Biosynthesis of Silver Nanoparticles Using Tabernaemontana ventricosa Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Preparation of Plant Extracts
2.2.1. Reflux Solvent Extraction
2.2.2. Fresh Extract
2.2.3. Powder Extract
2.3. Synthesis of AgNPs
2.4. UV-Visible Spectral Analysis
2.5. Preparation, Purification, and Quantification of Samples
2.6. Characterization of AgNPs
2.6.1. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray (EDX) Analysis
2.6.2. High-Resolution Transmission Electron Microscopy (HRTEM)
2.6.3. Fourier Transform Infrared (FTIR) Spectral Analysis
2.6.4. Nanoparticle Tracking Analysis (NTA)
2.7. Statistical Analyses
3. Results and Discussion
3.1. Visual Inspection of Synthesized AgNPs
3.2. UV-Visible Spectroscopy
3.3. Quantification of Synthesized AgNPs
3.4. Scanning Electron Microscopy (SEM)
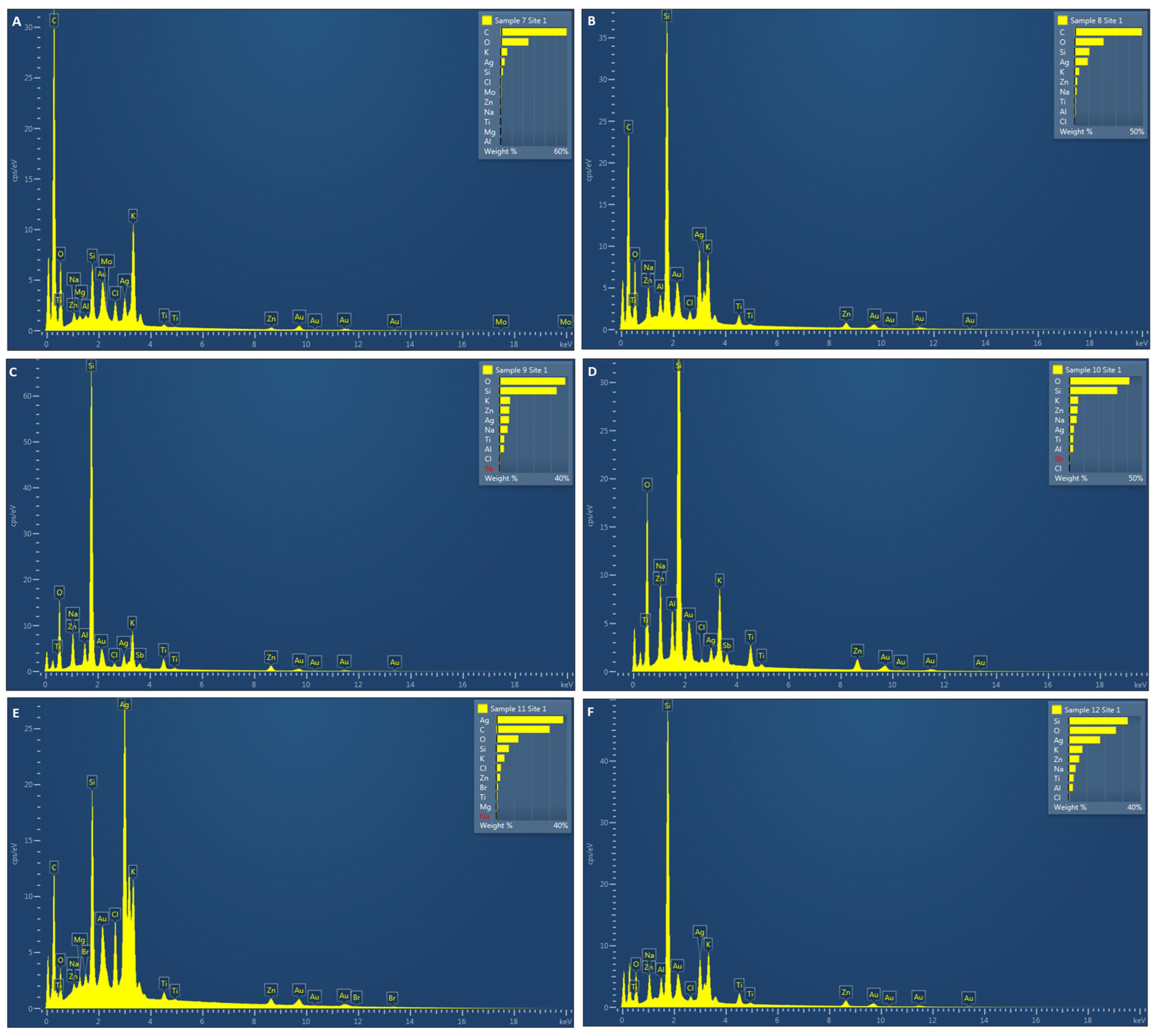
3.5. Energy-Dispersive X-ray (EDX) Analysis
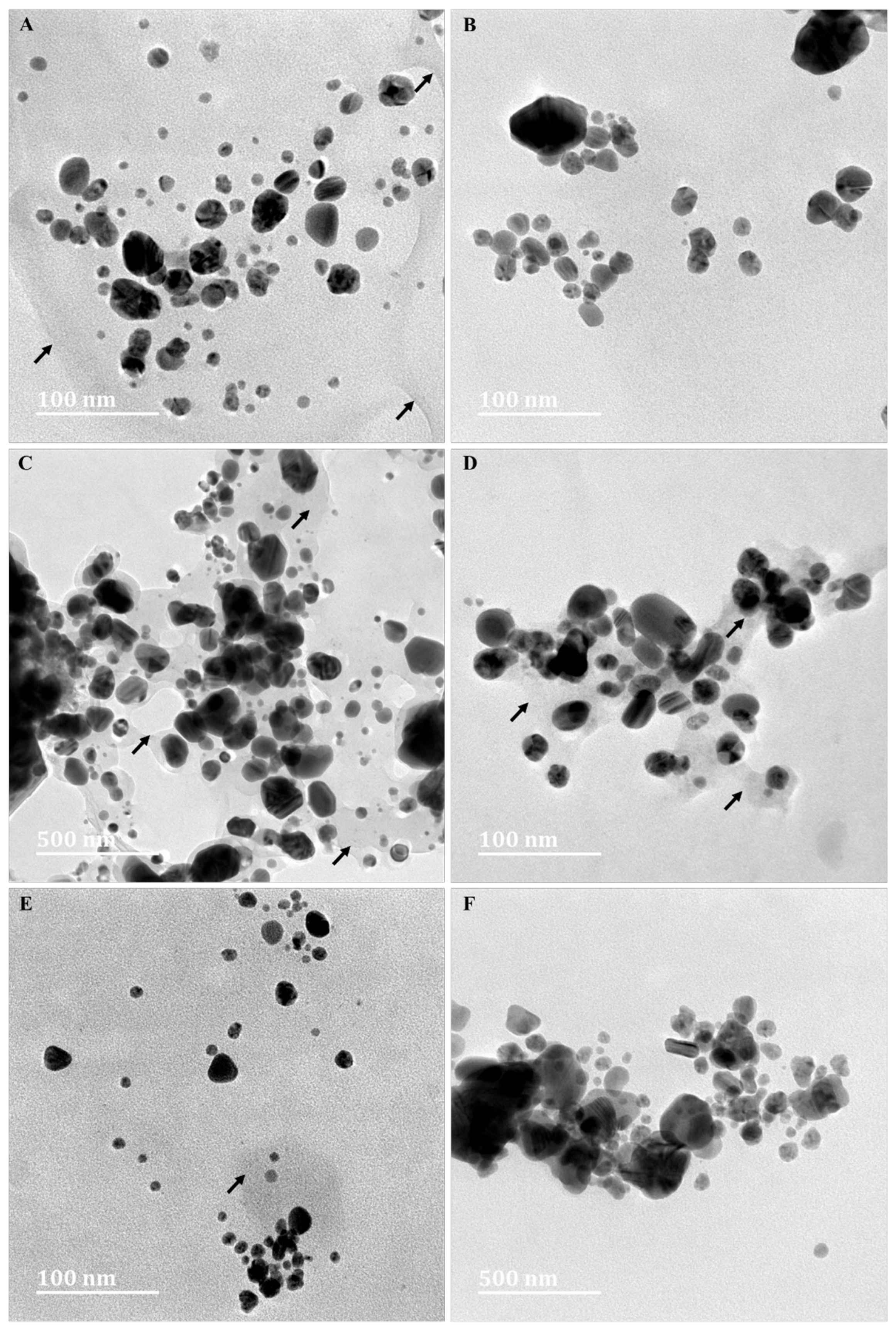
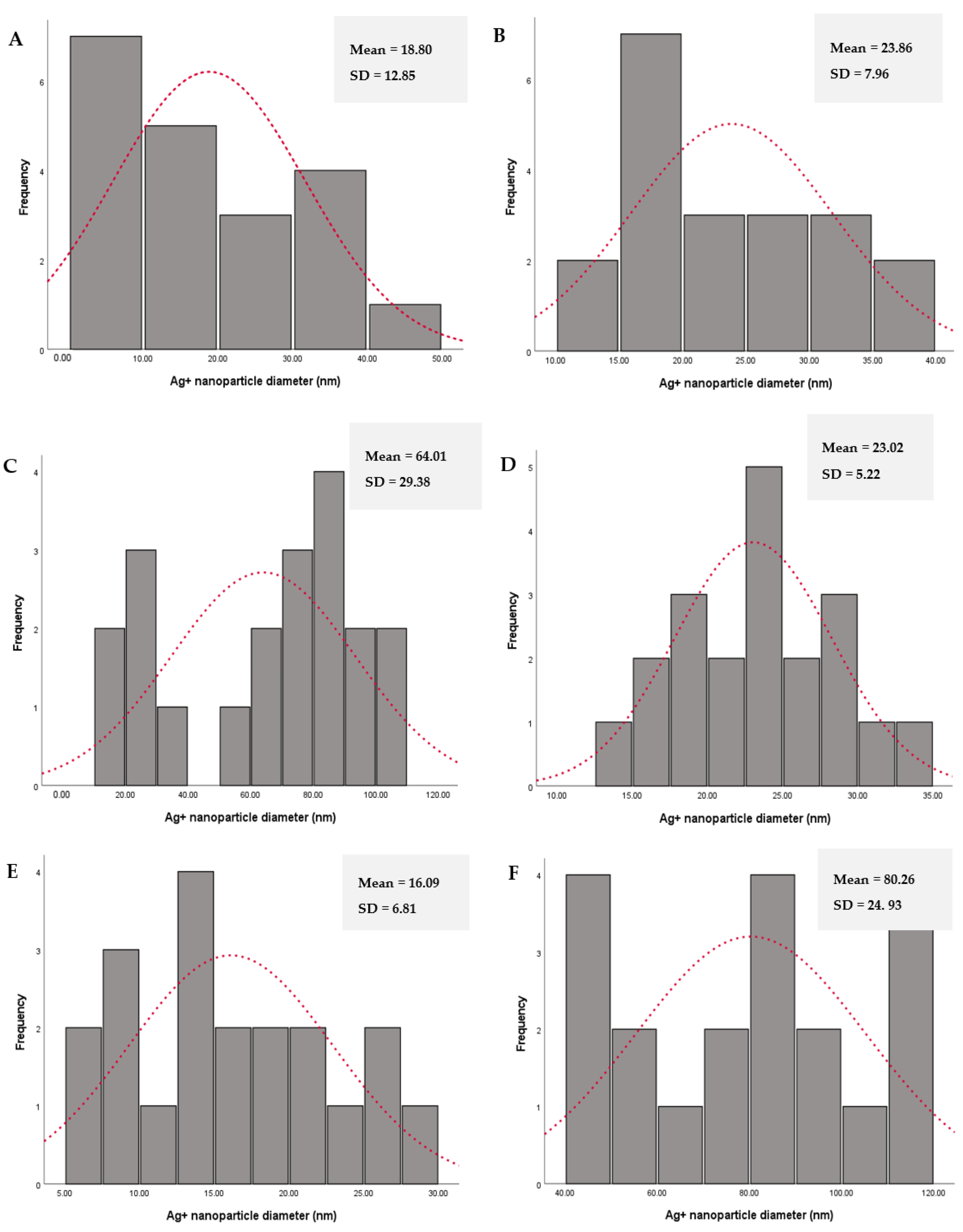
3.6. High-Resolution Transmission Electron Microscopy (HRTEM) Analyses
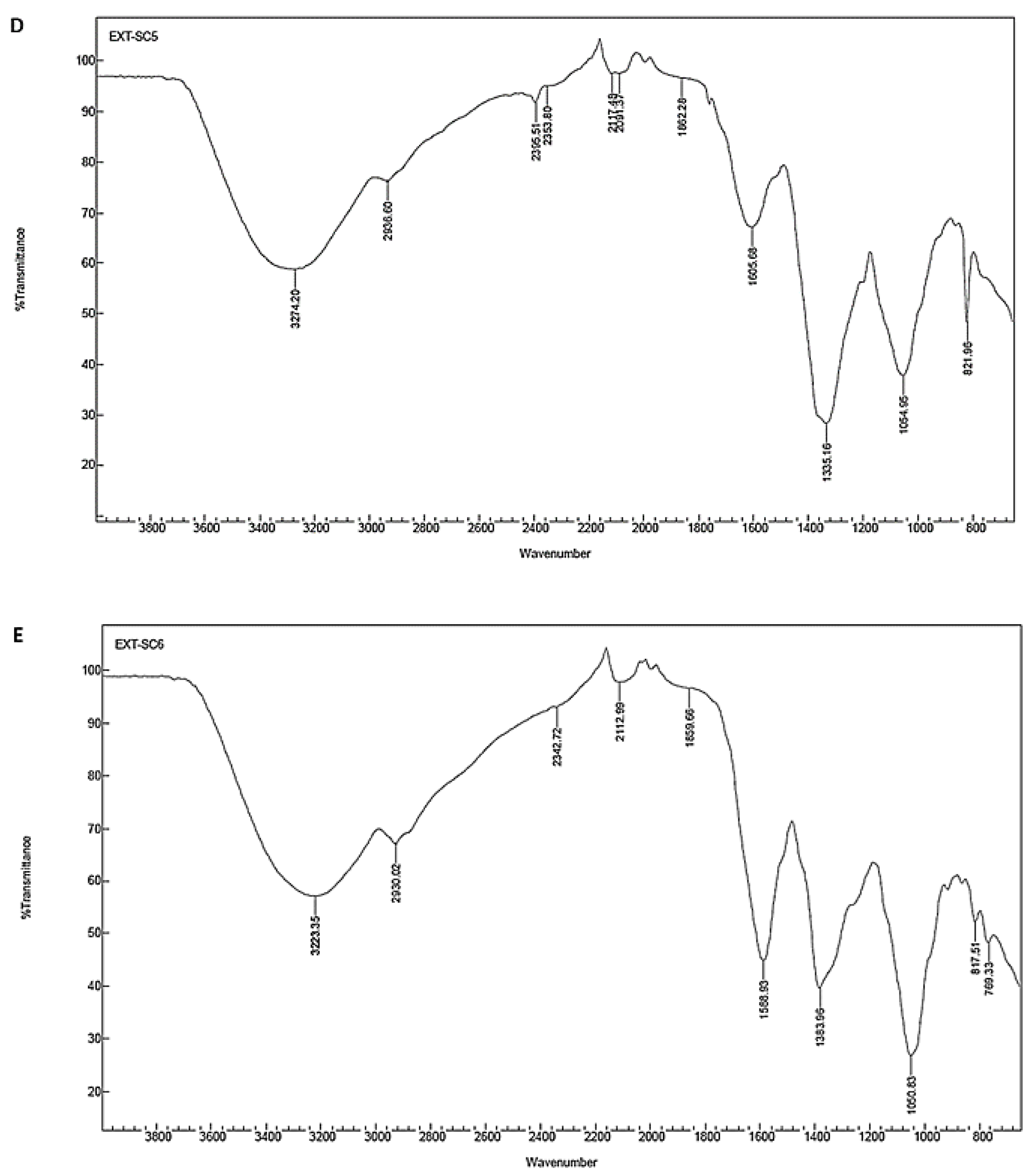
3.7. Fourier Transform Infrared (FTIR) Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baran, M.F.; Keskin, C.; Baran, A.; Hatipoğlu, A.; Yildiztekin, M.; Küçükaydin, S.; Kurt, K.; Ho¸sgören, H.; Sarker, M.M.R.; Sufianov, A.; et al. Green Synthesis of Silver Nanoparticles from Allium cepa L. Peel Extract, Their Antioxidant, Antipathogenic, and Anticholinesterase Activity. Molecules 2023, 28, 2310. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, P.; Aarti, C.; Kumari, P. Synthesis and characterization of silver nanoparticles using Tabernaemontana divaricata and its cytotoxic activity against MCF7 cell line. Int. J. Pharmacol. Pharm. Sci. 2014, 6, 86–90. [Google Scholar]
- Soliman, M.I.; Mohammed, N.S.; El-Sherbeny, G.; Safhi, F.A.; ALshamrani, S.M.; Alyamani, A.A.; Alharthi, B.; Qahl, S.H.; Al Kashgry, N.A.T.; Abd-Ellatif, S.; et al. Antibacterial, Antioxidant Activities, GC-Mass Characterization, and Cyto/Genotoxicity Effect of Green Synthesis of Silver Nanoparticles Using Latex of Cynanchum acutum L. Plants 2022, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Chouke, P.B.; Shrirame, T.; Potbhare, A.K.; Mondal, A.; Chaudhary, A.R.; Mondal, S.; Thakare, S.R.; Nepovimova, E.; Valis, M.; Kuca, K.; et al. Bioinspired metal/metal oxide nanoparticles: A road map to potential applications. Mater. Today Adv. 2022, 16, 100314. [Google Scholar] [CrossRef]
- Potbhare, A.K.; Umekar, M.S.; Chouke, P.B.; Bagade, M.B.; Tarik Aziz, S.K.; Abdala, A.A.; Chaudhary, R.G. Bioinspired graphenebased silver nanoparticles: Fabrication, characterization and antibacterial activity. Mater. Today Proc. 2019, 29, 720–725. [Google Scholar] [CrossRef]
- Lateef, A.; Folarin, B.I.; Oladejo, S.M.; Akinola, P.O.; Beukes, L.S.; Gueguim-Kana, E.B. Characterization, anti-microbial, anti-oxidant, and anti-coagulant activities of silver nanoparticles synthesized from Petiveria alliacea L. leaf extract. Prep. Biochem. Biotechnol. 2018, 48, 646–652. [Google Scholar] [CrossRef]
- Safavi, K. Evaluation of using nanomaterial in tissue culture media and biological activity. In Proceedings of the 2nd International Conference on Ecological, Environmental and Biological Sciences, Bali, Idonesia, 13–14 October 2012. [Google Scholar]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Sumra, A.A.; Zain, M.; Saleem, T.; Yasin, G.; Azhar, M.F.; Zaman, Q.U.; Budhram-Mahadeo, V.; Ali, H.M. Biogenic Synthesis, Characterization, and In Vitro Biological Evaluation of Silver Nanoparticles Using Cleome brachycarpa. Plants 2023, 12, 1578. [Google Scholar] [CrossRef]
- Kumar, K.; Chauhan, P.R.; Kumar, R.; Bharj, R.S. Irreversibility analysis in Al2O3-water nanofluid flow with variable property. Facta Univ.-Ser. Mech. 2022, 20, 503–518. [Google Scholar] [CrossRef]
- He, J.H.; Abd-Elazem, N.Y. The carbon nanotube-embedded boundary layer theory for energy harvesting. Facta Univ. Ser. Mech. 2022, 2, 211–235. [Google Scholar] [CrossRef]
- Lin, L.; Gong, W.Z.; Wang, S.Y. Hollow PET fibers containing silver particles as antibacterial materials. J. Text. Inst. 2011, 102, 419–423. [Google Scholar] [CrossRef]
- Khan, Y.; Numan, M.; Ali, M.; Khali, A.T.; Abbas, N.; Shinwar, Z.K. Bio-synthesized silver nanoparticles using different plant extracts as anti-cancer agent. J. Nanomed. Biother. Discov. 2017, 7, 2. [Google Scholar]
- Alowaiesh, B.F.; Alhaithloul, H.A.S.; Saad, A.M.; Hassanin, A.A. Green Biogenic of Silver Nanoparticles Using Polyphenolic Extract of Olive Leaf Wastes with Focus on Their Anticancer and Antimicrobial Activities. Plants 2023, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Sigamoney, M.; Shaik, S.; Govender, P.; Krishna, S.B.N. African leafy vegetables as bio-factories for silver nanoparticles: A case study on Amaranthus dubius C Mart. Ex Thell. S. Afri. J. Bot. 2016, 103, 230–240. [Google Scholar] [CrossRef]
- Chandra, H.; Kumari, P.; Bontempi, E.; Yadav, S. Medicinal plants: Treasure trove for green synthesis of metallic nanoparticles and their biomedical applications. Biocatal. Agric. Biotechnol. 2020, 24, 101518. [Google Scholar] [CrossRef]
- Habeeb Rahuman, H.B.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. Medicinal Plants Mediated the Green Synthesis of Silver Nanoparticles and Their Biomedical Applications. IET Nanobiotechnol. 2022, 16, 115–144. [Google Scholar] [CrossRef]
- Velgosová, O.; Mrazíková, A. Limitations and Possibilities of Green Synthesis and Long-Term Stability of Colloidal Ag Nanoparticles. In Proceedings of the 1st International Conference on Mechanical Engineering and Applied Science, Dhaka, Bangladesh, 22–23 February 2017; American Institute of Physics Inc.: College Park, MD, USA, 2017; Volume 1918. [Google Scholar]
- Velgosova, O.; Veselovský, L. Synthesis of Ag nanoparticle using R. officinalis, U. dioica and V. vitis-idaea extracts. Mater. Lett. 2019, 248, 150–152. [Google Scholar] [CrossRef]
- Singh, A.K. Engineered Nanoparticles: Structure, Properties and Mechanisms of Toxicity; Academic Press: London, UK, 2015. [Google Scholar]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Chouhan, N. Silver Nanoparticles: Synthesis, Characterization and Applications. In Silver Nanoparticles-Fabrication, Characterization and Applications; Maaz, K., Ed.; IntechOpen: Rajasthan, India, 2018. [Google Scholar]
- Akhtar, M.; Swamy, M.K.; Umar, A.; Al Sahli, A.A. Biosynthesis and characterization of silver nanoparticles from methanol leaf extract of Cassia didymobotyra and assessment of their antioxidant and antibacterial activities. J. Nanosci. Nanotechnol. 2015, 15, 9818–9823. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Green synthesis of silver nanoparticles using Carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Mallikarjuna, K.; Narasimha, G.; Dillip, G.R.; Praveen, B.; Shreedhar, B.; Shree Lakshmi, C.; Reddy, B.V.S.; Deva Prasad Raju, B. Green synthesis of silver nanoparticles using Ocimim leaf extract and their characterization. Dig. J. Nanomater. Biostructures. 2011, 6, 181–186. [Google Scholar]
- Vanaja, M.; Annadurai, G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl. Nanosci. 2013, 3, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Bar, H.; Bhui, D.K.R.; Sahoo, G.P.; Sarkar, P.; De, P.S.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surface. Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Tehri, N.; Kaur, R.; Maity, M.; Chauhan, A.; Hooda, V.; Vashishth, A.; Kumar, G. Biosynthesis, characterization, bactericidal and sporicidal activity of silver nanoparticles using the leaves extract of Litchi chinensis. Prep. Biochem. Biotechnol. 2020, 50, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Garg, S.; Ratan, J.K.; Giri, A.S. Silver Nanoparticles from Tabernaemontana Divaricate leaf extract: Mechanism of action and Bio-application for photo degradation of 4-Aminopyridine. Res. Sq. 2023, 30, 24856–24875. [Google Scholar] [CrossRef]
- Schmidt, E.; Lotter, M.; McCleland, W. Trees and Shrubs of Mpumalanga and Kruger National Park; Jacana Media: Johannesburg, South Africa, 2002; pp. 566–569. [Google Scholar]
- Schmelzer, G.B.; Gurib-Fakim, A. Plant Resources of Tropical Africa (PROTA). In Medicinal Plants, 1st ed.; Backhuys Publishers: Wageningen, The Netherlands, 2008; Volume 1, pp. 597–598. [Google Scholar]
- Mehrbod, P.; Abdalla, M.A.; Njoya, E.M.; Ahmed, A.S.; Fotouhi, F.; Farahmand, B.; Gado, D.A.; Tabatabaian, M.; Fasanmi, O.G.; Eloff, J.N.; et al. South African medicinal plant extracts active against influenza A virus. BMC Complement. Alterna. Med. 2018, 18, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Akwu, N.A.; Naidoo, Y.; Singh, M.; Nundkumar, N.; Daniels, A.; Lin, J. Two Temperatures Biogenic Synthesis of Silver Nanoparticles from Grewia lasiocarpa E. Mey. ex Harv. Leaf and Stem Bark Extracts: Characterization and Applications. J. BioNano. Sci. 2021, 11, 142–158. [Google Scholar] [CrossRef]
- Netai, M.M.; Stephen, N.; Musekiwa, C. Synthesis of silver nanoparticles using wild Cucumis anguria: Characterization and antibacterial activity. Afr. J. Biotechnol. 2017, 16, 1911–1921. [Google Scholar]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Saddal, S.K.; Telang, T.; Bhange, V.P.; Kopulwar, A.P.; Santra, S.R.; Soni, M. Green synthesis of silver nanoparticles using stem extract of Berberis aristata and to study its characterization and antimicrobial activity. J. Pharm. Res. 2018, 12, 840. [Google Scholar]
- Kumar, V.; Yadav, S.C.; Yadav, S.K. Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J. Chem. Technol. Biotechnol. 2010, 85, 1301–1309. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Khanra, K.; Panja, S.; Choudhuri, I.; Chakraborty, A.; Bhattacharyya, N. Antimicrobial and cytotoxicity effect of silver nanoparticle synthesized by Croton bonplandianum Baill. leaves. Nanomed. J. 2016, 3, 15–22. [Google Scholar]
- Zargar, M.; Hamid, A.A.; Bakar, F.A.; Shamsudin, M.N.; Shameli, K.; Jahanshiri, F.; Farahani, F. Green synthesis and anti-bacterial effect of silver nanoparticles using Vitex negundo L. Molecules 2011, 16, 6667–6676. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, S.; Vithiya, B.S.M.; Prasad, T.A.A. Green synthesis of silver nanoparticles by employing the Allium fistulosum, Tabernaemontana divaricate and Basella alba leaf extracts for antimicrobial applications. J. King Saud Univ. Sci. 2022, 34, 101939. [Google Scholar] [CrossRef]
- Mathew, S.; Victorio, C.P.; Sidhi, J.; Baby, B.T. Biosynthesis of silver nanoparticle using flowers of Calotropis gigantea (L.) WT Aiton and activity against pathogenic bacteria. Arab. J. Chem. 2020, 13, 9139–9144. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Siganl. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y.L. Silver nanoparticle synthesis using lignin as reducing and capping agents: A kinetic and mechanistic study. Int. J. Biol. Macromol. 2016, 82, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Valenti, L.E.; Giacomelli, C.E. Stability of silver nanoparticles: Agglomeration and oxidation in biological relevant conditions. J. Nanopart Res. 2017, 19, 156. [Google Scholar] [CrossRef]
- Song, J.Y.; Jang, H.K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Sivakumar, J.; Premkumar, C.; Santhanam, P.; Saraswathi, N. Biosynthesis of silver nanoparticles using Calotropis gigantean leaf. Afr. J. Basic Appl. Sci. 2011, 3, 265–270. [Google Scholar]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Heemasagar, D.; Jeeva, K.; Sureshkumar, M. Enhanced anti-microbial activity of honey with green synthesized AgNPs by using Tabernaemontana Coronaria (JACQ.) wild flower extract. Am. J. PharmaTech Res. 2014, 4, 51. [Google Scholar]
- Schripsema, J.; Hermans-Lokkerbol, A.; Van Der Heijden, R.; Verpoorte, R.; Svendsen, A.B.; Van Beek, T.A. Alkaloids of Tabernaemontana ventricosa. J. Nat. Prod. 1986, 49, 733–735. [Google Scholar] [CrossRef]
- Annadurai, G.; Gnanajobitha, G.; Kannan, C. Green synthesis of silver nanoparticle using Elettaria cardamomom and assessment of its anti-microbial activity. Int. J. Pharm. Sci. 2021, 3, 323–330. [Google Scholar]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81. [Google Scholar]
- Joshi, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Mishra, V.K. Plant-mediated synthesis of copper oxide nanoparticles and their biological applications. Nanomater. Plant Potential 2019, 221–237. [Google Scholar]
- Daniels, A.N.; Singh, M. Sterically stabilized siRNA: Gold nanocomplexes enhance c-MYC silencing in a breast cancer cell 869 model. Nanomedicine 2019, 14, 1387–1401. [Google Scholar] [CrossRef]
- Akinyelu, J.; Singh, M. Folate-tagged chitosan functionalized gold nanoparticles for enhanced delivery of 5-fluorouracil to cancer cells. Appl. Nanosci. 2019, 9, 7–17. [Google Scholar] [CrossRef]
- Oladimeji, O.; Akinyelu, J.; Daniels, A.; Singh, M. Modified Gold Nanoparticles for efficient Delivery of Betulinic Acid to Cancer Cell Mitochondria. Int. J. Mol. Sci. 2021, 22, 5072. [Google Scholar] [CrossRef]
- Preetam Raj, J.P.; Purushothaman, M.; Ameer, K.; Panicker, S.G. In-vitro anticancer and antioxidant activity of gold nanoparticles conjugate with Tabernaemontana divaricata flower SMs against MCF-7 breast cancer cells. Korean Chem. Eng. Res. 2016, 54, 5–80. [Google Scholar] [CrossRef]
- Anbukkarasi, M.; Thomas, P.A.; Sheu, J.R.; Geraldine, P. In vitro antioxidant and anticataractogenic potential of silver nanoparticles biosynthesized using an ethanolic extract of Tabernaemontana divaricata leaves. Biomed. Pharmacother. 2017, 91, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Mahmud, S.; Yasin, H.; Asif, R.; Qadeer, K.; Ahmad, I. Authentication of various commercially available crude drugs using different quality control testing parameters. Pak. J. Pharm. Sci. 2020, 33, 1641–1657. [Google Scholar] [PubMed]
- Patel, N. Biosynthesis and Anti-Bacterial Activity of Silver and Gold Nanoparticles from the Leaf and Callus Extracts of Amaranthus dubius, Gunnera perpensa, Ceratotheca triloba and Catharanthus roseus. Master Thesis, Durban University of Technology, South Africa, 2013. [Google Scholar]
- Firdhouse, M.J.; Lalitha, P. Phyto-assisted synthesis and characterization of silver nanoparticles from Amaranthus dubius. Int. J. Appl. Biol. Pharma. Tech. 2012, 3, 96–101. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef] [Green Version]





| Extracts | Leaves | Stems | Leaves | Stems |
|---|---|---|---|---|
| Dried Extract Yield (g) | Yield (%) | |||
| Methanol extract | 0.065 | 0.073 | 0.659 | 0.737 |
| Fresh extract | 0.007 | 0.009 | 0.023 | 0.027 |
| Powdered extract | 0.163 | 0.119 | 1.638 | 1.190 |
| Extracts | Elemental Silver (%) |
|---|---|
| Leaf methanol | 8.78 ± 1.32 |
| Stem methanol | 7.33 ± 3.23 |
| Fresh leaf | 14.10 ± 12.08 |
| Fresh stem | 7.10 ± 5.66 |
| Powdered leaf | 37.00 ± 2.04 |
| Powdered stem | 15.14 ± 3.34 |
| Extracts | Nanoparticle Diameter (nm) | Zeta Potential (mV) |
|---|---|---|
| Leaf methanol | 80.9 ± 8.8 | −3.5 ± 5.1 |
| Stem methanol | 70.2 ± 6.1 | −30.3 ± 0.1 |
| Fresh leaf | 125.0 ± 41.8 | 15.6 ± 5.6 |
| Fresh stem aqueous | 63.9 ± 63.9 | 7.2 ± 7.2 |
| Powdered leaf | 120.5 ± 36.2 | 3.5 ± 0.1 |
| Powdered stem | 147.4 ± 7.4 | 5.9 ± 0.0 |
| Plant Extract | Absorption Frequency (cm−1) | Types of Absorption/ Vibration | Appearance | Interference/ Functional Group | Compound Class |
|---|---|---|---|---|---|
| Leaf methanol | 3295.32 | Stretch | Strong broad | O–H | Alcohol |
| 3273.38 | Stretch | Strong broad | O–H | Alcohol | |
| 2924.68 | Stretch | Medium | C–H | Alkane | |
| 2394.69 | Stretch | Strong | O=C=O | Carbon dioxide | |
| 2105.80 | Stretch | Weak | C≡C | Alkyne | |
| 1623.91 | Stretch | Medium | C=C | Conjugated alkene | |
| 1336.46 | Bending | Medium | O–H | Alcohol | |
| 1050.28 | Stretch | Strong | C–O | Primary alcohol | |
| 802.05 | Bending | Medium | C=C | Alkene | |
| Stem methanol | 3304.50 | Stretch | Medium | N–H | Aliphatic primary amine |
| 3261.97 | Stretch | Strong broad | O–H | Alcohol | |
| 2922.18 | Stretch | Strong broad | N–H | amine salt | |
| 2340.00 | Stretch | Strong | O=C=O | Carbon dioxide | |
| 2110.75 | Stretch | Weak | C≡C | Alkyne | |
| 1601.17 | Stretch | Medium | C=C | Conjugated alkene | |
| 1334.31 | Bending | Medium | O–H | Alcohol | |
| 1043.12 | Stretch | Strong broad | CO–O–CO | anhydride | |
| 989.51 | Bending | Strong | C=C | alkene | |
| 922.32 | Bending | Strong | C=C | alkene | |
| 822.50 | Bending | Medium | C=C | alkene | |
| Fresh leaf | 3260.11 | Stretch | Strong broad | O–H | Alcohol |
| 2933.85 | Stretch | Strong broad | N–H | amine salt | |
| 2395.37 | Stretch | Strong | O=C=O | Carbon dioxide | |
| 2346.05 | Stretch | Strong | O=C=O | Carbon dioxide | |
| 2095.91 | Stretch | Strong | N=C=S | Isothiocyanate | |
| 1599.57 | Stretch | Strong | N–O | Nitro compound | |
| 1319.11 | Bending | Medium | O–H | Phenol | |
| 1044.99 | Stretch | Strong broad | S=O | sulfoxide | |
| 820.01 | Bending | Medium | C=C | alkene | |
| Fresh stem | 3274.20 | Stretch | Strong broad | O–H | Alcohol |
| 2936.60 | Stretch | Strong broad | N–H | amine salt | |
| 2395.51 | Stretch | Strong | O=C=O | Carbon dioxide | |
| 2353.80 | Stretch | Strong | O=C=O | Carbon dioxide | |
| 2117.48 | Stretch | Weak | C≡C | Alkyne | |
| 2091.37 | Stretch | Strong | N=C=S | Isothiocyanate | |
| 1862.28 | Bending | Weak | C–H | Aromatic compound | |
| 1605.68 | Stretch | Medium | C=C | Conjugated alkene | |
| 1335.16 | Stretch | Strong | S=O | Sulfonate/sulfonamide | |
| 1054.95 | Stretch | Strong | C–O | Primary alcohol | |
| 821.96 | Bending | Medium | C=C | Alkene | |
| Powder leaf | 3223.35 | Stretch | Strong broad | O–H | Alcohol |
| 2930.02 | Stretch | Strong broad | N–H | Amine salt | |
| 2342.72 | Stretch | Strong | O=C=O | Carbon dioxide | |
| 2112.99 | Stretch | Weak | C≡C | Alkyne | |
| 1859.66 | Bending | Weak | C–H | Aromatic compound | |
| 1588.93 | Bending | Medium | N–H | Amine | |
| 1383.96 | Bending | Medium | C–H | Aldehyde | |
| 1050.83 | Stretch | Strong | C–O | Primary alcohol | |
| 817.51 | Bending | Medium | C=C | Alkene | |
| 769.33 | Stretch | Strong | C–Cl | Halo compound | |
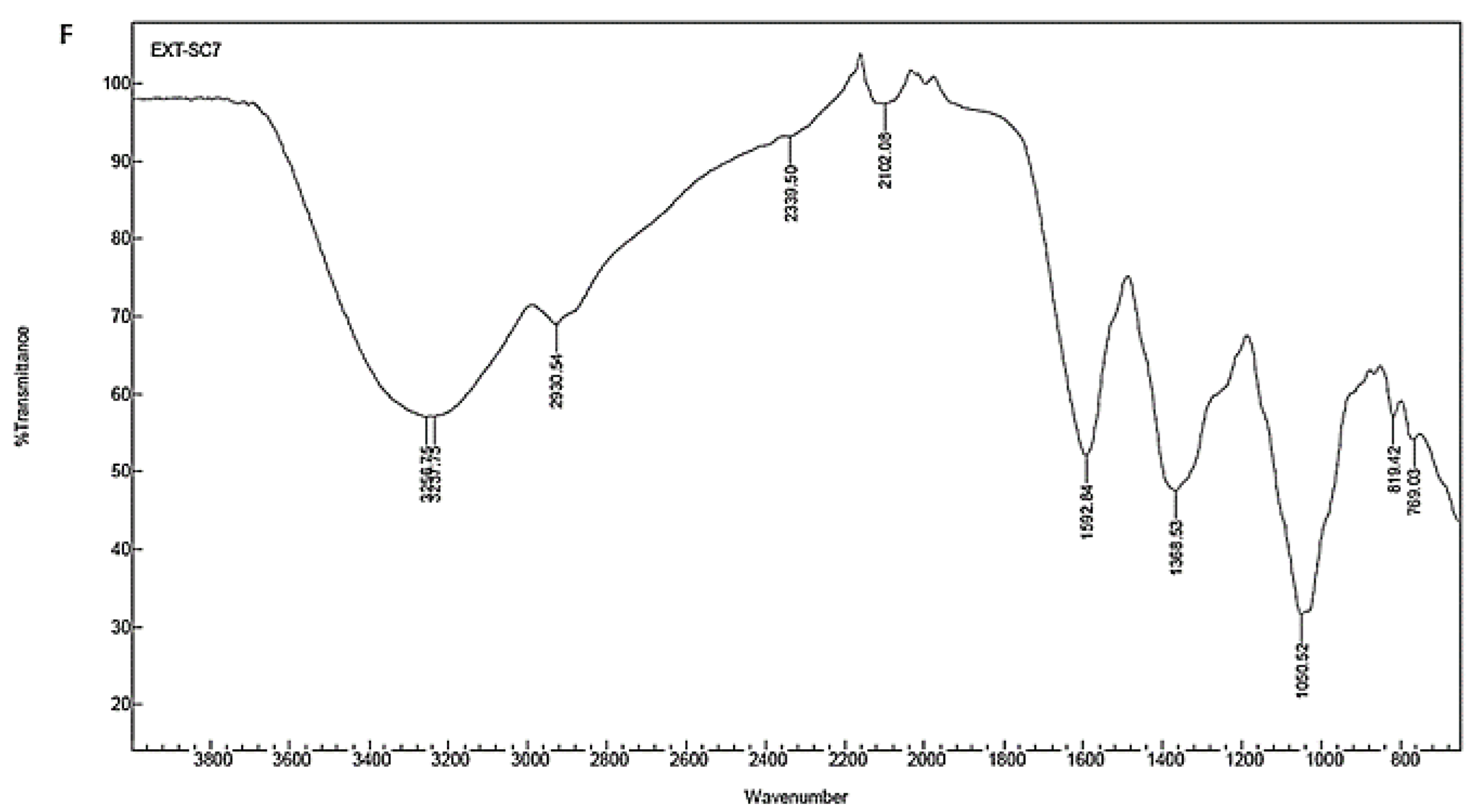
| Powder stem | 3256.75 | Stretch | Strong broad | O–H | Alcohol |
| 3237.75 | Stretch | Strong broad | O–H | Alcohol | |
| 2930.54 | Stretch | Strong broad | N–H | Amine salt | |
| 2339.50 | Stretch | Strong | O=C=O | Carbon dioxide | |
| 2102.08 | Stretch | Weak | C≡C | Alkyne | |
| 1592.84 | Bending | Medium | N–H | Amine | |
| 1368.53 | Bending | Medium | O–H | Alcohol | |
| 1050.52 | Stretch | Strong | C–O | Primary alcohol | |
| 819.44 | Bending | Medium | C=C | alkene | |
| 769.03 | Stretch | Strong | C–Cl | Halo compound |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidoo, C.M.; Naidoo, Y.; Dewir, Y.H.; Singh, M.; Daniels, A.N.; Lin, J. Biosynthesis of Silver Nanoparticles Using Tabernaemontana ventricosa Extracts. Appl. Sci. 2023, 13, 8395. https://doi.org/10.3390/app13148395
Naidoo CM, Naidoo Y, Dewir YH, Singh M, Daniels AN, Lin J. Biosynthesis of Silver Nanoparticles Using Tabernaemontana ventricosa Extracts. Applied Sciences. 2023; 13(14):8395. https://doi.org/10.3390/app13148395
Chicago/Turabian StyleNaidoo, Clarissa Marcelle, Yougasphree Naidoo, Yaser Hassan Dewir, Moganavelli Singh, Aliscia Nicole Daniels, and Johnson Lin. 2023. "Biosynthesis of Silver Nanoparticles Using Tabernaemontana ventricosa Extracts" Applied Sciences 13, no. 14: 8395. https://doi.org/10.3390/app13148395









