Approximate Entropy and Velocity of Center of Pressure to Determine Postural Stability: A Pilot Study
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Data Acquisition
2.4. Data Analysis
2.4.1. Signal Preprocessing
2.4.2. Approximate Entropy
2.4.3. Velocity
2.4.4. Statistical Analysis
3. Results
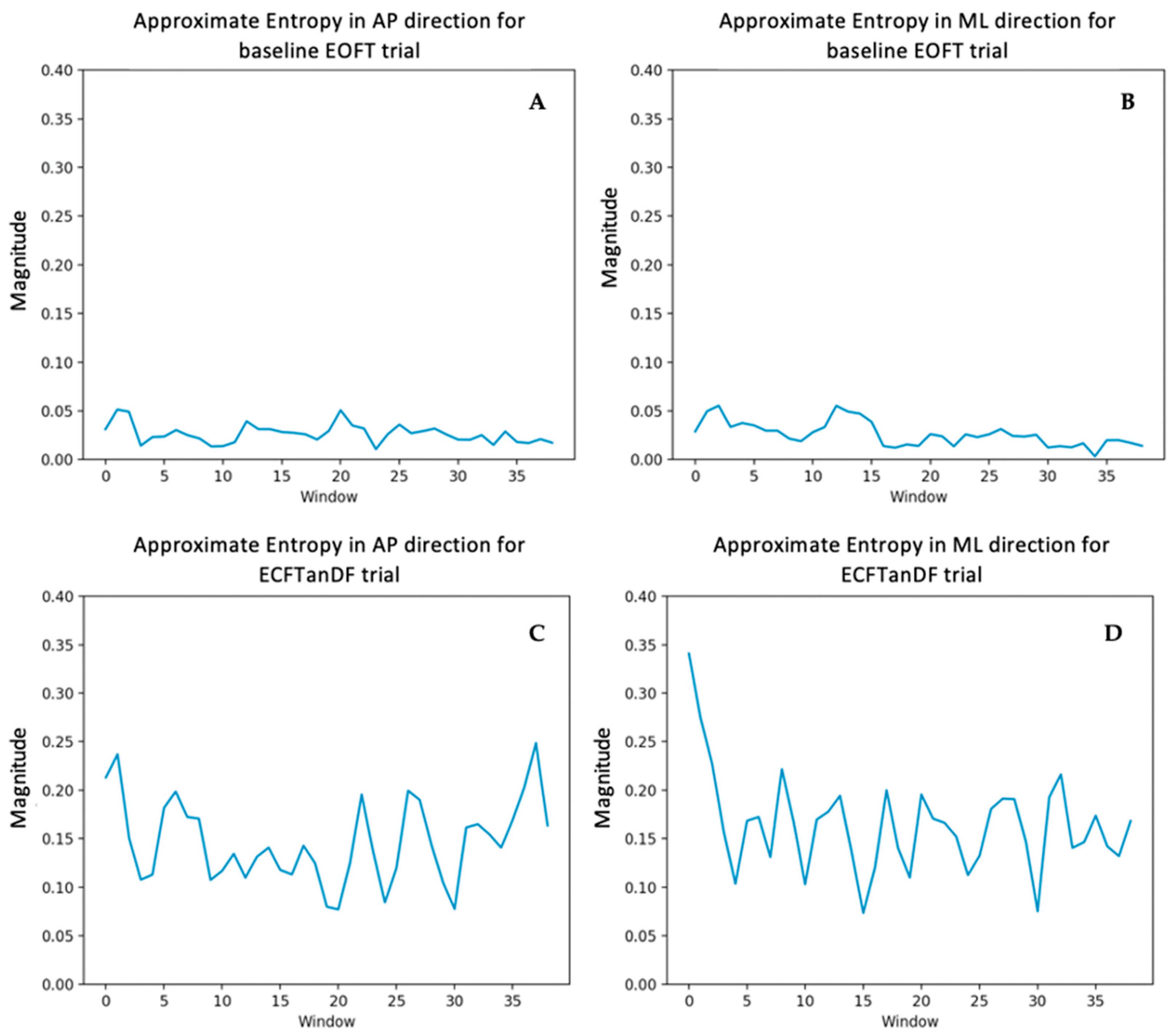
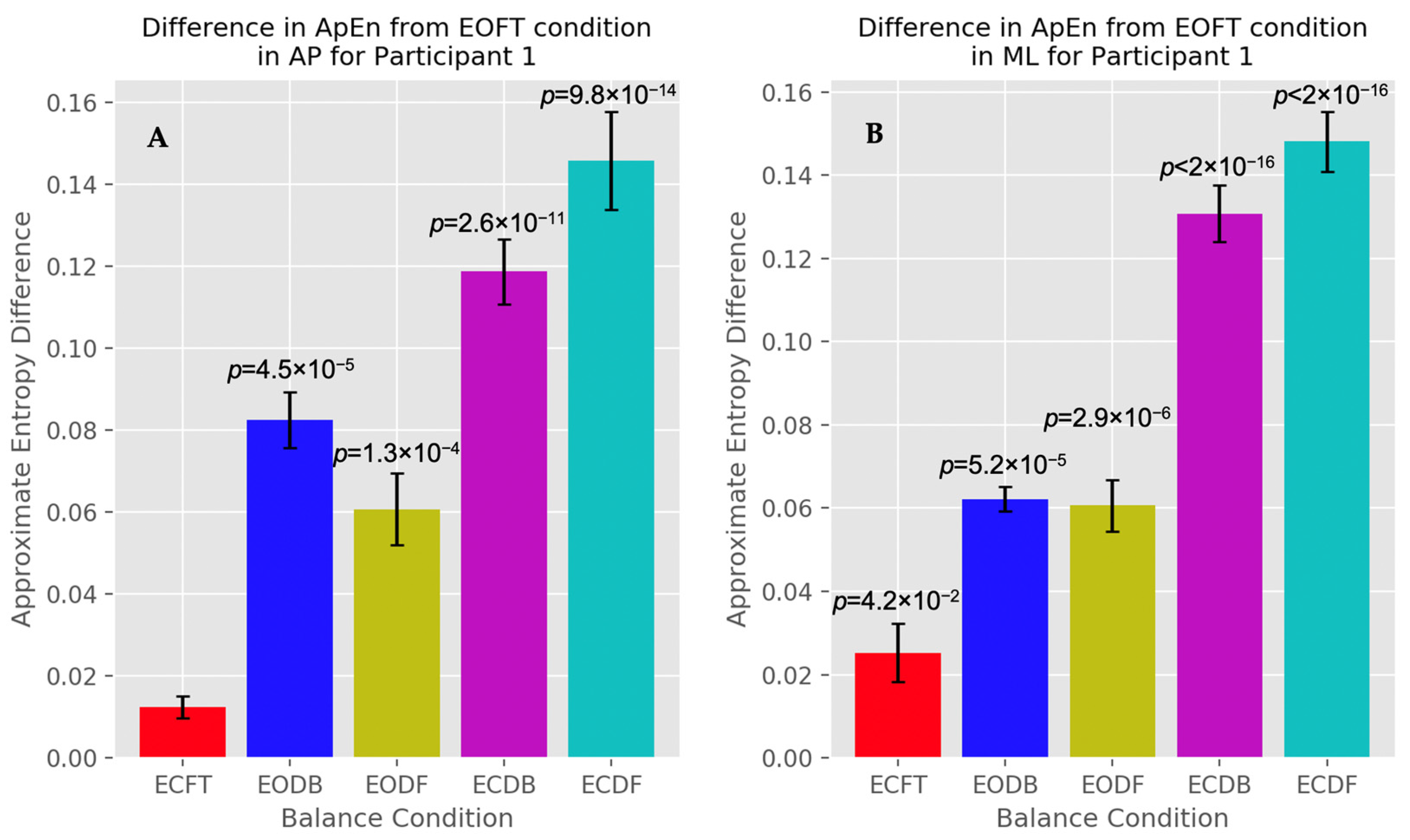
3.1. Approximate Entropy
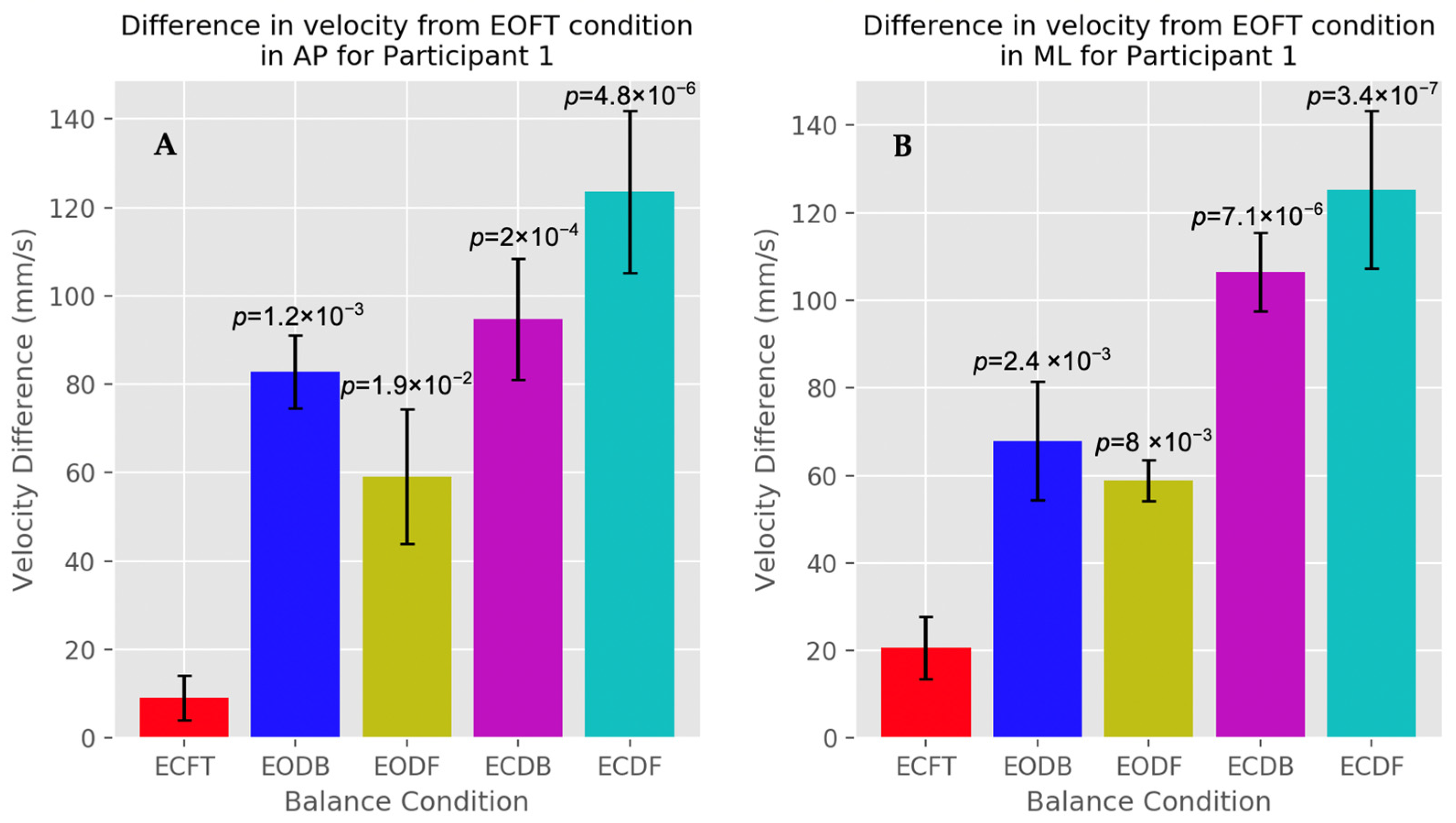
3.2. Velocity
4. Discussion
4.1. Center of Pressure Irregularity
4.2. Oscillation Velocity
4.3. Comparison of Analysis Methods
4.4. Limitations and Future Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What Is Balance? Clin. Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Cortical Control of Postural Responses. J. Neural Transm. 2007, 114, 1339. [Google Scholar] [CrossRef]
- Horak, F.B. Postural Orientation and Equilibrium: What Do We Need to Know about Neural Control of Balance to Prevent Falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef]
- Winter, D. Human Balance and Posture Control during Standing and Walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Massion, J. Postural Control System. Curr. Opin. Neurobiol. 1994, 4, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Bair, W.-N.; Rogers, M.W. Center of Pressure Control for Balance Maintenance during Lateral Waist-Pull Perturbations in Older Adults. J. Biomech. 2015, 48, 963–968. [Google Scholar] [CrossRef]
- Rochefort, C.; Walters-Stewart, C.; Aglipay, M.; Barrowman, N.; Zemek, R.; Sveistrup, H. Balance Markers in Adolescents at 1 Month Postconcussion. Orthop. J. Sports Med. 2017, 5, 2325967117695507. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, S.A.; Torres-Oviedo, G.; Safavynia, S.A.; Ting, L.H. Common Muscle Synergies for Control of Center of Mass and Force in Nonstepping and Stepping Postural Behaviors. J. Neurophysiol. 2011, 106, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Oba, N.; Sasagawa, S.; Yamamoto, A.; Nakazawa, K. Difference in Postural Control during Quiet Standing between Young Children and Adults: Assessment with Center of Mass Acceleration. PLoS ONE 2015, 10, e0140235. [Google Scholar] [CrossRef]
- Catena, R.D.; van Donkelaar, P.; Chou, L.-S. Different Gait Tasks Distinguish Immediate vs. Long-Term Effects of Concussion on Balance Control. J. Neuroeng. Rehabil. 2009, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Merchant-Borna, K.; Jones, C.M.C.; Janigro, M.; Wasserman, E.B.; Clark, R.A.; Bazarian, J.J. Evaluation of Nintendo Wii Balance Board as a Tool for Measuring Postural Stability After Sport-Related Concussion. J. Athl. Train. 2017, 52, 245–255. [Google Scholar] [CrossRef]
- Yu, E.; Abe, M.; Masani, K.; Kawashima, N.; Eto, F.; Haga, N.; Nakazawa, K. Evaluation of Postural Control in Quiet Standing Using Center of Mass Acceleration: Comparison Among the Young, the Elderly, and People with Stroke. Arch. Phys. Med. Rehabil. 2008, 89, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.; Inness, E.L. Force Plate Assessment of Quiet Standing Balance Control: Perspectives on Clinical Application within Stroke Rehabilitation. Rehabil. Process Outcome 2015, 4, RPO.S20363. [Google Scholar] [CrossRef]
- Versteeg, C.S.; Ting, L.H.; Allen, J.L. Hip and Ankle Responses for Reactive Balance Emerge from Varying Priorities to Reduce Effort and Kinematic Excursion: A Simulation Study. J. Biomech. 2016, 49, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Lafonda, D.; Duarteb, M.; Princea, F. Comparison of Three Methods to Estimate the Center of Mass during Balance Assessment. J. Biomech. 2004, 37, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Patla, A.E.; Ishac, M.; Gage, W.H. Motor Mechanisms of Balance during Quiet Standing. J. Electromyogr. Kinesiol. 2003, 13, 49–56. [Google Scholar] [CrossRef]
- Hsue, B.-J.; Miller, F.; Su, F.-C. The Dynamic Balance of the Children with Cerebral Palsy and Typical Developing during Gait. Part I: Spatial Relationship between COM and COP Trajectories. Gait Posture 2009, 29, 465–470. [Google Scholar] [CrossRef]
- Sarabon, N.; Jernej, R.; Loefler, S.; Kern, H. Sensitivity of Body Sway Parameters During Quiet Standing to Manipulation of Support Surface Size. J. Sports Sci. Med. 2010, 9, 431–438. [Google Scholar]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of Postural Steadiness: Differences between Healthy Young and Elderly Adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef]
- Corbeil, P.; Blouin, J.-S.; Bégin, F.; Nougier, V.; Teasdale, N. Perturbation of the Postural Control System Induced by Muscular Fatigue. Gait Posture 2003, 18, 92–100. [Google Scholar] [CrossRef]
- Creath, R.; Kiemel, T.; Horak, F.; Peterka, R.; Jeka, J. A Unified View of Quiet and Perturbed Stance: Simultaneous Co-Existing Excitable Modes. Neurosci. Lett. 2005, 377, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, Y.; Nozaki, D.; Masani, K.; Sato, T.; Nakazawa, K.; Yano, H. Reciprocal Angular Acceleration of the Ankle and Hip Joints during Quiet Standing in Humans. Exp. Brain Res. 2001, 136, 463–473. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nomura, T.; Casadio, M.; Morasso, P. Intermittent Control with Ankle, Hip, and Mixed Strategies during Quiet Standing: A Theoretical Proposal Based on a Double Inverted Pendulum Model. J. Theor. Biol. 2012, 310, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Günther, M.; Grimmer, S.; Siebert, T.; Blickhan, R. All Leg Joints Contribute to Quiet Human Stance: A Mechanical Analysis. J. Biomech. 2009, 42, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, S.; Shinya, M.; Nakazawa, K. Interjoint Dynamic Interaction during Constrained Human Quiet Standing Examined by Induced Acceleration Analysis. J. Neurophysiol. 2014, 111, 313–322. [Google Scholar] [CrossRef]
- Reimann, H.; Schöner, G. A Multi-Joint Model of Quiet, Upright Stance Accounts for the “Uncontrolled Manifold” Structure of Joint Variance. Biol. Cybern. 2017, 111, 389–403. [Google Scholar] [CrossRef]
- Morasso, P.; Cherif, A.; Zenzeri, J. Quiet Standing: The Single Inverted Pendulum Model Is Not so Bad after All. PLoS ONE 2019, 14, e0213870. [Google Scholar] [CrossRef]
- Tigrini, A.; Verdini, F.; Fioretti, S.; Mengarelli, A. Long Term Correlation and Inhomogeneity of the Inverted Pendulum Sway Time-Series under the Intermittent Control Paradigm. Commun. Nonlinear Sci. Numer. Simul. 2022, 108, 106198. [Google Scholar] [CrossRef]
- Valovich McLeod, T.C.; Hale, T.D. Vestibular and Balance Issues Following Sport-Related Concussion. Brain Inj. 2015, 29, 175–184. [Google Scholar] [CrossRef]
- Baracks, J.; Casa, D.J.; Covassin, T.; Sacko, R.; Scarneo, S.E.; Schnyer, D.; Yeargin, S.W.; Neville, C. Acute Sport-Related Concussion Screening for Collegiate Athletes Using an Instrumented Balance Assessment. J. Athl. Train. 2018, 53, 597–605. [Google Scholar] [CrossRef]
- Matuszak, J.M.; McVige, J.; McPherson, J.; Willer, B.; Leddy, J. A Practical Concussion Physical Examination Toolbox. Sports Health 2016, 8, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.T.; Guskiewicz, K.M.; Giuliani, C.; Marshall, S.; Mercer, V.S.; Stergiou, N. Recovery of Postural Control after Cerebral Concussion: New Insights Using Approximate Entropy. J. Athl. Train. 2006, 41, 305–313. [Google Scholar] [PubMed]
- Pincus, S. Approximate Entropy (ApEn) as a Complexity Measure. Chaos 1995, 5, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Fino, P.C.; Nussbaum, M.A.; Brolinson, P.G. Decreased High-Frequency Center-of-Pressure Complexity in Recently Concussed Asymptomatic Athletes. Gait Posture 2016, 50, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.T.; Mercer, V.S.; Stergiou, N. Approximate Entropy Detects the Effect of a Secondary Cognitive Task on Postural Control in Healthy Young Adults: A Methodological Report. J. NeuroEngineering Rehabil. 2007, 7, 42. [Google Scholar]
- Montesinos, L. On the Use of Approximate Entropy and Sample Entropy with Centre of Pressure Time-Series. J. Neuroeng. Rehabil. 2018, 15, 116. [Google Scholar] [CrossRef]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; Wiley: Hoboken, NJ, USA, 2020; Available online: https://www.wiley.com/en-us/Biomechanics+and+Motor+Control+of+Human+Movement%2C+4th+Edition-p-9780470398180 (accessed on 29 July 2020).
- Kirchner, M. Characterising Postural Sway Fluctuations in Humans Using Linear and Nonlinear Methods. Ph.D. Thesis, Goethe University, Frankfurt, Germany.
- Latash, M.L.; Ferreira, S.S.; Wieczorek, S.A.; Duarte, M. Movement Sway: Changes in Postural Sway during Voluntary Shifts of the Center of Pressure. Exp. Brain Res. 2003, 150, 314–324. [Google Scholar] [CrossRef]
- Singh, N.B.; Taylor, W.R.; Madigan, M.L.; Nussbaum, M.A. The Spectral Content of Postural Sway during Quiet Stance: Influences of Age, Vision and Somatosensory Inputs. J. Electromyogr. Kinesiol. 2012, 22, 131–136. [Google Scholar] [CrossRef]
- Woltring, H.J. On Optimal Smoothing and Derivative Estimation From Noisy Displacement Data in Biomechanics. Hum. Mov. Sci. 1985, 4, 229–245. [Google Scholar] [CrossRef]
- Combined Output from Multiple Force Plates—Nexus 2.6 Documentation—Vicon Documentation. Available online: https://docs.vicon.com/display/Nexus26/Combined+output+from+multiple+force+plates (accessed on 27 April 2020).
- Exell, T.; Kerwin, D.; Irwin, G.; Gittoes, M. Calculating Centre of Pressure from Multiple Force Plates for Kinetic ANalyses of Sprint Running. Port. J. Sport Sci. 2011, 11, 4. [Google Scholar]
- Lee, H.; Granata, K.P. Process Stationarity and Reliability of Trunk Postural Stability. Clin. Biomech. 2008, 23, 735–742. [Google Scholar] [CrossRef]
- Rocchi, L.; Chiari, L.; Cappello, A.; Horak, F.B. Identification of Distinct Characteristics of Postural Sway in Parkinson’s Disease: A Feature Selection Procedure Based on Principal Component Analysis. Neurosci. Lett. 2006, 394, 140–145. [Google Scholar] [CrossRef]
- Mengarelli, A.; Tigrini, A.; Verdini, F.; Rabini, R.A.; Fioretti, S. Multiscale Fuzzy Entropy Analysis of Balance: Evidences of Scale-Dependent Dynamics on Diabetic Patients with and without Neuropathy. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Hadamus, A.; Błażkiewicz, M.; Kowalska, A.J.; Wydra, K.T.; Grabowicz, M.; Łukowicz, M.; Białoszewski, D.; Marczyński, W. Nonlinear and Linear Measures in the Differentiation of Postural Control in Patients after Total Hip or Knee Replacement and Healthy Controls. Diagnostics 2022, 12, 1595. [Google Scholar] [CrossRef]
- Stergiou, N.; Decker, L.M. Human Movement Variability, Nonlinear Dynamics, and Pathology: Is There A Connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef]
- van Emmerik, R.E.A.; Ducharme, S.W.; Amado, A.C.; Hamill, J. Comparing Dynamical Systems Concepts and Techniques for Biomechanical Analysis. J. Sport Health Sci. 2016, 5, 3–13. [Google Scholar] [CrossRef]
- Cavanaugh, J.T.; Guskiewicz, K.M.; Stergiou, N. A Nonlinear Dynamic Approach for Evaluating Postural Control: New Directions for the Management of Sport-Related Cerebral Concussion. Sports Med. 2005, 35, 935–950. [Google Scholar] [CrossRef]
- Cavanaugh, J.T.; Guskiewicz, K.M.; Giuliani, C.; Marshall, S.; Mercer, V.; Stergiou, N. Detecting Altered Postural Control after Cerebral Concussion in Athletes with Normal Postural Stability. Br. J. Sports Med. 2005, 39, 805–811. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological Time-Series Analysis Using Approximate Entropy and Sample Entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]

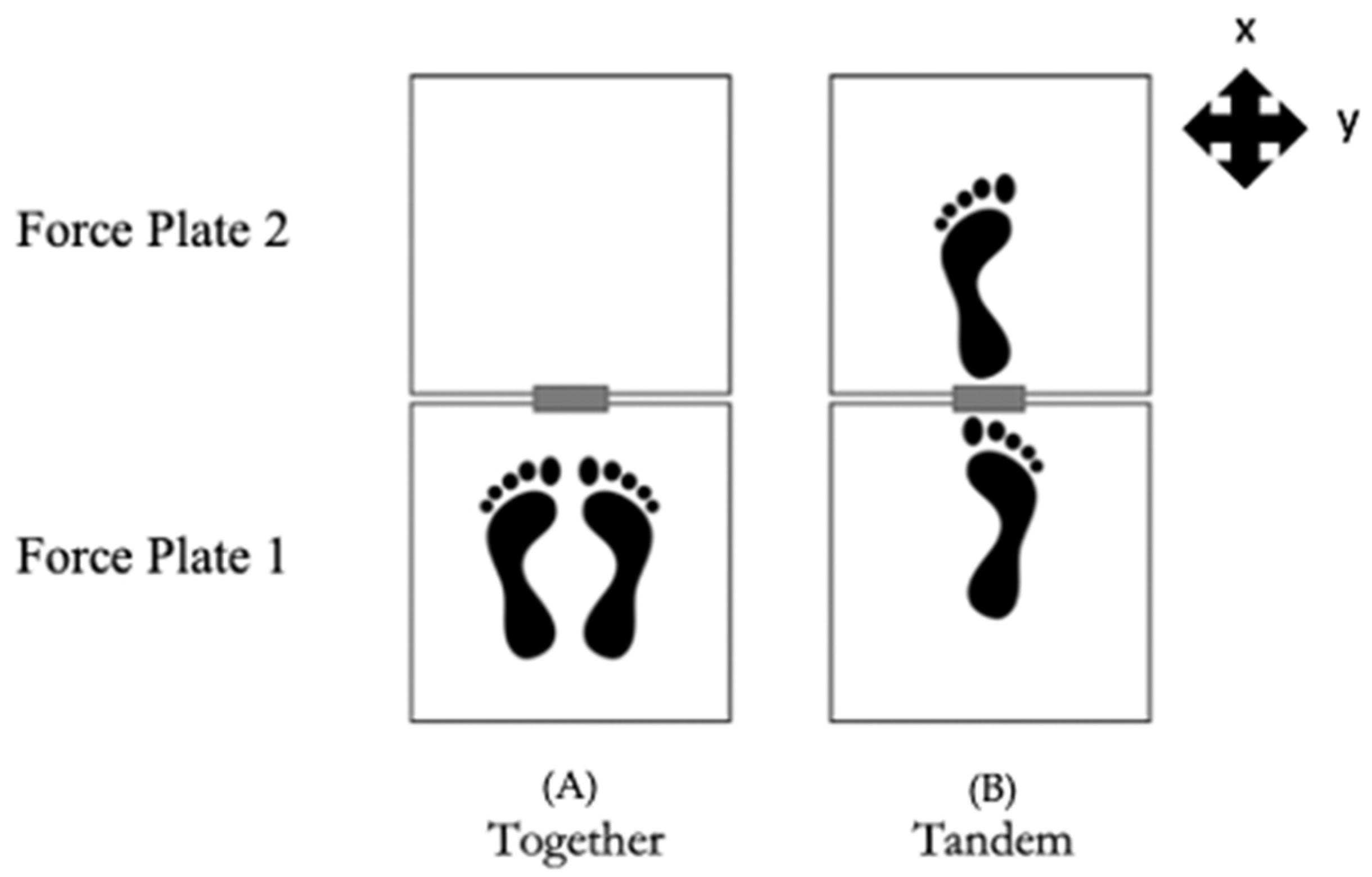




| Order | Balance Condition | Description |
|---|---|---|
| 1 | EOFT | Eyes open, feet together (most stable, baseline) |
| 2 | ECFT | Eyes closed, feet together |
| 3 | EOTanDB | Eyes open, feet tandem, dominant foot in back |
| 4 | ECTanDB | Eyes closed, feet tandem, dominant foot in back |
| 5 | EOTanDF | Eyes open, feet tandem, dominant foot in front |
| 6 | ECTanDF | Eyes closed, feet tandem, dominant foot in front |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tipton, N.; Alderink, G.; Rhodes, S. Approximate Entropy and Velocity of Center of Pressure to Determine Postural Stability: A Pilot Study. Appl. Sci. 2023, 13, 9259. https://doi.org/10.3390/app13169259
Tipton N, Alderink G, Rhodes S. Approximate Entropy and Velocity of Center of Pressure to Determine Postural Stability: A Pilot Study. Applied Sciences. 2023; 13(16):9259. https://doi.org/10.3390/app13169259
Chicago/Turabian StyleTipton, Natalie, Gordon Alderink, and Samhita Rhodes. 2023. "Approximate Entropy and Velocity of Center of Pressure to Determine Postural Stability: A Pilot Study" Applied Sciences 13, no. 16: 9259. https://doi.org/10.3390/app13169259
APA StyleTipton, N., Alderink, G., & Rhodes, S. (2023). Approximate Entropy and Velocity of Center of Pressure to Determine Postural Stability: A Pilot Study. Applied Sciences, 13(16), 9259. https://doi.org/10.3390/app13169259








