Radiation-Induced Thermal Runaway Propagation in a Cylindrical Li-Ion Battery Pack: Non-Monotonicity, Chemical Kinetics, and Geometric Considerations
Abstract
1. Introduction
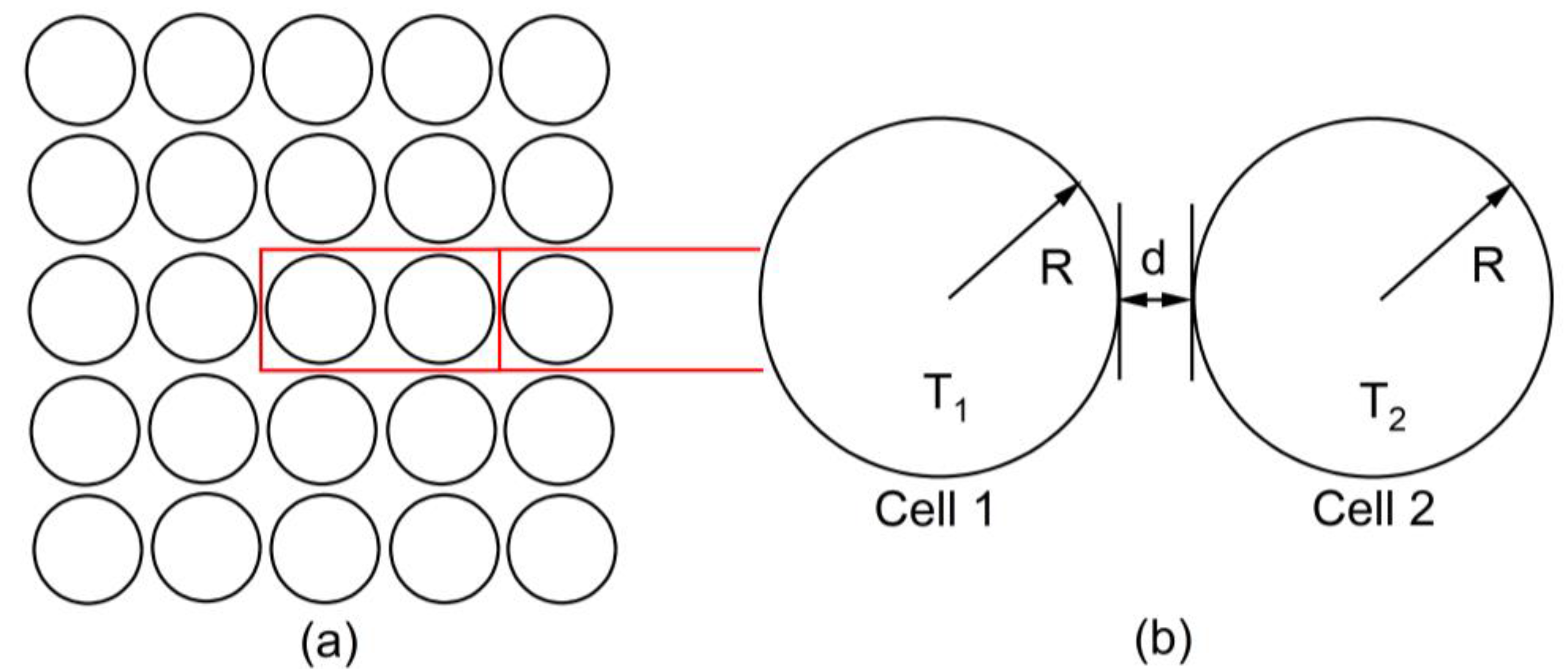
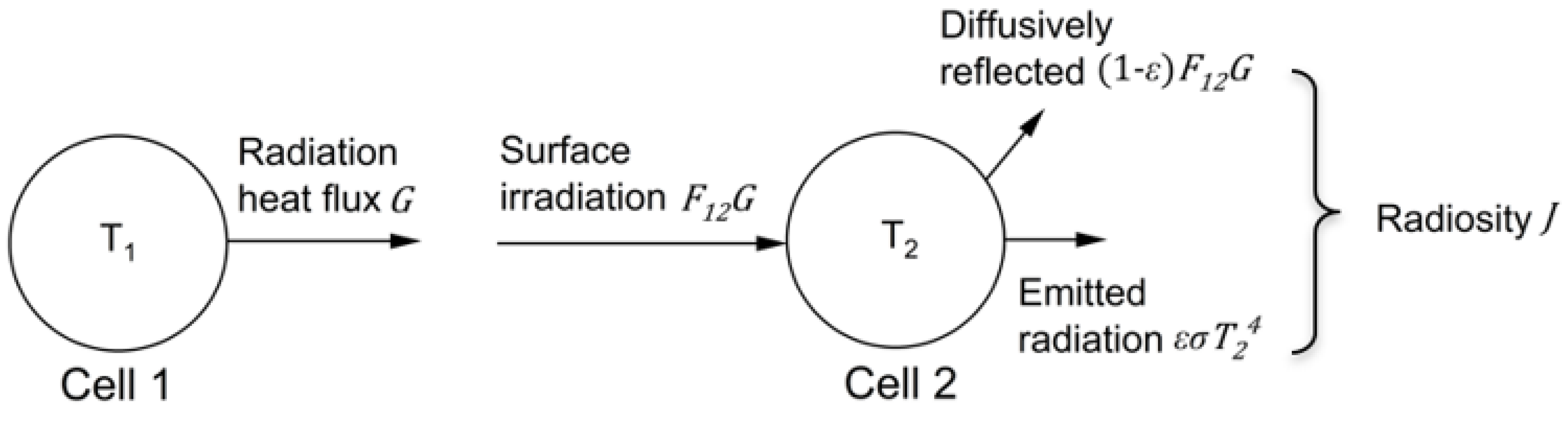
2. Materials and Methods
3. Results and Discussions
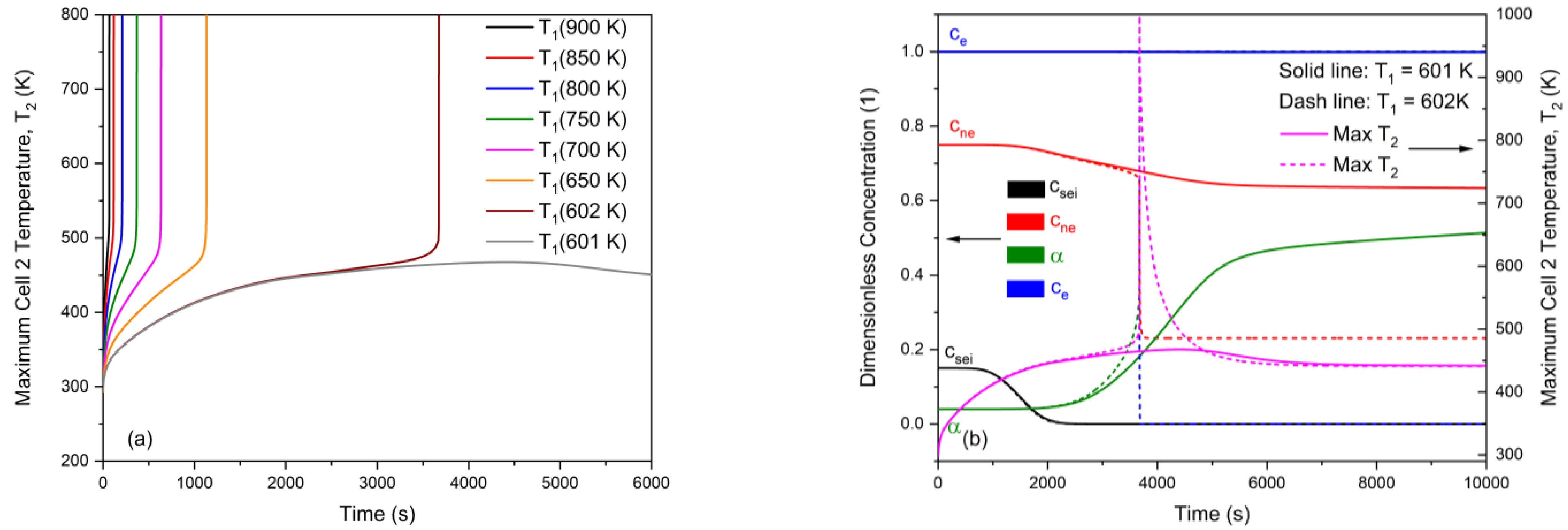
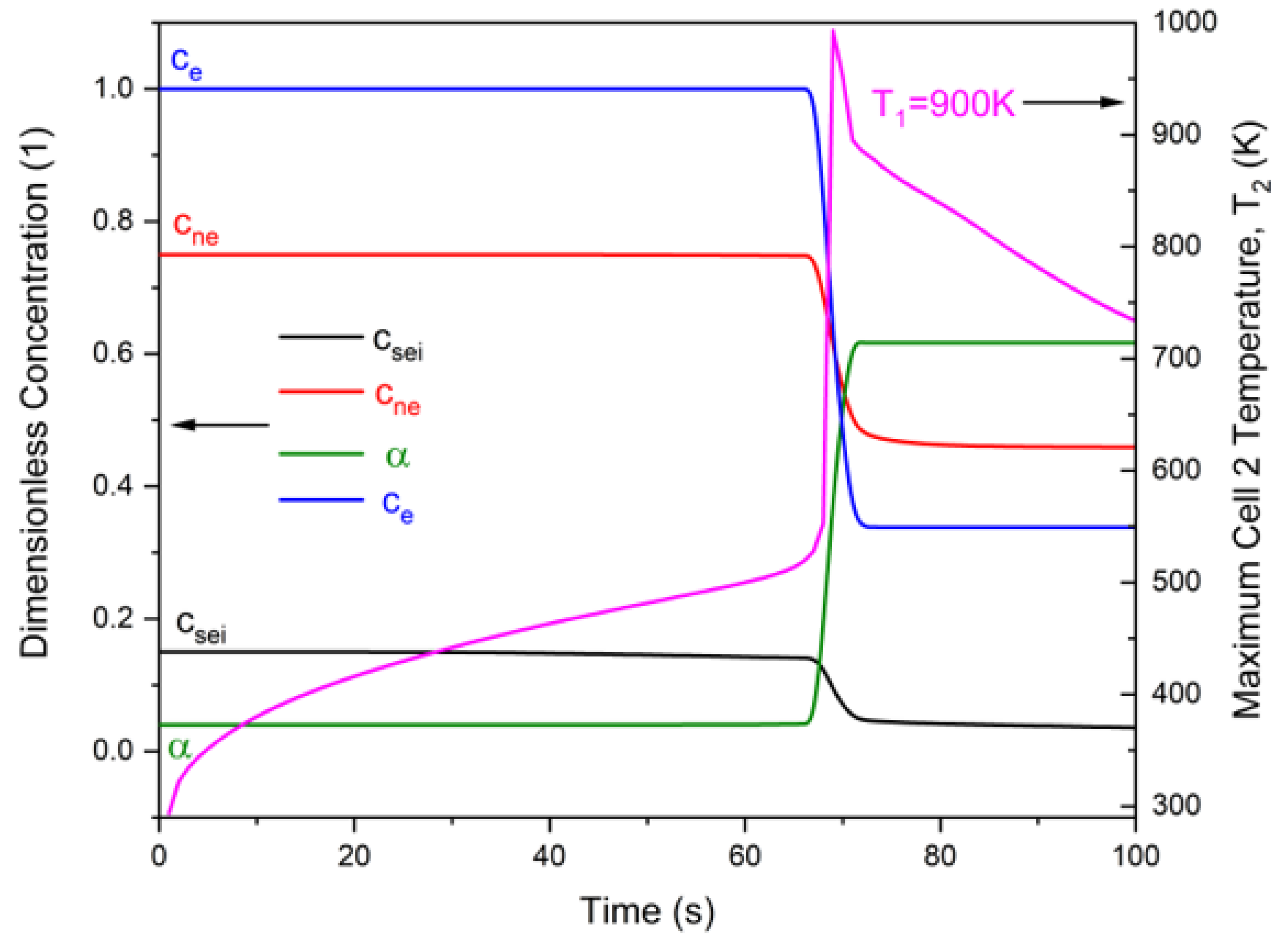
3.1. General Features of Radiation-Induced Thermal Runaway
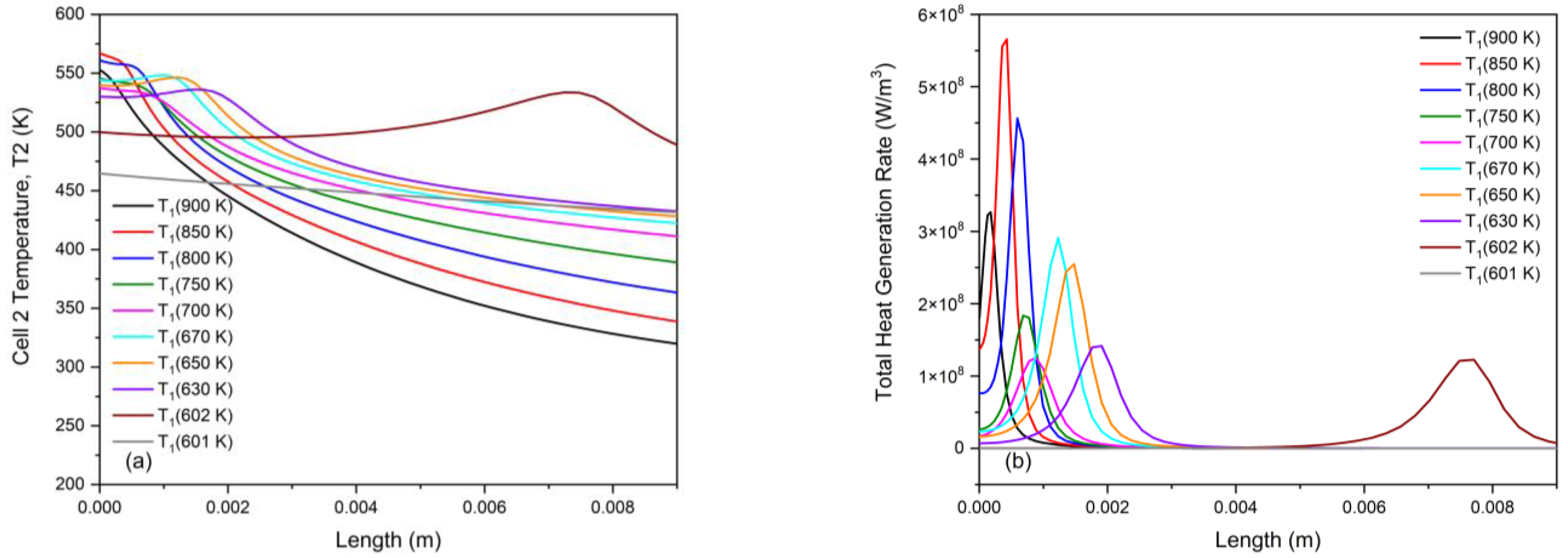
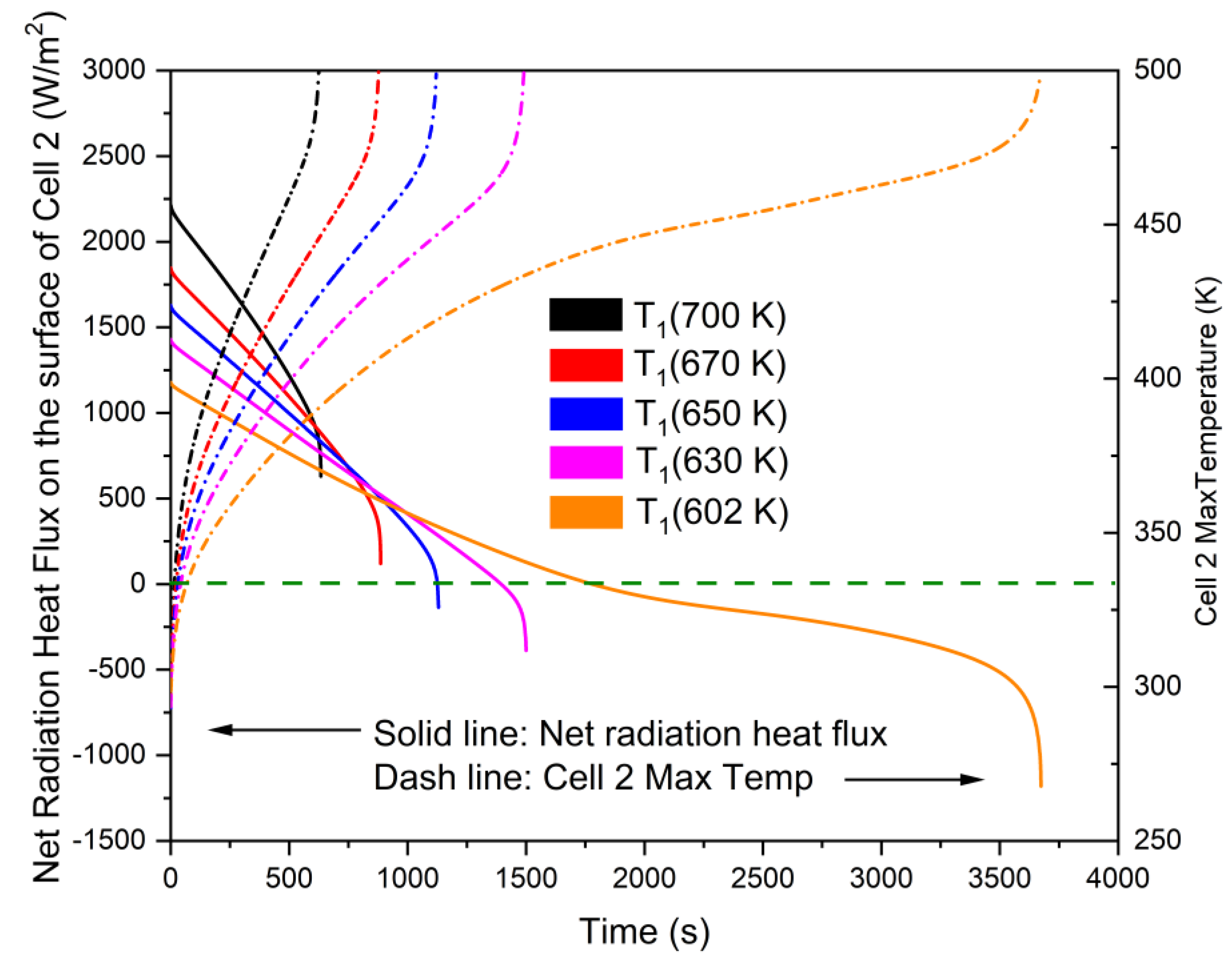
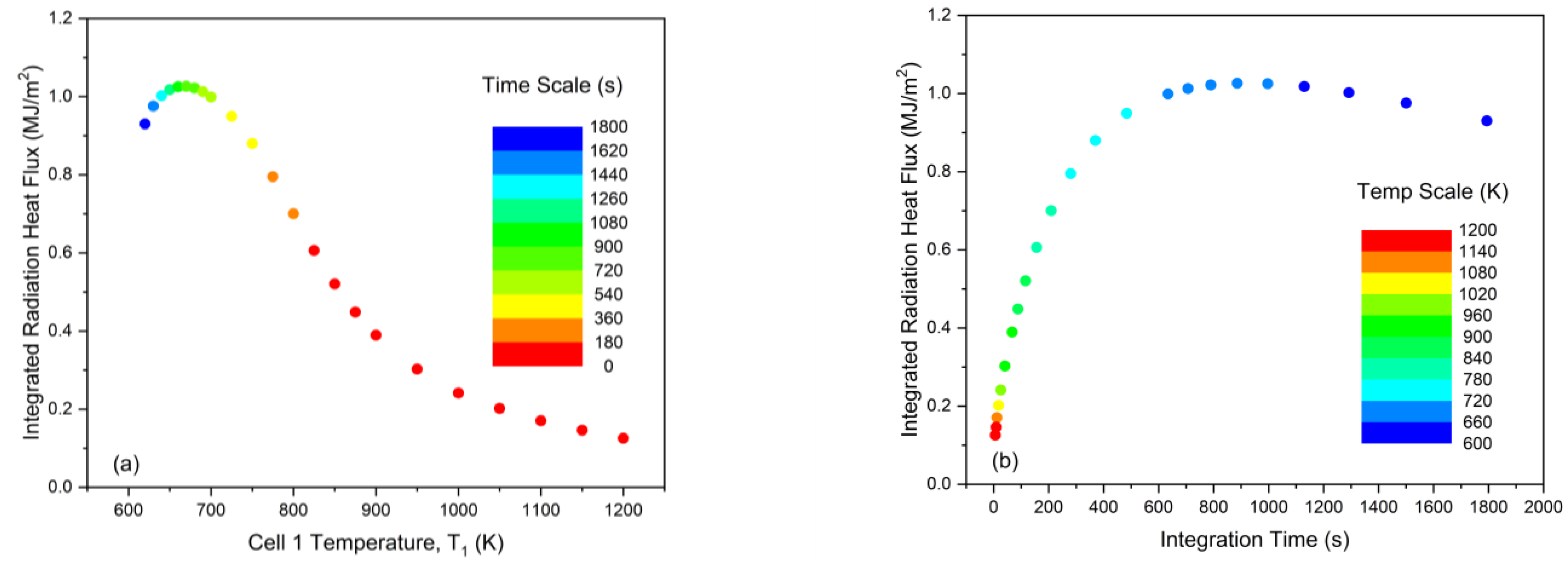
3.2. Non-Monotonicity in Radiation-Induced Thermal Runaway
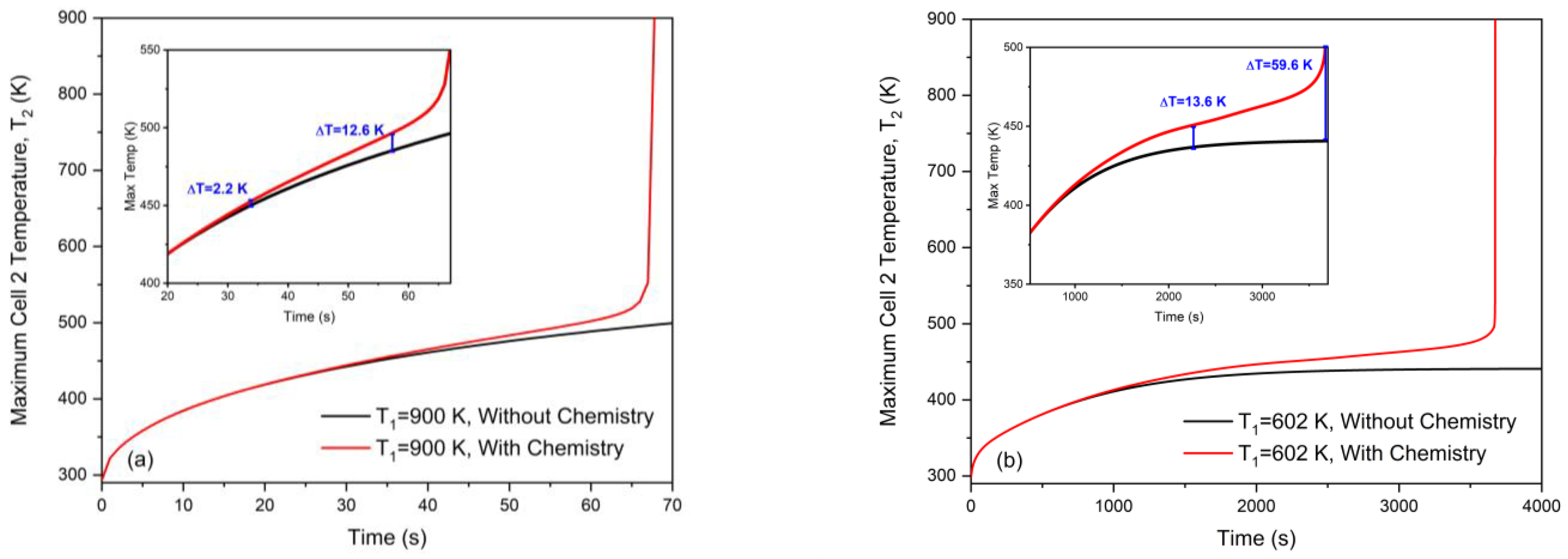
3.3. Role of Pre-Runaway Chemical Heat Release in Radiation-Induced Thermal Runaway
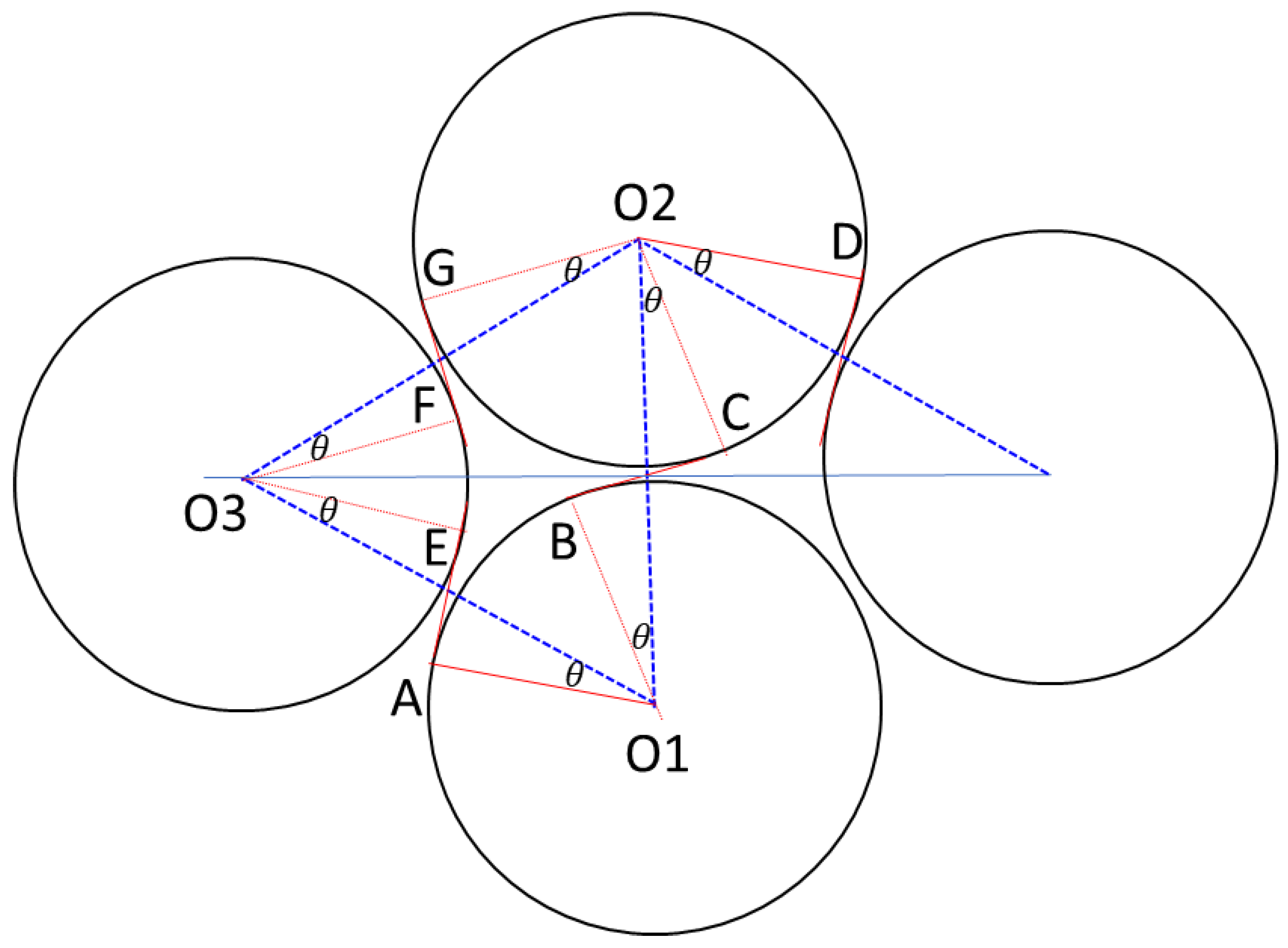
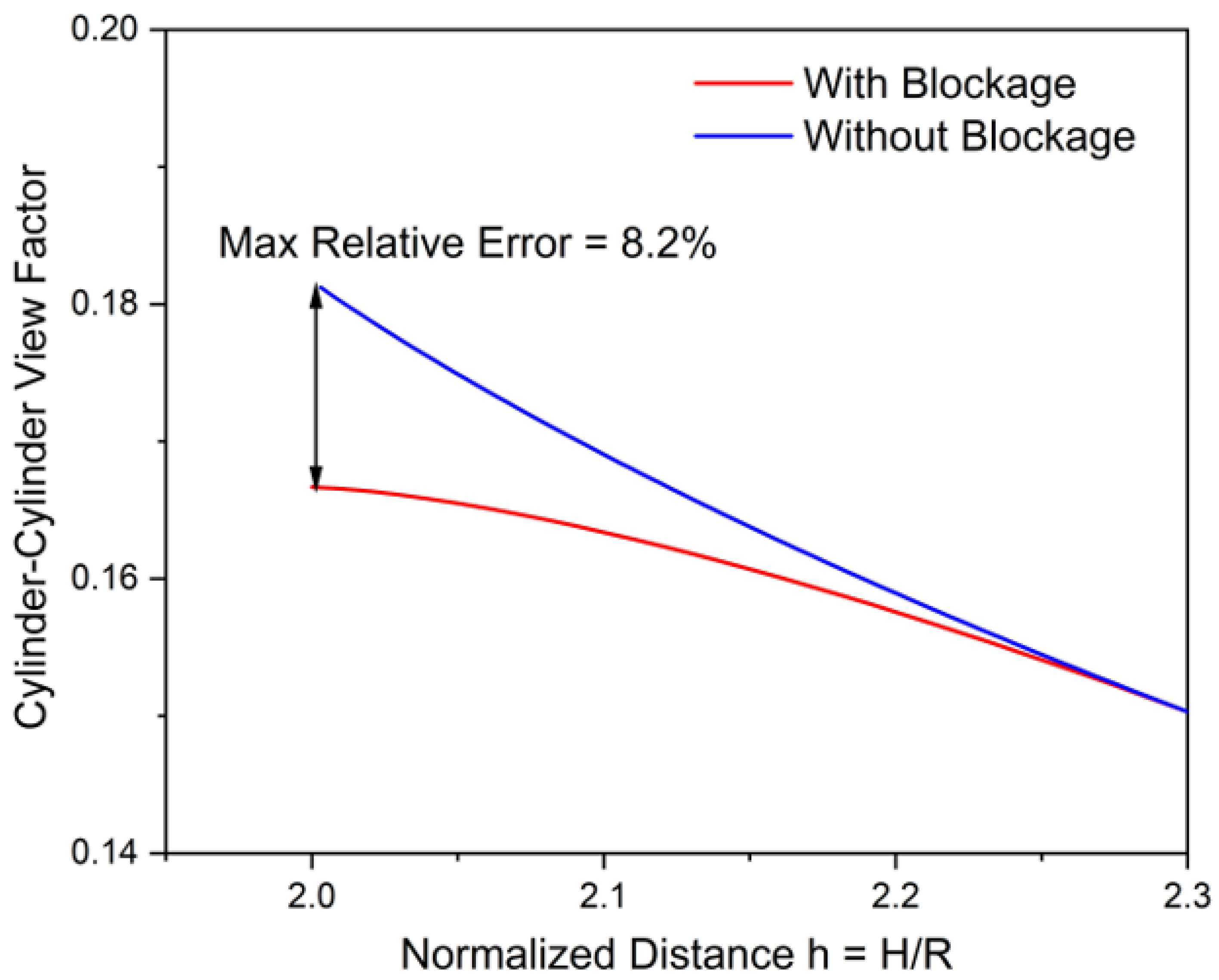
3.4. Effects from Blockage on Radiation Heat Flux in Practical Battery Packs
3.5. Effects of Radiation from Localized Hot Spots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Blueprint for Lithium Batteries, 2021–2030, US Department of Energy. Available online: https://www.energy.gov/eere/vehicles/articles/national-blueprint-lithium-batteries (accessed on 13 July 2023).
- Huang, Z.; Liu, J.; Zhai, H.; Wang, Q. Experimental investigation on the characteristics of thermal runaway and its propagation of large-format lithium ion batteries under overcharging and overheating conditions. Energy 2021, 233, 121103. [Google Scholar] [CrossRef]
- Duh, Y.S.; Theng, J.H.; Chen, C.C.; Kao, C.S. Comparative study on thermal runaway of commercial 14500, 18650 and 26650 LiFePO4 batteries used in electric vehicles. J. Energy Storage 2020, 31, 101580. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Liu, L.; Hsu, H.; Lu, L.; Wang, L.; He, X.; Ouyang, M. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Liu, L.; Feng, X.; Zhang, M.; Lu, L.; Han, X.; He, X.; Ouyang, M. Comparative study on substitute triggerring approaches for internal short circuit in lithium-ion batteries. Appl. Energy 2020, 259, 114143. [Google Scholar] [CrossRef]
- Huang, Z.; Li, H.; Mei, W.; Zhao, C.; Sun, J.; Wang, Q. Thermal Runaway Behavior of Lithium Iron Phosphate Battery during Penetration. Fire Technol. 2020, 56, 2405–2426. [Google Scholar] [CrossRef]
- Xu, J.; Mei, W.; Zhao, C.; Liu, Y.; Zhang, L.; Wang, Q. Study on thermal runaway mechanism of 1000 mAh lithium ion pouch cell during nail penetration. J. Therm. Anal. Calorim. 2021, 144, 273–284. [Google Scholar] [CrossRef]
- Ren, D.; Liu, X.; Feng, X.; Lu, L.; Ouyang, M.; Li, J.; He, X. Model-based thermal runaway prediction of lithium-ion batteries from kinetics analysis of battery components. Appl. Energy 2018, 228, 633–644. [Google Scholar] [CrossRef]
- Gong, J.; Wang, Q.; Sun, J. Thermal analysis of nickel cobalt lithium manganese with varying nickel content used for lithium-ion batteries. Thermochim. Acta 2017, 655, 176–180. [Google Scholar] [CrossRef]
- Lei, B.; Zhao, W.; Ziebert, C.; Uhlmann, N.; Rohde, M.; Seifert, H.J. Experimental Analysis of Thermal Runaway in 18650 Cylindrical Li-Ion Batteries Using an Accelerating Rate Calorimeter. Batteries 2017, 3, 14. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, T.; Huang, Z.; Wu, J.; Zhou, H.; Ma, M.; Xu, J.; Wang, Z.; Li, H.; Sun, J.; et al. Experimental study on thermal runaway of fully charged and overcharged lithium-ion batteries under adiabatic and side-heating test. J. Energy Storage 2021, 38, 102519. [Google Scholar] [CrossRef]
- Hatchard, T.D.; MacNeil, D.D.; Basu, A.; Dahn, J.R. Thermal model of cylindrical and prismatic lithium-ion batteries. J. Electrochem. Soc. 2001, 148, A755–A761. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, D.; Feng, X.; Wang, L.; Ouyang, M. Thermal kinetics comparison of delithiated LiNMC cathodes. J. Power Sources 2021, 514, 230582. [Google Scholar] [CrossRef]
- Xu, J.; Lan, C.; Qiao, Y.; Ma, Y. Prevent thermal runaway of lithium-ion batteries with minichannel cooling. Appl. Therm. Eng. 2017, 110, 883–890. [Google Scholar] [CrossRef]
- Offer, G.; Patel, Y.; Hales, A.; Diaz, L.; Marzook, M. Cool metric for lithium-ion batteries could spur progress. Nature 2020, 582, 485–487. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Z.; Ouyang, D.; Chen, S. Analysis and prediction of thermal runaway propagation interval in confined space based on response surface methodology and artificial neural network. J. Energy Storage 2022, 55, 105822. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Bian, H.; Wang, J.; Bai, W.; Gao, T.; Bai, J.; Zhou, Y. Critical conditions for the thermal runaway propagation of lithium-ion batteries in air and argon environments. J. Therm. Anal. Calorim. 2022, 147, 13699–13710. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Wang, J.; Yang, Y.; Zhu, Y.; Bai, W. Inhibition effect of liquid nitrogen on thermal runaway propagation of lithium ion batteries in confined space. J. Loss. Prev. Process Ind. 2022, 79, 104853. [Google Scholar] [CrossRef]
- Zhao, P.; Garcia, A.; Burton, T. Initiation and propagation of curved reaction front in solids: In-sights into solid combustion and battery thermal runaway, Combust. Flame 2022, 238, 111951. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Lu, L.; Ouyang, M.; Zheng, S.; Li, J.; He, X. An electrochemical-thermal coupled overcharge-to-thermal-runaway model for lithium-ion battery. J. Power Sources 2017, 364, 328–340. [Google Scholar] [CrossRef]
- Jia, Z.; Qin, P.; Li, Z.; Wei, Z.; Jin, K.; Jiang, L.; Wang, Q. Analysis of gas release during the pro-cess of thermal runaway of lithium-ion batteries with three different cathode materials. J. Energy Storage 2022, 50, 104302. [Google Scholar] [CrossRef]
- Mishra, D.; Shah, K.; Jain, A. Investigation of the impact of flow of vented gas on propagation of thermal runaway in a Li-ion battery pack. J. Electrochem. Soc. 2021, 168, 060555. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, P.; Ping, P.; Du, Y.; Li, K.; Sun, J. Combustion behavior of lithium iron phosphate battery induced by external heat radiation. J. Loss Prev. Process Ind. 2017, 49, 961–969. [Google Scholar] [CrossRef]
- Wang, Z.; He, T.; Bian, H.; Jiang, F.; Yang, Y. Characteristics of and factors influencing thermal runaway propagation in lithium-ion battery packs. J. Energy Storage 2021, 41, 102956. [Google Scholar] [CrossRef]
- Hatchard, T.D.; MacNeil, D.D.; Stevens, D.A.; Christensen, L.; Dahn, J.R. Importance of heat transfer by radiation in li-ion batteries during thermal abuse. Electrochem. Solid-State Lett. 2000, 3, 305. [Google Scholar] [CrossRef]
- Fang, J.; Cai, J.; He, X. Experimental study on the vertical thermal runaway propagation in cylindrical Lithium-ion batteries: Effects of spacing and state of charge. Appl. Therm. Eng. 2021, 197, 117399. [Google Scholar] [CrossRef]
- Tang, Z.; Song, A.; Wang, S.; Cheng, J.; Tao, C. Numerical Analysis of Heat Transfer Mechanism of Thermal Runaway Propagation for Cylindrical Lithium-ion Batteries in Battery Module. Energies 2020, 13, 1010. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Z.; Chen, S. Quantitative analysis on the heat transfer modes in the process of thermal runaway propagation in lithium-ion battery pack under confined and semi-confined space. Int. J. Heat Mass Transf. 2021, 176, 121483. [Google Scholar] [CrossRef]
- Allafi, W.; Zhang, C.; Uddin, K.; Worwood, D.; Dinh, T.Q.; Ormeno, P.A.; Li, K.; Marco, J. A lumped thermal model of lithium-ion battery cells considering radiative heat transfer. Appl. Therm. Eng. 2018, 143, 472–481. [Google Scholar] [CrossRef]
- Lopez, C.F.; Jeevarajan, J.A.; Mukherjee, P.P. Experimental analysis of thermal runaway and propagation in Li-ion battery modules. J. Electrochem. Soc. 2015, 162, A1905. [Google Scholar] [CrossRef]
- Mishra, D.; Jain, A. Multi-mode heat transfer simulations of the onset and propagation of thermal runaway in a pack of cylindrical Li-ion batteries. J. Electrochem. Soc. 2021, 168, 020504. [Google Scholar] [CrossRef]
- Feng, X.; Lu, L.; Ouyang, M.; Li, J.; He, X. A 3D thermal runaway propagation model for a large format lithium ion battery module. Energy 2016, 115, 194–208. [Google Scholar] [CrossRef]
- Mishra, D.; Shah, K.; Jain, A. Investigation of the Impact of Radiative Shielding by Internal Partitions Walls on Propagation of Thermal Runaway in a Matrix of Cylindrical Li-Ion Cells. J. Electrochem. Soc. 2021, 168, 120507. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Liu, L.; Zhao, P. Cell-to-cell variability in Li-ion battery thermal runaway: Experimental testing, statistical analysis, and kinetic modeling. J. Energy Storage 2022, 56, 106024. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, P.; Xu, M.; Wang, X. Computational identification of the safety regime of Li-ion battery thermal runaway. Appl. Energy 2020, 261, 114440. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, L.; Zhang, L.; Chen, Y. Mitigating battery thermal runaway through mild combustion. Chem. Eng. J. Adv. 2022, 9, 100208. [Google Scholar] [CrossRef]
- Bergman, L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Fan, L.; Khodadadi, J.M.; Pesaran, A.A. A parametric study on thermal management of an air-cooled lithium-ion battery module for plug-in hybrid electric vehicles. J. Power Sources 2013, 238, 301–312. [Google Scholar] [CrossRef]
- Coleman, B.; Ostanek, J.; Heinzel, J. Reducing cell-to-cell spacing for large-format lithium ion battery modules with aluminum or PCM heat sinks under failure conditions. Appl. Energy 2016, 180, 14–26. [Google Scholar] [CrossRef]
- Jia, Y.; Uddin, M.; Li, Y.; Xu, J. Thermal runaway propagation behavior within 18650 lithium-ion battery packs: A modeling study. J. Energy Storage 2020, 31, 101668. [Google Scholar] [CrossRef]






| Parameter (Unit) | Value |
|---|---|
| Battery radius, R (mm) | 9 |
| Battery density, | 2060 |
| Heat capacity, | 1000 |
| Radial thermal conductivity, | 0.8 |
| Reaction term | Equations | |
|---|---|---|
| SEI decomposition | ||
| Anode–electrolyte reaction | ||
| Cathode–electrolyte reaction | ||
| Electrolyte decomposition | ||
| Overall heat generation | ||
| Symbol | Equations | Physical Description |
|---|---|---|
| 1.667 × 1015 (1/s) | SEI–decomposition frequency factor | |
| 2.5 × 1013 (1/s) | Anode–electrolyte frequency factor | |
| 6.667 × 1013 (1/s) | Cathode–electrolyte frequency factor | |
| 5.14 × 1025 (1/s) | Electrolyte decomposition frequency factor | |
| 1.3508 × 105 (J/mol) | SEI–decomposition activation energy | |
| 1.3508 × 105 (J/mol) | Anode–electrolyte activation energy | |
| 1.396 × 105 (J/mol) | Cathode–electrolyte activation energy | |
| 2.74 × 105 (J/mol) | Electrolyte decomposition activation energy | |
| 0.15 | Initial value of | |
| 0.75 | Initial value of | |
| 0.04 | Initial value of | |
| 1 | Initial value of | |
| 0.033 | Initial value of | |
| 1 | Reaction order for | |
| 1 | Reaction order for | |
| 1 | Reaction order for | |
| 1 | Reaction order for | |
| 1 | Reaction order for | |
| 2.57 × 105 (J/kg) | Reaction heat of SEI–decomposition | |
| 1.714 × 106 (J/kg) | Reaction heat of anode–electrolyte | |
| 3.14 × 105 (J/kg) | Reaction heat of cathode–electrolyte | |
| 1.55 × 105 (J/kg) | Reaction heat of electrolyte decomposition | |
| 6.104 × 102 (kg/m3) | Specific carbon content in jellyroll | |
| 1.221 × 103 (kg/m3) | Specific positive active content in jellyroll | |
| 4.069 × 102 (kg/m3) | Specific electrolyte content in jellyroll | |
| 8.314 (J/mol/K) | Universal gas constant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chen, Y.; Ge, H.; Jain, A.; Zhao, P. Radiation-Induced Thermal Runaway Propagation in a Cylindrical Li-Ion Battery Pack: Non-Monotonicity, Chemical Kinetics, and Geometric Considerations. Appl. Sci. 2023, 13, 8229. https://doi.org/10.3390/app13148229
Zhang L, Chen Y, Ge H, Jain A, Zhao P. Radiation-Induced Thermal Runaway Propagation in a Cylindrical Li-Ion Battery Pack: Non-Monotonicity, Chemical Kinetics, and Geometric Considerations. Applied Sciences. 2023; 13(14):8229. https://doi.org/10.3390/app13148229
Chicago/Turabian StyleZhang, Liwen, Yi Chen, Haiwen Ge, Ankur Jain, and Peng Zhao. 2023. "Radiation-Induced Thermal Runaway Propagation in a Cylindrical Li-Ion Battery Pack: Non-Monotonicity, Chemical Kinetics, and Geometric Considerations" Applied Sciences 13, no. 14: 8229. https://doi.org/10.3390/app13148229
APA StyleZhang, L., Chen, Y., Ge, H., Jain, A., & Zhao, P. (2023). Radiation-Induced Thermal Runaway Propagation in a Cylindrical Li-Ion Battery Pack: Non-Monotonicity, Chemical Kinetics, and Geometric Considerations. Applied Sciences, 13(14), 8229. https://doi.org/10.3390/app13148229








