Simulation and Experimental Study on the Responses of Subcellular Structures in Tumor Cells Induced by 5 ns Pulsed Electric Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishment of Cell Mechatronic Model
2.2. Experimental Device
2.3. Cell Culture
2.4. Cell Fluorescence Experiment
2.5. Statistical Analysis
3. Results
3.1. Simulation Result
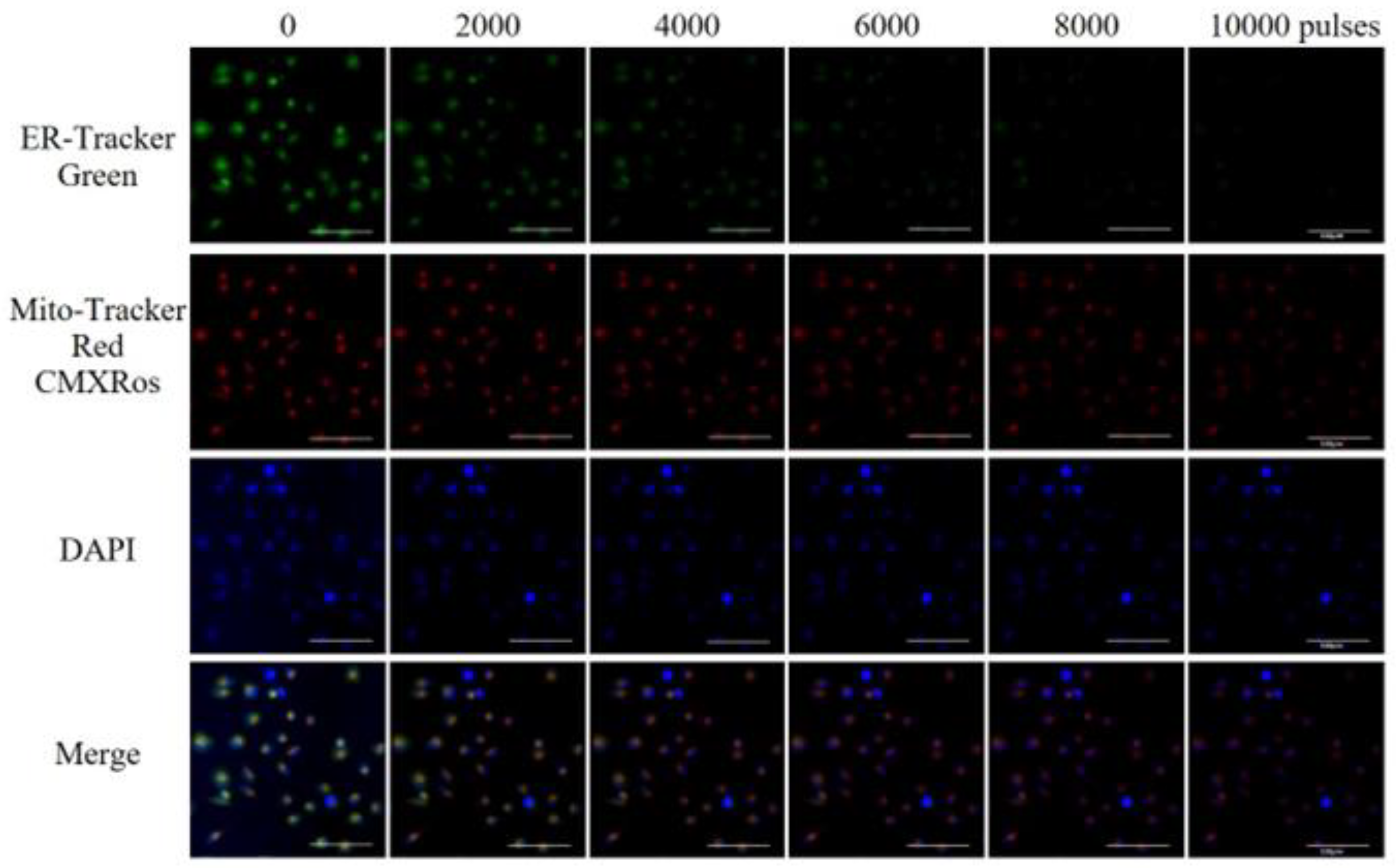
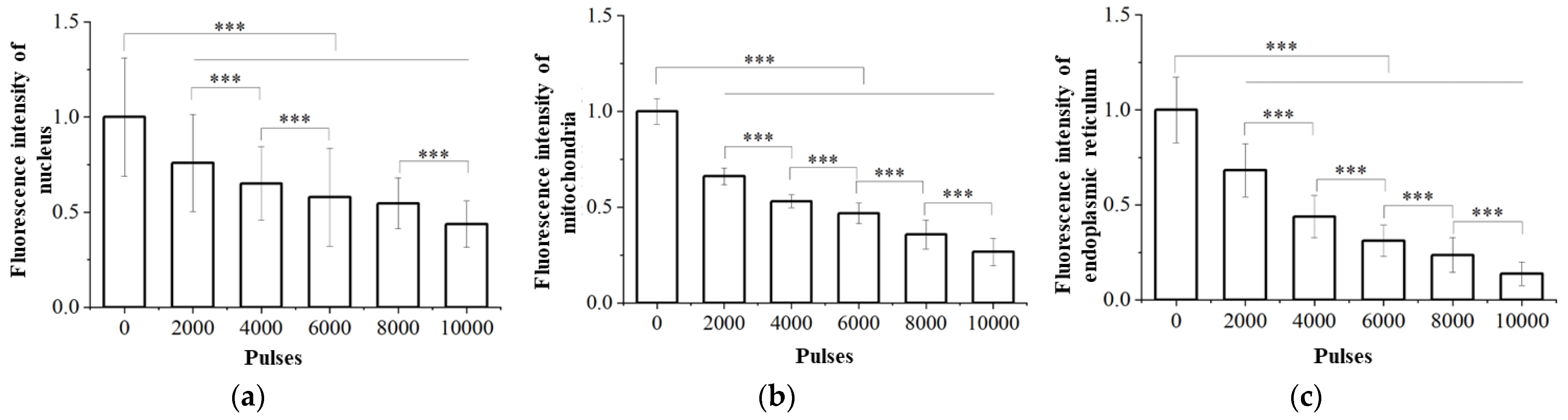
3.2. Damage of Subcellular Structure
4. Discussion
5. Conclusions
- Due to the extremely short pulse duration and rich high-frequency components of high-voltage nsPEFs, they can pass through the cell membrane with large equivalent capacitance and reach the membrane structures inside the cell as the target site.
- Under the action of high-voltage nsPEFs, the cell membrane structure undergoes electric perforation and is subjected to Maxwell stress. The stress on the nuclear membrane is greater than that on the cell membrane, which causes the nuclear membrane to stretch along the electric field direction.
- High-voltage nsPEF stimulation causes fibrous actin bundles to depolymerize, increasing the degree of strain on cells after stress and damaging the integrity and order of the cell skeletons. The integrity of the cell skeleton will recover to some extent, but the order will not be restored after being disrupted.
- High-voltage nsPEFs can damage the subcellular structures of tumor cells. Under the electric field, the nuclear membrane is perforated and damaged, affecting the nuclear matrix; the membrane potential of mitochondria is dissipated, the membrane structure is damaged, caspase apoptosis protein is activated, and ATP is depleted; endoplasmic reticulum perforation, calcium ion release, and endoplasmic reticulum stress occur.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 13 February 2023).
- Friebel, E.; Kapolou, K.; Unger, S.; Tugues, S.; Neidert, M.C.; Becher, B. Single-Cell Mapping of Human Brain Cancer Reveals Tumor-Specific Instruction of Tissue-Invading Leukocytes. Cell 2020, 181, 1626. [Google Scholar] [CrossRef] [PubMed]
- Specht, L.; Yahalom, J.; Illidge, T.; Berthelsen, A.K.; Constine, L.S.; Eich, H.T.; Girinsky, T.; Hoppe, R.T.; Mauch, P.; Mikhaeel, N.G.; et al. Modern Radiation Therapy for Hodgkin Lymphoma: Field and Dose Guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int. J. Radiat. Oncol. Biol. Phys. 2013, 89, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.H.; Hong, J.Y.; Park, H.J.; Lee, S.K. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int. J. Cancer 2017, 141, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahl, D.R.; Matthew, H.; Tao, Y.; Pollom, E.L.; Caoili, E.M.; Schipper, T.L.; Feng, M. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J. Clin. Oncol. 2016, 34, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubner, M.G.; Brace, C.L.; Ziemlewicz, T.J.; Hinshaw, J.L.; Lee, F.T., Jr. Microwave ablation of hepatic malignancy. Semin. Interv. Radiol. 2013, 30, 56–66. [Google Scholar]
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.F.; Yung, D.; Skanes, A.; Khaykin, Y.; et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. Mass. Med. Soc. 2021, 384, 305–315. [Google Scholar] [CrossRef]
- Solyanik, G.I. Multifactorial nature of tumor drug resistance. Exp. Oncol. 2010, 32, 181–185. [Google Scholar]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef] [Green Version]
- Maleki Vareki, S.; Garrigós, C.; Duran, I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit. Rev. Oncol. Hematol. 2017, 116, 116–124. [Google Scholar] [CrossRef]
- Williams, A.D.; Payne, K.K.; Posey, A.D., Jr.; Hill, C.; Jose, C.G.; June, C.H.; Tchou, J. Immunotherapy for Breast Cancer: Current and Future Strategies. Curr. Surg. Rep. 2017, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Yao, C.; Sun, C.; Guo, F. Dependence on electric field intensities of cell biological effects induced by microsecond pulsed electric fields. IEEE Trans. Dielectr. Electr. Insul. 2012, 18, 2083–2088. [Google Scholar] [CrossRef]
- Onik, G.; Rubinsky, B. Irreversible electroporation: First patient experience focal therapy of prostate cancer. In Irreversible Electroporation; Springer: Berlin/Heidelberg, Germany, 2010; pp. 235–247. [Google Scholar]
- Yao, C.; Hu, X.; Mi, Y.; Li, C.; Sun, C. Window effect of pulsed electric field on biological cells. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1259–1266. [Google Scholar] [CrossRef]
- Tang, J.; Yin, H.; Ma, J.; Bo, W.; Yang, Y.; Xu, J.; Liu, Y.; Gong, Y. Terahertz electric field-induced membrane electro-poration by molecular dynamics simulations. J. Membr. Biol. 2018, 251, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Liu, H.; Zhao, Y.; Mi, Y.; Dong, S.; Lv, Y. Analysis of dynamic processes in single-cell electroporation and their effects on parameter selection based on the finite-element model. IEEE Trans. Plasma Sci. 2017, 45, 889–900. [Google Scholar] [CrossRef]
- Pucihar, G.; Miklavcic, D.; Kotnik, T. A time-dependent numerical model of transmembrane voltage inducement and electroporation of irregularly shaped cells. IEEE Trans. Biomed. Eng. 2009, 56, 1491–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Rubinsky, B. Microfabricated electroporation chip for single cell membrane permeabilization. Sens. Actuators A Phys. 2001, 89, 242–249. [Google Scholar] [CrossRef]
- Ivey, J.W.; Latouche, E.L.; Sano, M.B.; Rossmeisl, J.H.; Davalos, R.V.; Verbridge, S.S. Targeted cellular ablation based on the morphology of malignant cells. Sci. Rep. 2015, 5, 1757. [Google Scholar] [CrossRef] [Green Version]
- Nesin, O.M.; Pakhomova, O.N.; Xiao, S.; Pakhomov, A.G. Manipulation of cell volume and membrane pore comparison following single cell permeabilization with 60- and 600-ns electric pulses. Biochim. Biophys. Acta (BBA) Biomembr. 2011, 1808, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Ivorra, A.; Rubinsky, B. In Vivo electrical impedance measurements during and after electroporation of rat liver. Bioelectrochemistry 2007, 70, 287–295. [Google Scholar] [CrossRef]
- Beebe, S.J.; Sain, N.M.; Ren, W. Induction of cell death mechanisms and apoptosis by nanosecond pulsed electric fields (nsPEFs). Cells 2013, 2, 136–162. [Google Scholar] [CrossRef] [Green Version]
- Pakhomov, A.G.; Xiao, S.; Pakhomova, O.N.; Semenov, I.; Kuipers, M.A.; Ibey, B.L. Disassembly of actin structures by nanosecond pulsed electric field is a downstream effect of cell swelling. Bioelectrochemistry 2014, 100, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, J.; Kolb, J.F. Time domain dielectric spectroscopy of nanosecond pulsed electric field induced changes in dielectric properties of pig whole blood. Bioelectrochemistry 2015, 103, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Schoenbach, K.H.; Joshi, R.P.; Kolb, J.F.; Chen, N.; Stacey, M.; Blackmore, P.F.; Buescher, E.S.; Beebe, S.J. Ultrashort electrical pulses open a new gateway into biological cells. Proc. IEEE 2004, 92, 1122–1137. [Google Scholar] [CrossRef]
- Beebe, S.J.; Fox, P.M.; Rec, L.J.; Somers, K.; Stark, R.H.; Schoenbach, K.H. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: Apoptosis induction and tumor growth inhibition. IEEE Trans. Plasma Sci. 2002, 30, 286–292. [Google Scholar] [CrossRef]
- Beebe, S.J.; Chen, Y.J.; Sain, N.M.; Schoenbach, K.H.; Xiao, S. Transient features in nanosecond pulsed electric fields differentially modulate mitochondria and viability. PLoS ONE 2012, 7, e51349. [Google Scholar] [CrossRef]
- Vernier, P.T.; Sun, Y.; Marcu, L.; Salemi, S.; Craft, C.M.; Gundersen, M.A. Calcium bursts induced by nanosecond electric pulses. Biochem. Biophys. Res. Commun. 2003, 310, 286–295. [Google Scholar] [CrossRef]
- Stacey, M.; Fox, P.; Buescher, S.; Kolb, J. Nanosecond pulsed electric field induced cytoskeleton, nuclear membrane and telomere damage adversely impact cell survival. Bioelectrochemistry 2011, 82, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Graybill, P.M.; Davalos, R.V. Cytoskeletal disruption after electroporation and its significance to pulsed electric field therapies. Cancers 2020, 12, 1132. [Google Scholar] [CrossRef]
- Haberkorn, I.; Siegenthaler, L.; Buchmann, L.; Neutsch, L.; Mathys, A. Enhancing single-cell bioconversion efficiency by harnessing nanosecond pulsed electric field processing. Biotechnol. Adv. 2021, 53, 107780. [Google Scholar] [CrossRef]
- Neu, J.C.; Krassowska, W. Asymptotic model of electroporation. Phys. Rev. E 1999, 59, 3471. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, L.; Liu, X. Nonlinear dispersive cell model for microdosimetry of nanosecond pulsed electric fields. Sci. Rep. 2020, 10, 19456. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Xiao, S.; Joshi, R.P. Simulations of voltage transients across intracellular mitochondrial membranes due to nanosecond electrical pulses. IEEE Trans. Plasma Sci. 2014, 42, 3113–3120. [Google Scholar] [CrossRef]
- Guo, F.; Qian, K.; Deng, H.; Li, X. Multiphysics analysis of nsPEF induced electrodeformation in a dispersive cell model. Appl. Phys. Lett. 2021, 118, 083701. [Google Scholar] [CrossRef]
- Weaver, J.C. Electroporation of cells and tissues. IEEE Trans. Plasma Sci. 2000, 28, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Prasad, R.; Roy, S.; Mukherjee, A.; Prosenjit, S. The protease activated receptor2 promotes Rab5a mediated generation of pro-metastatic microvesicles. Sci. Rep. 2018, 8, 7357. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.Y.; Huang, K.W. Irreversible electroporation: A novel ultrasound-guided modality for non-thermal tumor ablation. J. Med. Ultrasound 2017, 25, 195–200. [Google Scholar] [CrossRef]
- Rao, X.; Chen, S.; Alfadhl, Y.; Chen, X.; Sun, L.; Yu, L.; Zhou, J. Pulse width and intensity effects of pulsed electric fields on cancerous and normal skin cells. Sci. Rep. 2022, 12, 18039. [Google Scholar] [CrossRef]
- Zaklit, J.; Craviso, G.L.; Leblanc, N.; Yang, L.; Vernier, P.T.; Chatterjee, I. Adrenal chromaffin cells exposed to 5-ns pulses require higher electric fields to porate intracellular membranes than the plasma membrane: An experimental and modeling study. J. Membr. Biol. 2017, 250, 535–552. [Google Scholar] [CrossRef]
- Retelj, L.; Pucihar, G.; Miklavčič, D. Electroporation of intracellular liposomes using nanosecond electric pulses—A theoretical study. IEEE Trans. Biomed. Eng. 2013, 60, 2624–2635. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Semenov, I.; Casciola, M.; Xiao, S. Neuronal excitation and permeabilization by 200-ns pulsed electric field: An optical membrane potential study with FluoVolt dye. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 1273–1281. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Pakhomova, O.N. The interplay of excitation and electroporation in nanosecond pulse stimulation. Bioelectrochemistry 2020, 136, 107598. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Wu, X.; Xu, J.; Zheng, W.; Ma, C.; Chen, W.J.; Zhang, Q. Effect of the mechanical properties of the cell membrane on the transition energy barrier of electroporation. J. Appl. Phys. 2022, 131, 084701. [Google Scholar] [CrossRef]
- Yadollahpour, A.; Rezaee, Z. Electroporation as a new cancer treatment technique: A review on the mechanisms of action. Biomed. Pharmacol. J. 2015, 7, 53–65. [Google Scholar] [CrossRef]
- Chafai, D.E.; Sulimenko, V.; Havelka, D.; Kubínová, L.; Dráber, P.; Cifra, M. Reversible and Irreversible Modulation of Tubulin Self-Assembly by Intense Nanosecond Pulsed Electric Fields. Adv. Mater. 2019, 31, 1903636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghöfer, T.; Eing, C.; Flickinger, B.; Hohenberger, P.; Wegner, L.H.; Frey, W.; Nick, P. Nanosecond electric pulses trigger actin responses in plant cells. Biochem. Biophys. Res. Commun. 2009, 387, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.K.; Tan, W.; Ho, C.M. Cell relaxation after electrodeformation: Effect of latrunculin A on cytoskeletal actin. J. Biomech. 2005, 38, 529–535. [Google Scholar] [CrossRef]
- Thompson, G.L.; Roth, C.; Tolstykh, G.; Kuipers, M. Role of cytoskeleton and elastic moduli in cellular response to nanosecond pulsed electric fields. In Terahertz and Ultrashort Electromagnetic Pulses for Biomedical Applications; SPIE: San Francisco, CA, USA, 2013. [Google Scholar]
- Chen, N.; Schoenbach, K.H.; Kolb, J.F.; Swanson, R.J.; Garner, A.L.; Yang, J.; Joshi, R.P.; Beebe, S.J. Leukemic cell intracellular responses to nanosecond electric fields. Biochem. Biophys. Res. Commun. 2004, 317, 421–427. [Google Scholar] [CrossRef]
- Chen, N.; Garner, A.L.; Chen, G.; Jing, Y.; Deng, Y.; Swanson, R.J.; Kolb, J.F.; Beebe, S.J.; Joshi, R.P.; Schoenbach, K.H. Nanosecond electric pulses penetrate the nucleus and enhance speckle formation. Biochem. Biophys. Res. Commun. 2007, 364, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Kolb, J.F.; Swanson, R.J.; Schoenbach, K.H.; Beebe, S.J. Apoptosis initiation and angiogenesis inhibition: Melanoma targets for nanosecond pulsed electric fields. Pigment Cell Melanoma Res. 2010, 23, 554–563. [Google Scholar] [CrossRef]
- Napotnik, T.B.; Polajžer, T.; Miklavčič, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, R.; McDaniel, A.; Anand, S.; Cha, J.; Mallon, Z.; Berridge, J.C.; Uecker, D. Nano-Pulse Stimulation is a physical modality that can trigger immunogenic tumor cell death. J. Immunother. Cancer 2017, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Jing, Y.; Burcus, N.I.; Lassiter, B.P.; Tanaz, R.; Heller, R.; Beebe, S.J. Nano-pulse stimulation induces potent immune responses, eradicating local breast cancer while reducing distant metastases. Int. J. Cancer 2018, 142, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Beebe, S.J.; Blackmore, P.F.; White, J.; Joshi, R.P.; Schoenbach, K.H. Nanosecond pulsed electric fields modulate cell function through intracellular signal transduction mechanisms. Physiol. Meas. 2004, 25, 1077. [Google Scholar] [CrossRef]
- Furumoto, Y.; Sato, D.; Teranishi, K.; Shimomura, N.; Hamada, Y.; Miyake, M.; Oyadomari, S. Activation of Endoplasmic Reticulum Stress Response by Applying of Nanosecond Pulsed Electric Fields for Medical Application. In Proceedings of the 2018 IEEE International Power Modulator and High Voltage Conference, Jack Lake Lodge, WY, USA, 3 June 2018. [Google Scholar]
- Taibi, A.; Perrin, M.L.; Albouys, J.; Jacques, J.; Yardin, C.; Durand-Fontanier, S.; Bardet, S.M. 10 ns PEFs induce a histological response linked to cell death and cytotoxic T-lymphocytes in an immunocompetent mouse model of peritoneal metastasis. Clin. Transl. Oncol. 2021, 23, 1220–1237. [Google Scholar] [CrossRef] [PubMed]
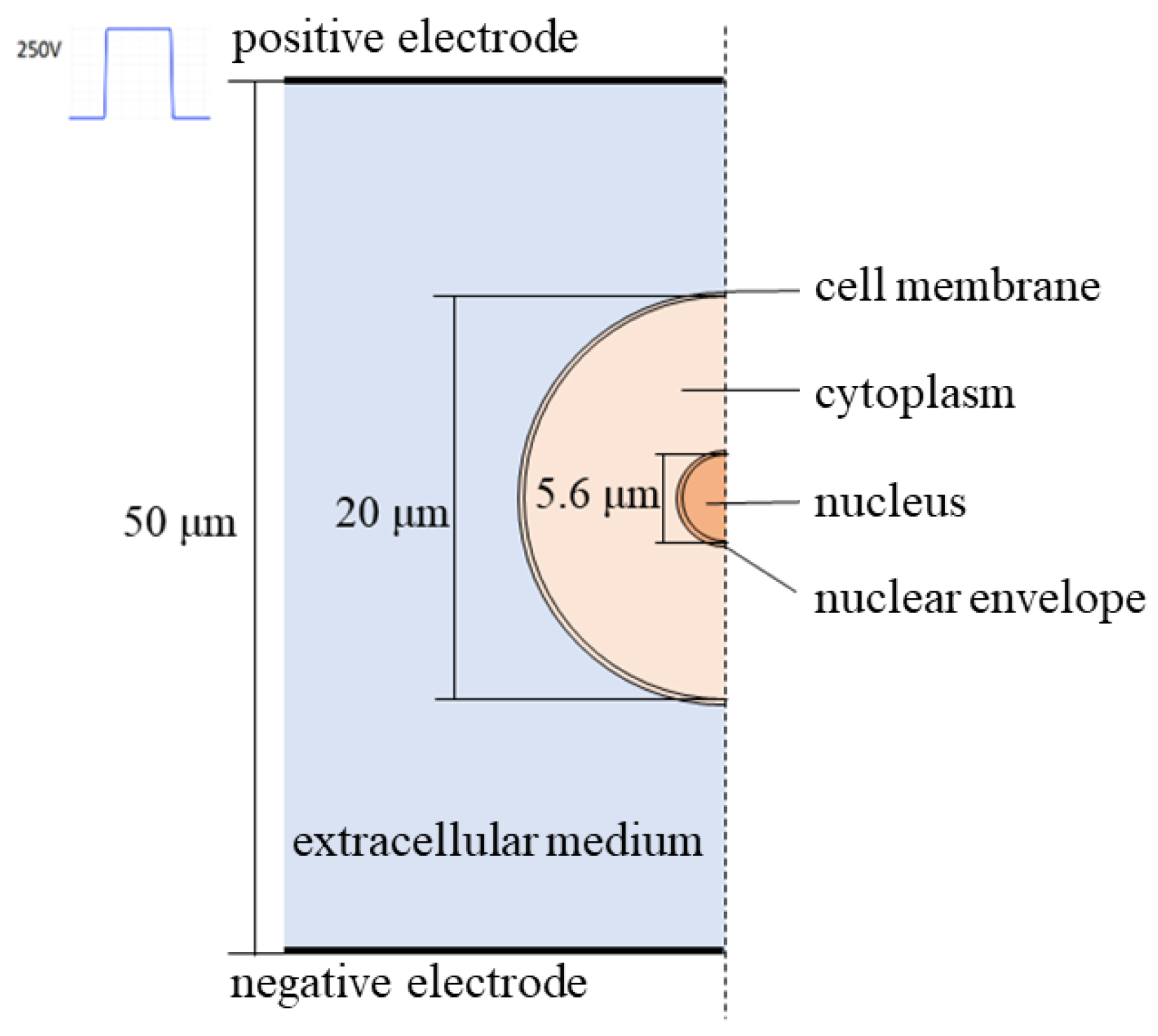
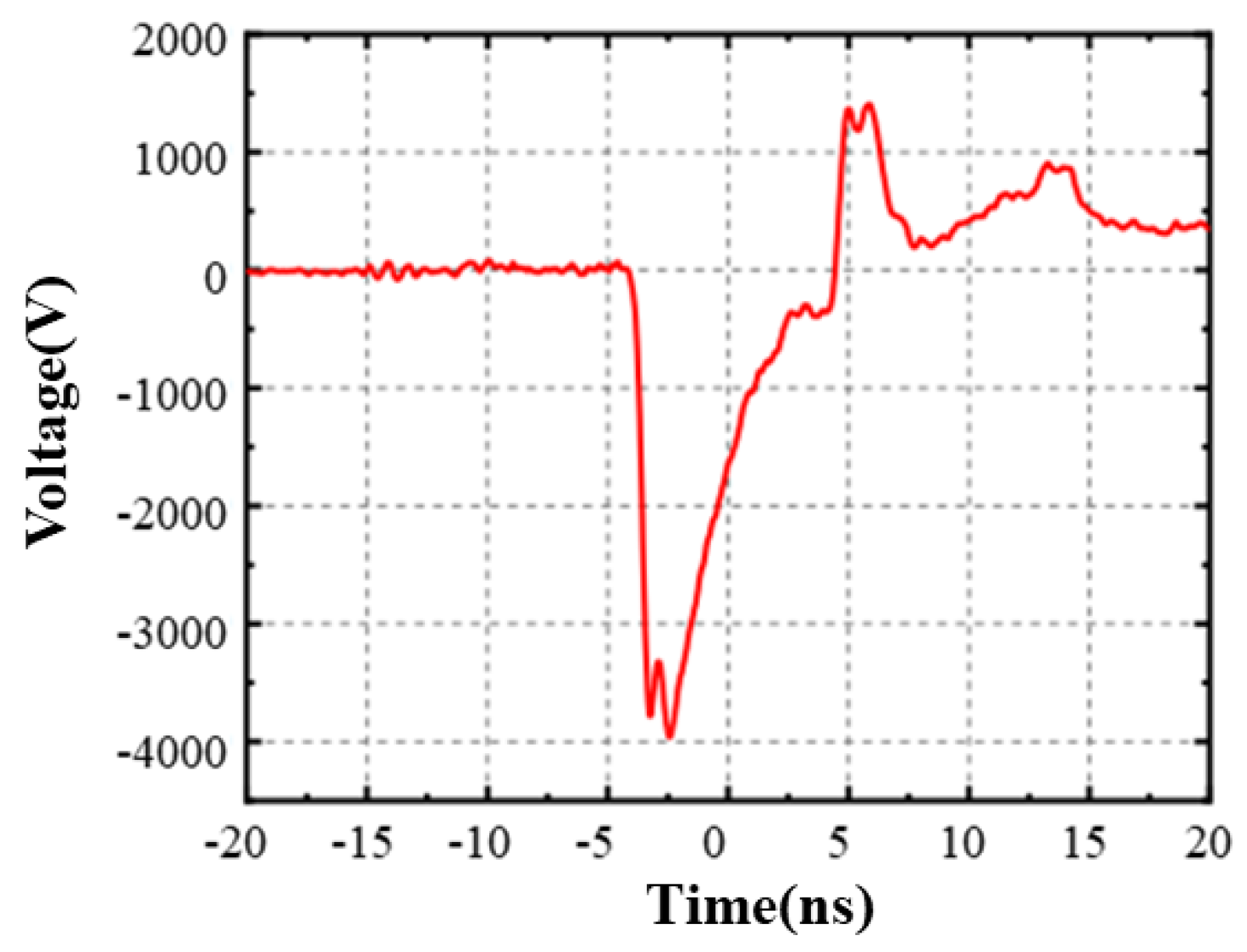



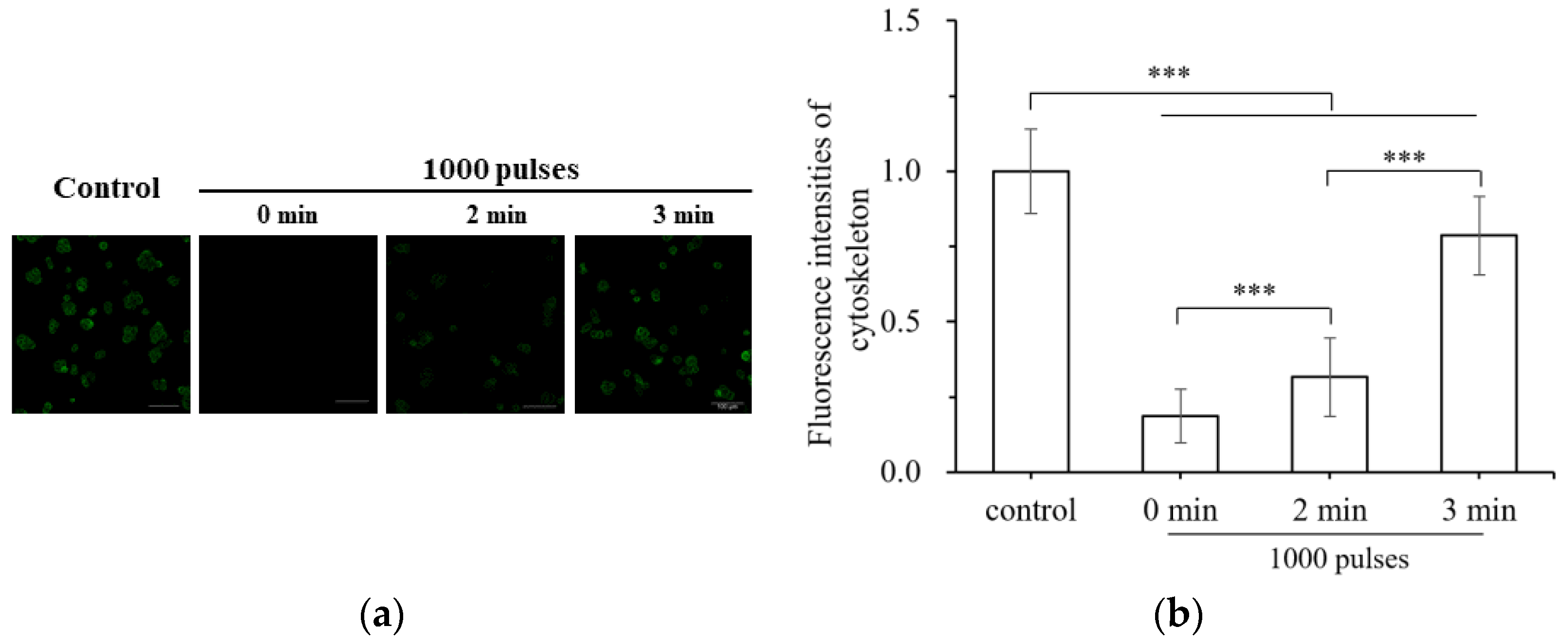
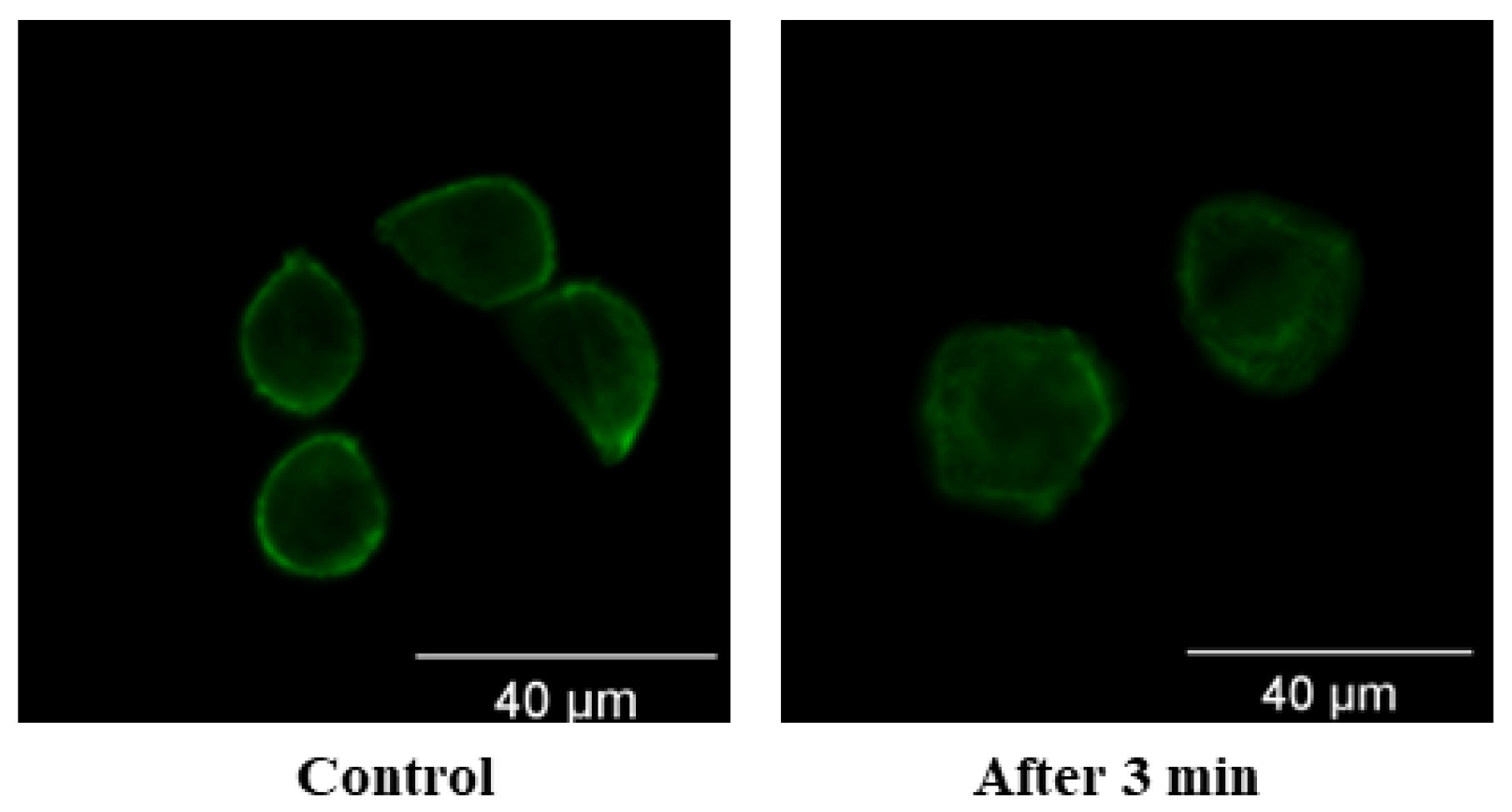

| Parameter | Simulation Calculation Value | |
|---|---|---|
| Geometrical parameter/μm | Cell radius | 10 |
| Cell membrane thickness | 0.004 | |
| Nuclear radius | 2.8 | |
| Nuclear membrane thickness | 0.004 | |
| Conductivity/(S·m−1) | Extracellular fluid | 0.3 |
| Cell membrane | 3.0 × 10−7 | |
| Cytoplasm | 1.3 | |
| Nuclear membrane | 3.0 × 10−5 | |
| Nucleoplasm | 1.3 | |
| Relative dielectric constant | Extracellular fluid | 72 |
| Cell membrane | 5 | |
| Cytoplasm | 72 | |
| Nuclear membrane | 5 | |
| Nucleoplasm | 72 | |
| Dispersion parameter | First-order relaxation time τ1/s | 3.0 × 10−9 |
| Second-order relaxation time τ2/s | 4.6 × 10−10 | |
| First-order relaxation amplitude Δε1/(F·m−1) | 2.3 × 10−11 | |
| Second-order relaxation amplitude Δε2/(F·m−1) | 7.4 × 10−12 | |
| High-frequency dielectric constant ε∞ /(F·m−1) | 13.9 × 10−12 | |
| Electroporation parameters | Electroporation parameters α/(m−2·s−1) | 1.0 × 109 |
| Initial pore density N0/m−2 | 1.5 × 109 | |
| Characteristic voltage Uep/V | 0.258 | |
| Electroporation constant q | 2.46 | |
| Aperture rp/nm | 0.8 | |
| Microporous energy barrier w0 | 2.65 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.; Ma, X.; Qian, K.; Wang, Y.; Dong, S. Simulation and Experimental Study on the Responses of Subcellular Structures in Tumor Cells Induced by 5 ns Pulsed Electric Fields. Appl. Sci. 2023, 13, 8142. https://doi.org/10.3390/app13148142
Yao C, Ma X, Qian K, Wang Y, Dong S. Simulation and Experimental Study on the Responses of Subcellular Structures in Tumor Cells Induced by 5 ns Pulsed Electric Fields. Applied Sciences. 2023; 13(14):8142. https://doi.org/10.3390/app13148142
Chicago/Turabian StyleYao, Chenguo, Xin Ma, Kun Qian, Yancheng Wang, and Shoulong Dong. 2023. "Simulation and Experimental Study on the Responses of Subcellular Structures in Tumor Cells Induced by 5 ns Pulsed Electric Fields" Applied Sciences 13, no. 14: 8142. https://doi.org/10.3390/app13148142





