1. Introduction
With the increasing demand for ready-to-eat food products and globalization, concerns for food quality and safety are expanding. Meat-based perishable food products are subjected to bacterial contamination causing undesirable reactions [
1]. The sliced meat products are rich in fats, proteins, and some (added) carbohydrates that may be metabolized by spoilage microorganisms, resulting in off-odors, off-flavors, gas formation, discoloration, changes in texture, and slime-formation. These changes make the product unsuitable for consumption. Although cooking eliminates the majority of the microorganisms, products may be contaminated during handling operations after the cooking process, for instance, during packaging or slicing by shop assistants [
2,
3]. Microbial surface contamination of cooked meat products has an influence on the potential shelf-life stability, creating important food preservation challenges [
2]. Vacuum and modified-atmosphere packaging (MAP) allows for an extension of the shelf-life period of meat-based products with minimal changes in the chemical and physical properties of the products, such as sliced cooked ham [
2,
3]. In order to inhibit the growth of microorganisms responsible for food spoilage in ready-to-eat sliced ham, bioactive compounds can be incorporated into product formulation, coated on its surface, or incorporated into the packaging material [
1]. Cooked ham packaged in a modified atmosphere is a very popular product, subjected to abundant microbial contamination throughout its shelf life that can lead to deterioration of both textural properties and microbial purity [
3,
4]. Consumers are happy to purchase sliced ham packed in a vacuum or in MAP. However, many of them decide to buy cooked ham that is sliced in shops, and there can be situations where it is contaminated by the staff while being cut [
3]. The assessment of appropriate secondary shelf life, defined as the time after package opening or after buying a meat product sliced in a butcher shop by a shop assistant during which the food product retains a required level of quality, is pivotal for reducing domestic food waste [
4]. The need to produce safe and healthy meat-based products not based on chemical additives has resulted in the control of both spoilage and pathogenic microorganisms. This requirement has been led by consumer demand for safe food products while, in addition, leading to a huge decrease in processing, cutting out many preservatives, and an extension in shelf-life [
5]. Active food packaging plays an important role in the preservation of the quality of food products and ensuring their safety [
6]. Packaging materials covered with active coatings are designed to release active substances into the product and, by doing so, can extend the shelf life of the food while maintaining its safety and quality [
6,
7]. The fabrication of active films, which are based on a surface covering method, contains a number of biobased carriers and antibacterial substances, including those based on plant extracts to create active layers, that can result in an increase in both the primary or secondary shelf-life of foodstuffs [
3,
4,
5].
Uncaria tomentosa, known as a cat’s claw, is a medicinal plant used as complementary medicine to treat many diseases. Extensive research carried out over many years has demonstrated its antibacterial, antiviral, anti-inflammatory, antioxidant, anticonceptive, and immunostimulant effects, as well as not being toxic [
8,
9].
Fomitopsis betulina is a plant known as a birch polypore. It is a medicinal and edible mushroom (when young). The effectiveness of
F. betulina originates mostly from the triterpenoids, especially lanostane derivatives present in the fungus [
9,
10]. The results of the previous work [
9] have shown that
Fomitopsis betulina extract was unable to inhibit
S. aureus growth. A lower than 1-log reduction in the number of bacterial cells was noted for 5% and 10% plant extracts. A greater than 2-log reduction in the number of this strain was only observed in the case of a 50% extract of
F. betulina. It should be underlined that
U. tomentosa extract had a much lower influence on the
S. aureus cells than the
F. betulina extract. When analyzing the effect of
F. betulina and
U. tomentosa extracts on
B. subtilis cells, it was demonstrated that all of the extracts showed lower than a 2-log reduction but higher than a 1-log reduction in the number of bacterial cells. The results of the previous study have also confirmed that
F. betulina and
U. tomentosa extracts had no activity against
E. coli cells [
9]. An excellent example is an earlier work carried out by the authors [
11], who analyzed the antibacterial effect of a methyl-hydroxy-propyl-cellulose (MHPC) active layer (G) containing 5% of geraniol in the coating carrier. The authors observed a 3-log reduction in the number of
S. aureus cells, a 4-log reduction in the number of
E. coli cells, and a 2-log reduction in the number of
P. syringae cells. It should also be underlined that a characteristic aroma of geraniol in the active layer was noticeable. The findings of the previous work demonstrated that a decrease in the amount of geraniol (by 99.75%) in the MHPC carrier (G1) had a significant effect on the activity of the coating G1 against Gram-positive and Gram-negative strains. This coating slightly decreased the number of
S. aureus strains (1 log). The G1 layer was found not to be active against
E. coli and
P. syringae cells; however, a lowering of the scent of the coating G1 compared to G was observed. The authors confirmed [
12] that the coating containing 0.082 g of ZnO nanoparticles in 100 mL of MHPC carrier inhibited the growth of both Gram-positive and Gram-negative bacteria. The results of an earlier study [
11] confirmed that the addition of the 0.041 g of nanoparticles (decreased amount) to the 100 mL of MHPC carrier containing 0.0125 g of geraniol (decreased amount compared to the MHPC containing 5% of geraniol) led to obtaining the active layer, which inhibited the growth of
S. aureus cells. Additionally, a 4-log reduction in the number of
E. coli and
P. syringae cells was noticed.
Zinc oxide nanoparticles are widely used as antimicrobial agents. They have ideal properties such as biocompatibility, high safety, long-term efficiency, stability, and an uncomplicated production process, making them a proper option for active coatings/packaging applications. Additionally, the US Food and Drug Administration (FDA) has affirmed the safety of these nanoparticles [
12,
13]. The antibacterial activity of ZnO NPs against different bacterial strains such as Gram-negative
Klebsiella sp.,
E. coli, and Gram-positive
S. aureus, including its AMR variant (MRSA), vancomycin-resistant enterococci, and
B. subtilis, was confirmed [
12,
13,
14,
15,
16,
17,
18,
19]. The various advantages of ZnO NPs may be attributed to their unique features, including their semiconducting behavior, high transparency in the visible light range, and high UV absorption capabilities. Ultraviolet radiation can lead to a degradation in the antimicrobial activity of packaging coverings by inactivation of the active agent introduced into the coating. The addition of an active agent which is resistant to UV in an active layer, or the addition of a substance with shielding properties, may prevent inactivation of the antimicrobial layer after UV irradiation [
12,
14,
17,
18,
19].
As an assumption [
11,
12], it may be concluded that the addition of ZnO nanoparticles into the
F. betulina or
U. tomentosa (separately) extracts can lead to a synergistic effect between these plant extracts and nanoparticles, and as a result, it may increase their antibacterial activity. The coatings containing nano ZnO as an additive will be resistant to ultraviolet irradiation due to their UV-shielding properties; they will maintain the plant extract’s active compound effectiveness [
12,
17,
18,
19].
The first purpose of the work was to obtain coatings based on F. betulina and U. tomentosa extracts with the addition of a decreased number of nanoparticles of ZnO (to 0.021 g per 100 mL of coating carrier) that could be active against selected Gram-positive and Gram-negative bacterial strains. The ZnO nanoparticles were used as a synergetic agent to increase plant extracts’ antimicrobial activity and to prevent their inactivation when added to the coatings. Another aim of the study was to determine the influence of packaging covered with the obtained active layers on the microbial purity and texture of the sliced cooked ham.
2. Materials and Methods
2.1. Materials
The microorganisms used to verify antibacterial properties of coatings in this research were purchased from a collection from the Leibniz Institute DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen). There was a Bacillus atrophaeus DSM 675 IZT, a Staphylococcus aureus DSMZ 346, a Pseudomonas syringae van Hall 1902 DSM 21482, and an Escherichia coli DSMZ 498 strain.
Polypropylene films (A4, 20 μm) were obtained from a MarDruk company (Andrychów, Poland). The solvent dispersion 70 GU279686 (Hubergroup, Warsaw, Poland) was used as coating carrier (CC), as well as a Zinc Oxide AA 44899, (~70 nm) powder (Thermo Fisher GmbH, Kandel Germany), Uncaria tomentosa, and Fomitopsis betulina (Planteon, Borków Stary, Poland), which were used as antibacterial substances. The 99.8% ethanol (EUROCHEM BGD Sp. z o.o. Tarnów, Poland) was used as a plant solvent extract. Agar-agar, TSB (Triptic Soy Broth), TSA (Triptic Soy Agar), and MacConkey agar (Merck, Darmstadt, Germany) were used to determine the antimicrobial properties of all analyzed coatings. To carry out the microbial analysis of sliced, cooked ham pre- and post-storage, a number of chosen tests were performed. Samples were tested to verify the coliform bacteria count through the use of Violet Red Bile Glucose Agar (VRBG) (Merck KGAA, Darmstadt, Germany). As a verification of the total bacterial count and the S. aureus count of the sliced cooked ham, PPS (PPS: 0.85% m/v NaCl, 0.1% m/v peptone), PCA (BTL, Łódź, Poland) and Baird-Parker mediums (Merck KGAA, Darmstadt, Germany) were used. These mediums were made in accordance with instructions set by the manufacturer. All mediums, barring VRBG agar, were then weighed and later suspended in 1 L of distilled water; this was then autoclaved at 121 °C for 15 min. After being weighed, the VRBG agar was then suspended in 1 L of distilled water and heated to a resulting boiling temperature.
2.2. Extracts Preparation
The dry plants
Uncaria tomentosa and
Fomitopsis betulina were introduced (separately) into a TM6 Thermomix (VORWERK, Wrocław, Poland). The dry plants were ground to a powder (7600 rpm, 20 s). As next step, 100 g of each plant powder was introduced (separately) into 100 mL of 99.8% ethanol. The ethanol solutions containing the
U. tomentosa and
F. betulina powders were then introduced into a Microwave (Amica, Wronki, Poland) for 5 min at 70 °C. Next, the samples were introduced into a shaker (Ika, Staufen im Breisgau, Germany) and extracted for 1 h at 70 °C (150 rpm). The extraction process was carried out according to the methods described by the authors [
9,
20,
21]. After the extraction, the plants were separated from the extracts using Büchner funnel, then the samples were filtered through a 0.2 μm filter and evaporated to obtain the dry mass of the samples 13.74% (
F. betulina) and 21.72% (
U. tomentosa). Then the extracts were used in the next stage of the experiments.
2.3. Coating Preparation
(1. Coating Zn) A total of 0.06 g of ZnO nanoparticles was introduced into 50 mL of ethanol. As a first step, the dispersion was mixed for 1 h using a magnetic stirrer (500 rpm, Ika, Warsaw, Poland). Next, it was sonicated for 30 min. (sonication parameters: amplitude: 20%; cycle: 0.5). A ZnO nanoparticle dispersion was introduced into the 50 mL of the coating carrier (CC) and mixed for 10 min using a magnetic stirrer (500 rpm).
(2. Coatings Ut and Fb) A total of 0.03 g of ZnO nanoparticles was introduced into 45 mL of ethanol. Initially, the dispersion was mixed for 1 h using a magnetic stirrer (500 rpm); the dispersion was then sonicated for 30 min (sonication parameters: amplitude: 20%; cycle: 0.5), while at the same time, the second nano ZnO dispersion was prepared as described above. Then 5 mL of U. tomentosa extract (Ut) was introduced into the first dispersion, and 5 mL of F. betulina (Fb) extract was introduced into the second dispersion. As next step of the experiment, the dispersions were mixed for 15 min using a magnetic stirrer (500 rpm). A 50 mL of the ZnO nanoparticle dispersions containing Ut and Fb extracts (separately) was introduced (separately) into the 50 mL of the coating carrier (CC) and mixed for 10 min using a magnetic stirrer (500 rpm).
Polypropylene (PP) films were coated using an Unicoater 409 (Erichsen, Hemer, Germany) at a temperature of 24 °C with a 40 μm diameter roller. The coatings were dried for 10 min at a temperature of 50 °C. PP films that were not coated were control samples (PP). Having been cut into square shapes (3 cm × 3 cm), the film samples were tested for their antibacterial properties.
2.4. Antibacterial Analysis
The antibacterial activity of the layers containing the Ut or Fb extracts with the addition of ZnO nanoparticles against Gram-negative bacteria:
E. coli P. syringae and Gram-positive
B. atrophaeus, S. aureus strains, compared to the non-coated PP film samples was performed according to the ASTM E 2180-01 standard [
22]. As first step, agar slurry was prepared by dissolving 0.85 g of NaCl and 0.3 g of agar-agar in 100 mL of deionized water (one agar slurry was prepared for each analyzed microbial strain). Agar slurries were sterilized by autoclaving for 15 min at 121 °C and equilibrated at 45 °C. Then 1 mL of bacterial broth culture (1 × 10
8 cells/mL; for each strain separately) was placed into 100 mL of the molten agar slurry to obtain the final concentration of microorganisms (1 × 10
6 cells/mL). Square samples of the PP film (as control samples) and the samples covered with the UT and Fb coatings (test samples) were placed into sterile Petri dishes (separately). Then 1 mL of inoculated agar slurry was pipetted onto the control and test samples (in triplicates) to obtain a 1 mm film (in-depth). The samples were incubated for 24 h at 37 °C (
E. coli, S. aureus, B. atrophaeus) and at 28 °C (
P. syringae). The relative humidity within the incubator (Memmert GmbH, Schwabach, Germany) was 80%. After incubation, the samples were treated from Petri dishes to the sterile bags containing 100 mL of TSB and introduced into the BagMixer
®400 VW (Interscience, Saint Nom la Bretêche, France). The samples were then mixed for 2 min. After mixing, serial dilutions of the initial TSB mediums containing control or test samples (separately) were performed. Each dilution was spread in duplicates on agar mediums as follows: (a) the dilutions of the samples containing
B. atrophaeus and
P. syringae were spread into TSA agar; (b) the dilutions of the samples containing
S. aureus were spread into TSA agar containing 9% of NaCl; (c) the dilutions of the samples containing
E. coli were spread into MacConkey agar. The plates were incubated for 48 h at 37 °C (
E. coli,
S. aureus, B. atrophaeus) and at 28 °C (
P. syringae).
2.5. SEM Analysis of the Coatings
The PP films and PP coated with the active layers Zn (containing ZnO nanoparticles as active substance), Ut (containing U. tomentosa extract and ZnO nanoparticles as active agents), and Fb (containing F. betulina extract and ZnO nanoparticles as active compounds) were analyzed using a scanning electron microscope (SEM). As first step, the samples were placed on pin stubs and coated with a thin layer of gold in a sputter coater at room temperature (Quorum Technologies Q150R S, Laughton, East Sussex, UK). Microscopic analysis was carried out by the use of a Vega 3 LMU microscope (Tescan, Brno-Kohoutovice, Czech Republic), described earlier. These tests were of vital importance as they would clearly show if the PP films had been thoroughly and homogeneously coated with the active layers. Analysis took place at 24 °C through the use of a tungsten filament with an accelerating voltage of 10 kV being used to create SEM images of the non-active (uncoated) and active (covered) layers that had been put on PP foil. The specimens were then examined from above.
2.6. Bags and Spacers Preparation
PP film was covered with the active layers (Ut or Fb) containing plant extracts with the addition of ZnO nanoparticles (separately) from one side for the creation of bags while taken from both sides for the creation of spacers. A control sample (C) was created from PP film that was not coated with any layer. The films that included both the coated and non-coated films were then cut into pieces to prepare bags and for the creation of foil spacers which were square shaped. For the creation of the bags, both coated and non-coated films were welded together (HSE-3, RDM Test Equipment, Hertfordshire, Great Britain) in air conditions deemed normal. The welding parameters were set at pressure—4 kN; temperature—117 °C; time—4 s.
2.7. Packaging and Storage
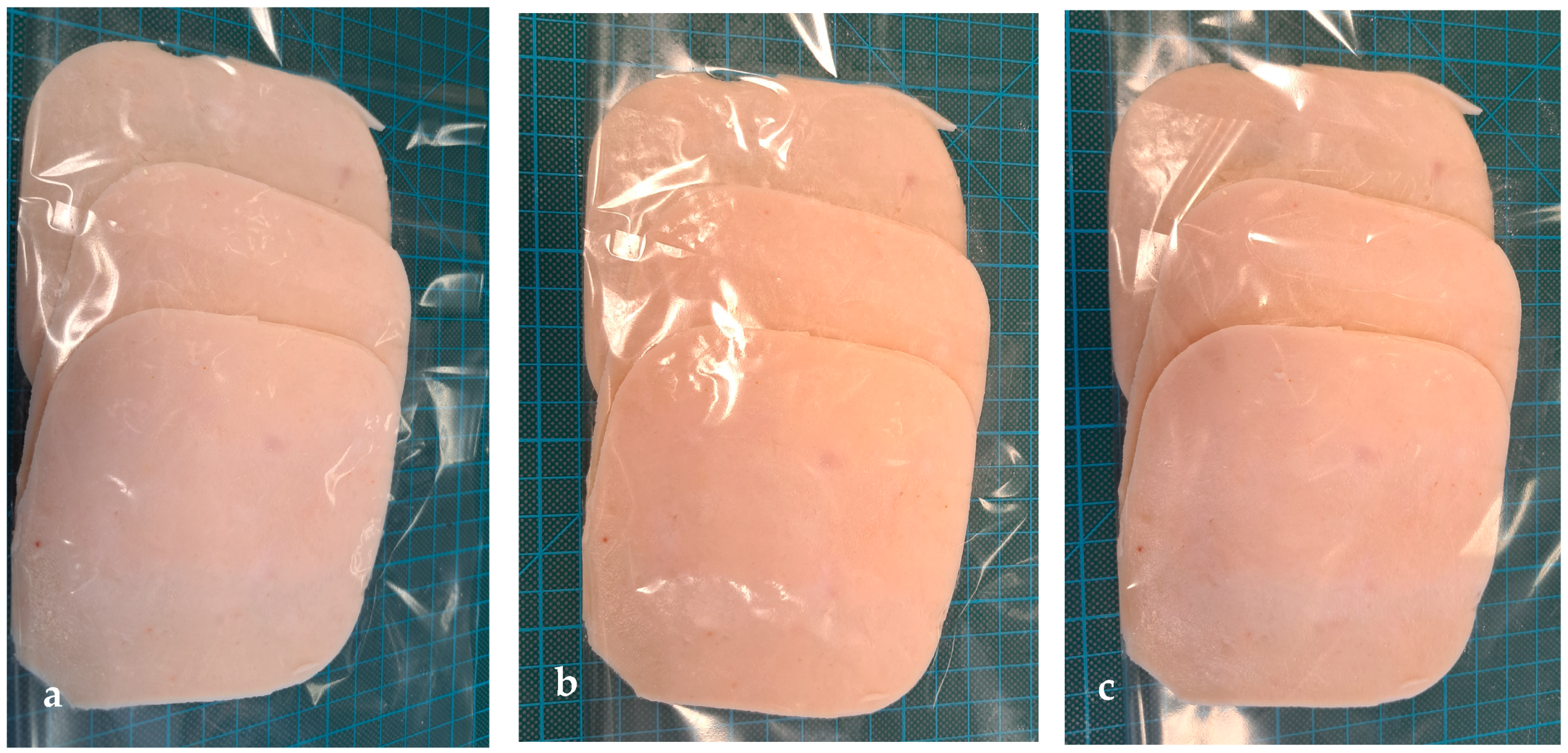
Two bars of cooked ham with the same expiration date (±2 days) were purchased in a local market (Szczecin, Poland). The producer suggested ham consumption within 2–3 days after package opening. The package was opened, and cooked ham was cut into 20 mm slices and 3 mm slices by a shop assistant; the portions were packed into polyethylene bags and immediately transported to the Center of Bioimmobilisation and Innovative Packaging Materials (CBIMO). The sliced ham was then aseptically introduced into bags. All of the cooked ham slices were separated by the use of a square spacer (
Figure 1a) and placed into bags, which included (
Figure 1b,c):
Six PP bags (C) (control samples), these sliced pieces of ham were separated by un-coated PP spacers;
Six PP bags coated with Ut coating, these sliced pieces of ham were separated by PP spacers, coated with the Ut layer on each side;
Six PP bags covered with Fb coating; these sliced pieces of ham were separated by PP spacers that were coated with the Fb layer on each side.
The precooked ham slices were placed in contact with the active layers on each side.
Both the active and non-active bags were welded and sealed (HSE-3, RDM Test Equipment, Hertfordshire, Great Britain) in air conditions deemed normal. Welding parameters were set at pressure—4 kN; temperature –117 °C; time—4 s.
The 18 bags (six of each packaging material) containing sliced cooked ham were then refrigerated at 5 °C. The 9 samples were later examined on 48 h (three bags of each packaging material) and 96 h of storage (three bags of each packaging material). The 20 mm slices were used for textural analysis. The 3 mm slices were used for dry mass analysis, microbiological analysis, SEM, and L*a*b* tests.
2.8. The Microbiological Analysis
Microbiological analysis was performed for sliced cooked ham before and after storage (in active and non-active packaging). For each microbiological analysis, 10 ± 0.1 g of individual cooked ham slices were aseptically introduced into a sterile stomacher bag in physiological saline peptone solution (PPS: 0.85% m/v NaCl; 0.1% m/v peptone). Then ham slices were homogenized in a Bag Mixer (Interscience, Saint-Nom-la-Brèteche, France) for 120 s, and appropriate decimal dilutions were prepared in PPS. The total count was determined according to PN-EN ISO 4833-2:2013-12 [
23] as follows: serial dilutions of the initial, homogenized ham samples (in PPS) were performed; then the 0.1 mL of the initial inoculum and its dilutions were carefully, uniformly spread over the surface of the PCA medium (in duplicates, 90 mm Petri dishes). The plates were left for 15 min for the inoculum to be absorbed into the agar. Then the plates were inverted and introduced into the incubator (Memmert GmbH, Schwabach, Germany). The samples were then incubated for 72 h at 30 °C. After incubation, agar plates with fewer than 300 colonies were selected, and the colonies were counted. The
S. aureus count analysis was performed according to PN-EN ISO 6888-1 [
24] as follows: serial dilutions of the initial homogenized ham samples (in PPS) were performed; then the 1 mL of the initial inoculum and its dilutions were uniformly spread over the surface of the Baird-Parker agar medium (in duplicates, 90 mm Petri dishes). The plates were left for 15 min for the inoculum to be absorbed into the agar. Then the plates were incubated for 48 h at 37 °C. The total coliforms bacteria count was carried out according to PN-ISO 4832:2007 [
25] as follows: serial dilutions of the initial homogenized ham samples were performed; then the 1 mL of the initial ham sample and its dilutions were pipetted from test tubes to the Petri dishes; VRBG medium was weighted, suspended in 1 L of distilled water, heated to a resulting boiling temperature (it was very important not to overheat the medium), and equilibrated at 45 °C. Then 15 mL of VRBG medium was pipetted to the Petri dishes containing the samples and their dilutions and mixed carefully. The mediums were left to solidify. As next step, 4 mL of VRBG medium was pipetted on the surface of each sample and left to solidify. The samples were incubated for 24 h at 37 °C.
2.9. The Textural Analysis
An analysis of the texture of the cooked ham (20 mm slices) took place in accordance with the PN-ISO 11036:1999 standard: “Sensory analysis. Methodology. Texture profiling” [
26]. Zwick/Roell Z 2.5 was used (Wrocław, Poland). An analysis was carried out of the ham samples after delivery from a retail point and after our own storage tests.
2.10. Dry Mass Tests
The dry mass was noted in the case of the cooked ham both before being placed in bags and on 48 h and 96 h storage time. A Weight Dryer (Radwag, Warsaw, Poland) was used to ascertain dry mass. The samples from each bag were tested in duplicates.
2.11. L*a*b* Tests
The color of the cooked ham was determined through the average of 9 individual measurements from ham slice spots randomly selected by the use of a colorimeter (NR 20 XE, EnviSense) with other related data software. The measurement of color was carried out through an aperture (8 mm diameter) using a CIE L*a*b* color space which utilized a standard 10 observer and Illuminant D65. The selected parameters (that would account for the results) were ∆L (darkness and lightness difference) and ∆Elab (total color aberration). The EnviSense protocol was used to calculate parameters.
2.12. SEM Analysis of the Sliced Ham
Before being placed into bags and after 48 h and 96 h storage, an SEM analysis was carried out on the cooked ham. In addition, SEM micrographs were taken to visualize the cooked ham micro-texture. The samples of ham were set at 18 h at 4 °C (2% glutaraldehyde in a 0.1 M sodium cacodylate, pH 7.4). Further, the samples were washed with 0.1 M sodium cacodylate and later dehydrated in serial concentrations of ice-cold (–20 °C) methanol (20%, 40%, 60%, 80%, and 100%) at 2 h intervals. The samples of ham were then put in a Petri dish for 5 min. The food samples were then placed on pin stubs, and a thin layer of gold in a sputter coater was used to coat them at room temperature (Quorum Technologies Q150R S, Laughton, East Sussex, UK). The cooked ham samples were analyzed with a scanning electron microscope (SEM). The microscopic analysis of the samples was carried out through the use of a Vega 3 LMU microscope (Tescan, Brno-Kohoutovice, Czech Republic) already described above.
2.13. Statistical Analysis
The texture and microbiological analysis results were checked for statistical significance through the use of an analysis of variance (ANOVA) followed by an ANOVA test (one-way). Where p < 0.05, the values were seen as significantly different. All analyses were carried out through the use of GraphPad Prism 8 (GraphPad Software, Version 9, San Diego, CA, USA).
4. Discussion
The results of the current study demonstrated that the amount of the ZnO nanoparticles should be increased (up to 0.06 g in 100 mL of the coating carrier) to maintain the bacteriolytic effect of the coating against selected Gram-negative and Gram-positive bacterial strains. The results of the previous work [
12] demonstrated that a methyl-hydroxy-propyl-cellulose (MHPC) coating containing ZnO nanoparticles (0.082 g in 100 mL of the coating carrier) inhibited the growth of
S. aureus, E. coli, B. cereus, and
P. aeruginosa. It is worth mentioning that accelerated irradiation of this coating did not influence its antibacterial properties due to the shielding properties of ZnO nanoparticles [
12,
13,
14,
15,
16,
17,
18,
19]. The decreased amount of the nanoparticles in MHPC coating (0.041 g in 100 mL of the coating carrier) led to obtaining the active layer that inhibited the growth of
S. aureus but only decreased the number of
E. coli and
P. syringae cells, confirming that its antibacterial activity was lower [
11]. The previous work [
11] also indicated that the coating containing a decreased amount of ZnO (0.041 g/100 mL of the coating carrier) and decreased amount of geraniol was more active against
S. aureus, E. coli, and
P. syringae strains than the coatings containing a decreased amount of geraniol or decreased amount of nano ZnO, confirming the synergistic effect of these active agents. The analysis of the antibacterial properties of
U. tomentosa and
F. betulina showed that the activity of these two extracts was very low [
9]. A lower than 1-log reduction in the number of
S. aureus cells was noticed for 5% of extracts of described plants. While analyzing the antimicrobial properties of
F. betulina extracts on
B. subtilis cells, it was demonstrated that they demonstrated lower than a 2-log reduction but higher than a 1-log reduction in the number of living cells.
U. tomentosa extract was found to be even less active. Unfortunately, both extracts were not effective against
E. coli and
P. syringae strains. The findings of the current work indicated that the coatings containing 5% of
U. tomentosa or
F. betulina extracts with the addition of a decreased amount of the nanoparticles of ZnO (0.03 g/100 mL of the coating carrier) demonstrated high antibacterial activity. The synergistic effect between active agents caused a complete inhibition of the growth of
S. aureus, B. atropaheus, and
E. coli strains and a reduction in the number of
P. syringae cells. Additionally, SEM analysis confirmed that the PP film was homogenously and thoroughly covered with the active Ut and Fb layers. However, small pores were observed on the Ut coating, which might have led to a faster release of the active substances from this coating than from the Fb coating or which might have been caused by the release of the active compounds from the Ut layer. Comparing the coatings Ut and Fb, it may be suggested that the Ut active layers were able to display a greater influence on the growth of microorganisms than Fb coating, but for a shorter time.
The results led to the assumption that packaging materials coated with Ut and Fb layers could be used to preserve cooked ham during short-term storage in air conditions.
The described findings demonstrated that coatings containing 5% of the
U. tomentosa or 5% of the
F. betulina extracts with the addition of a decreased amount of nano ZnO did not inhibit the growth of mesophilic bacterial strains for a sliced cooked ham that was stored for 96 h, but decreased their number when compared to the ham stored in uncovered bags. Comparing the influence of active layers on the total count after 48 h, it may be mentioned that the most effective packaging material was the bags covered with the Ut coating. Comparing SEM micrographs of the “0” samples and ham slices after storage, it was observed that the most similar microstructures were the surfaces of the ham before storage and the ham slices stored in bags with Ut coating, confirming its effectiveness. The results of this study determined that the number of mesophilic microbes from ready-prepared, sliced cooked ham samples purchased from a local market (sample “0”) was almost 4 log
10 CFU/g, which is seen as an unsuitable number of microorganisms for the sliced ham to be consumed [
28]. The expiration date (±2 days) of the product could have been one of the reasons. Moreover, the producer suggested ham consumption within 2–3 days after package opening, meaning that a total count should be on the level acceptable for consumption after 48 h of ham storage. Unfortunately, according to Grzybowski et al. [
28], ham samples were not acceptable for consumption after 2 days of storage. However, according to another author [
29], an acceptable level of the total count of cooked ham products is generally set to 6 log
10 CFU/g. It means that ham slices stored in uncoated and in active packaging were still acceptable to be consumed after 48 h of storage. While after 96 h of storage, the only suitable samples for consumption were the ham slices stored in bags covered with the Ut coating. It should be added that the ham was purchased in a local market and sliced by the shop assistant after the package opening. The high level of mesophilic bacteria observed for the sample “0” proved that the ham must have been contaminated during slicing. It may be predicted and even suggested that the number of bacteria in sample “0” was too high to be decreased by the active substances which were released from the layers. Considering the presence of pathogenic microorganisms, it should be emphasized that neither the coliform bacteria nor
S. aureus cells were detected in the cooked ham slices after 48 h and 96 h of storage in PP bags coated with the Ut and Fb layer. From this point of view, these ham slices were still suitable for consumption even after 96 h of storage. In summary, the influence of Ut coating on the quality of sliced, cooked ham was noted, confirming that packaging covered with this active layer can be used for short-term storage. It may be predicted that if ham slices are not contaminated during slicing, the samples will be stored for a longer time than the time suggested by producers. Similar results were obtained in the previous study [
2]. The bags and spacers coated with the layer containing 50% of the mixture of
Glycyrrhiza L. and
Scutelaria baicalensis extracts were proved to be the best packaging material for ready-prepared chicken sausages which were purchased from a local butcher’s shop and which were contaminated during slicing. This active packaging material preserved and maintained the quality of sausage slices after short-term storage (72 h) at 5 °C. Spampinato et al. [
4] performed similar experiments. The authors purchased sliced cooked ham with an expiration date of ±3 days. According to the authors, the producer suggested ham consumption within 1–3 days after the package opening. It has to be underlined that the ham analyzed by the authors was not sliced by the shop assistant, but it was already sliced. The results of the authors’ study showed that the total count ranged between 5.8 and 9.0 log
10 CFU/g at 0 days. Furthermore, after 4, 8, and 12 days of fridge storage (without active packaging), total aerobic bacteria significantly increased. Additionally, the absence of viable
Salmonella sp. in the product after 12 days of storage was satisfying for the authors; nevertheless, the presence of other opportunistic pathogens, such as
Klebsiella pneumoniae and
E. coli, was noted. The experiments of the authors revealed that, at the opening of packages, sliced ham presented a high microbial load and quite rich microbiota. It can be explained by other authors who suggested [
30] that it is possible that post-cooking contamination of cooked ham occurred during the packaging phases of manufacturing, with the bacteria occurring in the environment of the facility, entering into contact and being packaged with virtually sterile sliced cooked ham.
The results of the study led to the assumption that bags and spacers coated with a Ut layer, even if though not recommended for the long-term safe keeping of sliced cooked ham, may be used for short-term storage. Many consumers buy ready-to-eat sliced ham that is sliced by shop assistants in a local market. It has to be underlined that ham is very often contaminated during the slicing process. Therefore, a packaging material, such as PP film, paper, or biopolymer foil coated with a Ut layer, could be used to preserve sliced cooked ham during transport and short-term storage (48–96 h). The findings of this work were similar to the results presented by Shiji et al. [
31], who introduced chicken sausages into active packaging for short-term storage. The authors proved that after four days of storage at 4 °C, the active pouches containing silver nanoparticles as active agents were more effective than polyethylene pouches. Similar results were obtained by Shahrampour et al. [
32], who confirmed that the use of active packaging for sausages storage at 4 °C led to detect a lower number of mesophilic microorganisms than the total count isolated from the samples which were stored in the uncoated polyethylene packaging. Rüegg et al. [
6] prepared polyethylene terephthalate (PET) foil covered with active coatings containing thyme and rosemary essential oils as active agents. The authors used antimicrobial packaging to preserve a sliced cooked chicken breast during short-term storage (6 days). As was shown by the authors, the active layers reduced the number of mesophilic bacteria compared to the uncovered foil, though only slightly. Furthermore, it was confirmed that the MAP packaging system had a greater effect on microorganisms than the antimicrobial coverings, which is why MAP is recommended for long-term storage.
The texture is one of the most important parameters determining the freshness and quality of cooked ham. A product that is too soft or is not cohesive enough can create doubts in the consumers as to its quality and freshness. All the forces holding the food products together are represented by cohesiveness. Additionally, gummy, tough, or stringy food product is not suitable for consumers as it presents excessively strong resistance during mastication [
2]. The results of this study determined that the cohesiveness of sliced cooked ham increased for active and uncoated packaging materials (when compared to the sample “0” after 96 h of storage. It is tempting to deduce that the storage of ham slices in uncovered PP bags and in the bags covered with the active layer had a positive effect on the texture of the ham. The contrary findings were noted by Patiño et al. [
33], who proved that cohesiveness tended to decrease during storage which may be caused by the interaction of proteins and fats with the rest of the components, affecting the texture of the sausages. Furthermore, Araújo et al. [
34] suggested that to increase the parameter values of the sausage samples during storage, an additive such as collagen powder should be added. The results of the current work indicated that the springiness of the ham samples increased for all analyzed packaging materials (when compared to the sample “0”) after 48 h storage. Moreover, a decrease in this parameter was observed, except in the slices that were stored for 96 h in the bags coated with the Ut layer, confirming that the active coating containing
U. tomentosa extract influenced the springiness improvement.
The present work indicated that, unfortunately, chewiness and gumminess increased in the samples stored in uncoated PP bags after 48 h and 96 h of storage, and this is a clear disadvantage. These findings were confirmed by SEM analysis, which showed a more compact microstructure of the cooked ham. Comparing the ham slices, which were stored in active packaging for 48 h, it has to be mentioned that the changes in chewiness values were not observed (sample “0”). In the case of gumminess, the decrease in this parameter was observed, but only for the samples stored in bags with a Ut layer. The bags covered with Fb coating did not influence the gumminess of the ham samples. This was backed up by a microbial purity analysis that showed that the bags coated with the Ut layer was the best packaging for the short-term (48 h) storage of the sliced cooked ham. It is also worth mentioning the disadvantage of the active bags with Ut and Fb layers; it was observed that water loss in sliced ham samples from these bags was higher than from uncoated bags. As was already suggested, the high water loss can release antibacterial substances from the active layers and thus could improve their activity. Analyzing the chewiness and gumminess for the ham slices stored in bags coated with Ut and Fb layers after 96 h of storage, the increase in these parameter values was noted. Simultaneously, the dry mass also increased. As was suggested by Zeraatpisheh et al. [
35], when water loss increases, the meat proteins converge because of the formation of new crosslinks; consequently, the chewiness and gumminess of the samples are elevated. Gumminess is associated with food hardness; therefore, its increase is a clear disadvantage. Thus, in cooked ham stored in uncoated bags or in bags covered with a Fb layer with increased gumminess after storage, ham sample hardness may let to difficulties in swallowing [
2]. While the ham stored in active packaging coated with Ut layer with decreased (after 48 h) or unchanged (after 96 h) gumminess, ham slices would still be acceptable for consumption.
The findings demonstrated in this work confirmed that the highest ∆E
lab values were noticed for cooked ham portions stored in the uncoated bags and into bags covered with Fb coating for 48 h and 96 h. It was also indicated that the highest ∆L values were noted for these portions. This indicates that the ham samples taken from this package were the lightest. Furthermore, it should be mentioned that these parameter values increased for samples stored in all analyzed packaging materials. The results were confirmed by Azlin-Hasim et al. [
36], who indicated that L* values increased in meat samples stored for 6 and 12 days in packaging containing Ag nanoparticles as antimicrobial substances.