.28 |
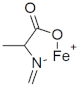 M+ = 155.9738 C4H6FeNO2 (−2.83 ppm) |  M+ = 169.9895 C5H8 FeNO2 (−2.3 ppm) | 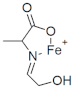 M+ = 185.9848 C5H8FeNO3 (−0.03 ppm) | |
|---|---|---|---|
| Label incorporation data | |||
| Glu [13C-U]/Ala | C3[13C]H6FeNO2 156.9765 (−6.98 ppm) C4H6FeNO2 155.9742 (−0.26 ppm) | C3[13C]2H8FeNO2 171.99 (nd) 170.99 (nd) | C3[13C]2H8FeNO3 187.9913 (−1.15 ppm) |
| Ala [13C-3]/Glu | C3[13C]H6FeNO2 156.9731 (−28 ppm) | C4[13C]H8FeNO2 170.9924 (−4.95 ppm) C3[13C]2H8FeNO2 171.99 (nd) | C4[13C]H8 FeNO3 186.98 (nd) |
 M+ = 114.0549 C5H8NO2 (−0.48 ppm) | 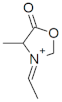 M+ = 128.0709 C6H10NO2 (1.98 ppm) | 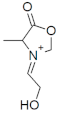 M+ = 144.0655 C6H10NO3 (−0.14 ppm) | |
|---|---|---|---|
| Label incorporation data | |||
| Glu [13C-U]/Ala/FeCl2 | C3[13C]2H8NO2 (nd) C4[13C]1H8NO2 (nd) C5H8NO2 (−5.74 ppm) | C3[13C]3H10NO2 131.0778 (21ppm) C4[13C]2H10NO2 (nd) | C3[13C]3H10NO3 147 (2.15 ppm) C4[13C]2H10NO3 |
| Ala [13C-3]/Glu/FeCl2 | C4[13C]H8NO2 115.0573 (−8.78pm) | C4[13C]2H10 NO2 (nd) C5[13C]1H10 NO2 (nd) C3[13C]3H10 NO2 | C5[13C]H9NO3 145 (−5.22 ppm) |
| [M + X]+ | Elemental Composition | Error ppm | Glu [13C-U] | Elemental Composition | Error ppm | Ala [13C-3] | Elemental Composition | Error ppm |
|---|---|---|---|---|---|---|---|---|
| 102.0548 | C4H8NO2 | −1.52 | 103.058 | C3[13C]H8NO2 | −3.01 | 103.0581 | C3[13C]H8NO2 | −2.04 |
| 114.0549 | C5H8NO2 | −0.48 | nd b | 115.0573 | C4[13C]H8NO2 | −8.78 | ||
| 116.0706 | C5H10NO2 | −0.04 | 118.0772 | C3[13C]2H10NO2 | −0.97 | 117.0736 | C4[13C]1H10NO2 | −3.07 |
| 128.0709 | C6H10NO2 | 1.98 | 131.0778 | C3[13C]3H10NO2 | nd | 167.0321 | C5[13C]H9KNO2 | nd |
| 132.0656 | C5H10NO3 | 0.61 | 134.0721 | C3[13C]2H10NO3 | −0.96 | 155.0503 | C4[13C]1H9NaNO3 | −3.35 |
| 142.0866 | C7H12NO2 | 2.43 | 146.097 | C3[13C]4H12NO2 | nd | 143.088 | C6[13C]1H12NO2 | nd |
| 144.0655 | C6H10NO3 | 3.2 | 147.0759 | C3[13C]3H10NO3 | 2.15 | 183.0238 | C5[13C]H9KNO3 | −5.22 |
| 155.9738 | C4H6FeNO2 | −2.83 | 156.9765 | C3[13C]1H6FeNO2 | −6.98 | 156.9731 | C3[13C]1H6FeNO2 | nd |
| 158.0815 | C7H12NO3 | 2.09 | nd | nd | nd | 159.0818 | C6[13C]H12NO3 | nd |
| 169.9895 | C5H8FeNO2 | −2.3 | 171.99 | nd | nd | 170.9924 | C4[13C]H8FeNO2 | −4.95 |
| 174.0766 | C7H12NO4 | 0.189 | nd | nd | nd | 175.0794 | C6[13C]H12NO4 | −0.22 |
| 185.9848 | C5H8FeNO3 | −0.03 | 187.9913 | C3[13C]2H8FeNO3 | −1.15 | nd | ||
| 227.1016 | C10H14N2O4 | −2.12 | 231.1147 | C6[13C]4H14N2O4 | −5.85 | nd | ||
| 328.1491 | C14H22N3O6 | −3.69 | 333.1695 | C9[13C]5H22N3O6 | 7.25 | nd | ||
| 215.1032 | C9H14N2O4 | {1.084 | 218.1132 | C6[13C]3H14N2O4 | −10.99 | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.S.; Yaylayan, V. Amino-Acid-Derived Oxazolidin-5-Ones as Chemical Markers for Schiff Base Formation in Glycation Reactions. Appl. Sci. 2023, 13, 7658. https://doi.org/10.3390/app13137658
Kim ES, Yaylayan V. Amino-Acid-Derived Oxazolidin-5-Ones as Chemical Markers for Schiff Base Formation in Glycation Reactions. Applied Sciences. 2023; 13(13):7658. https://doi.org/10.3390/app13137658
Chicago/Turabian StyleKim, Eun Sil, and Varoujan Yaylayan. 2023. "Amino-Acid-Derived Oxazolidin-5-Ones as Chemical Markers for Schiff Base Formation in Glycation Reactions" Applied Sciences 13, no. 13: 7658. https://doi.org/10.3390/app13137658
APA StyleKim, E. S., & Yaylayan, V. (2023). Amino-Acid-Derived Oxazolidin-5-Ones as Chemical Markers for Schiff Base Formation in Glycation Reactions. Applied Sciences, 13(13), 7658. https://doi.org/10.3390/app13137658







