Two-Dimensional Metal–Organic Frameworks and Their Derivative Electrocatalysts for Water Splitting
Abstract
:1. Introduction
2. Fabrication Strategy of 2D MOF and Their Derivatives
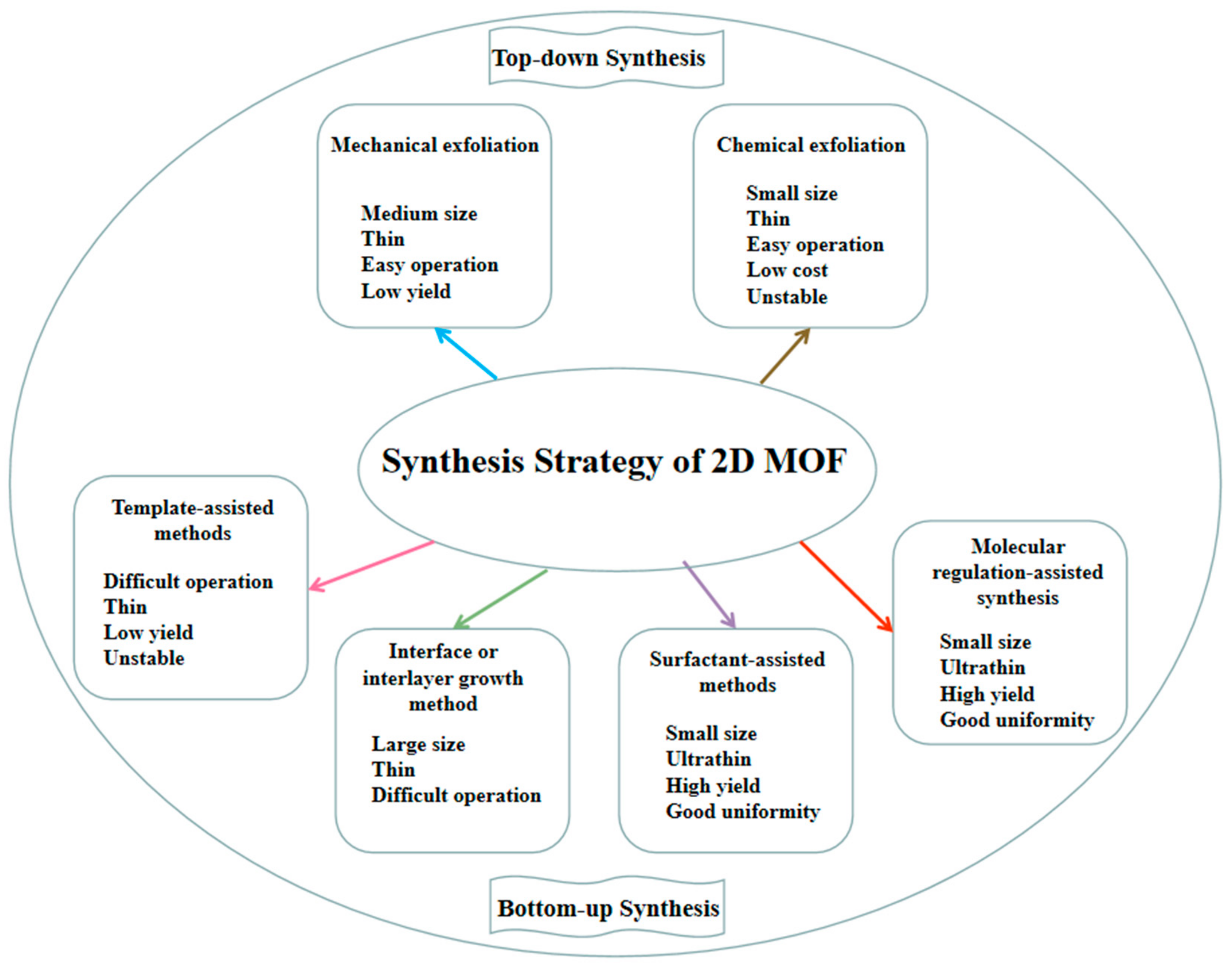
2.1. Fabrication Strategy of 2D MOF
2.1.1. Top-Down Synthesis
Mechanical Exfoliation
Chemical Exfoliation
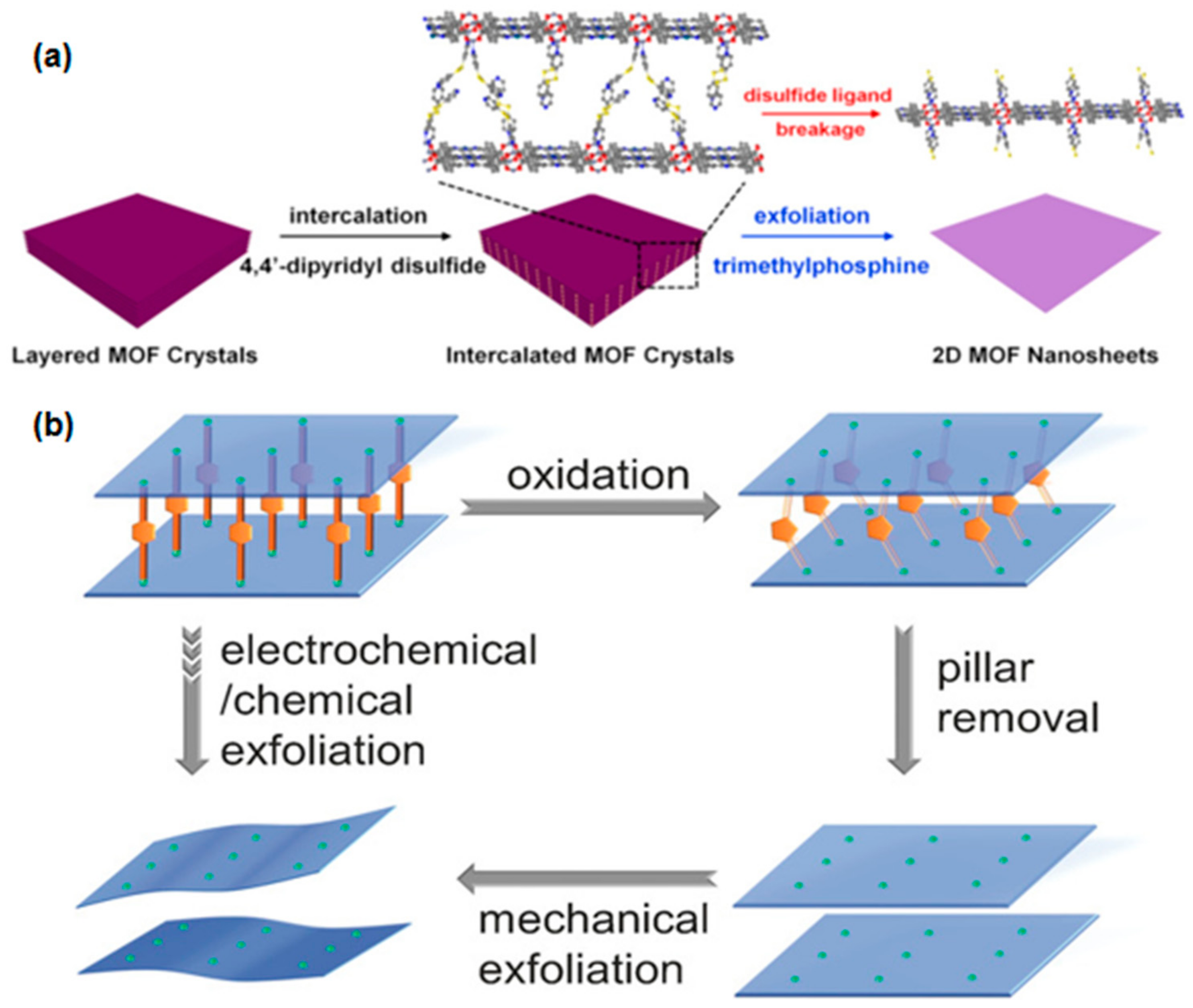
2.1.2. Bottom-Up Fabrication
Formwork-Assisted Methods
Interface or Interlayer Growth Method
Surface-Active Agent-Assisted Methods
Molecular Modulation-Assisted Synthesis
2.2. Fabrication Strategy of 2D MOF Derivative
3. Application of 2D MOFs and Their Derivatives for Water Splitting
3.1. Hydrogen Evolution Reaction
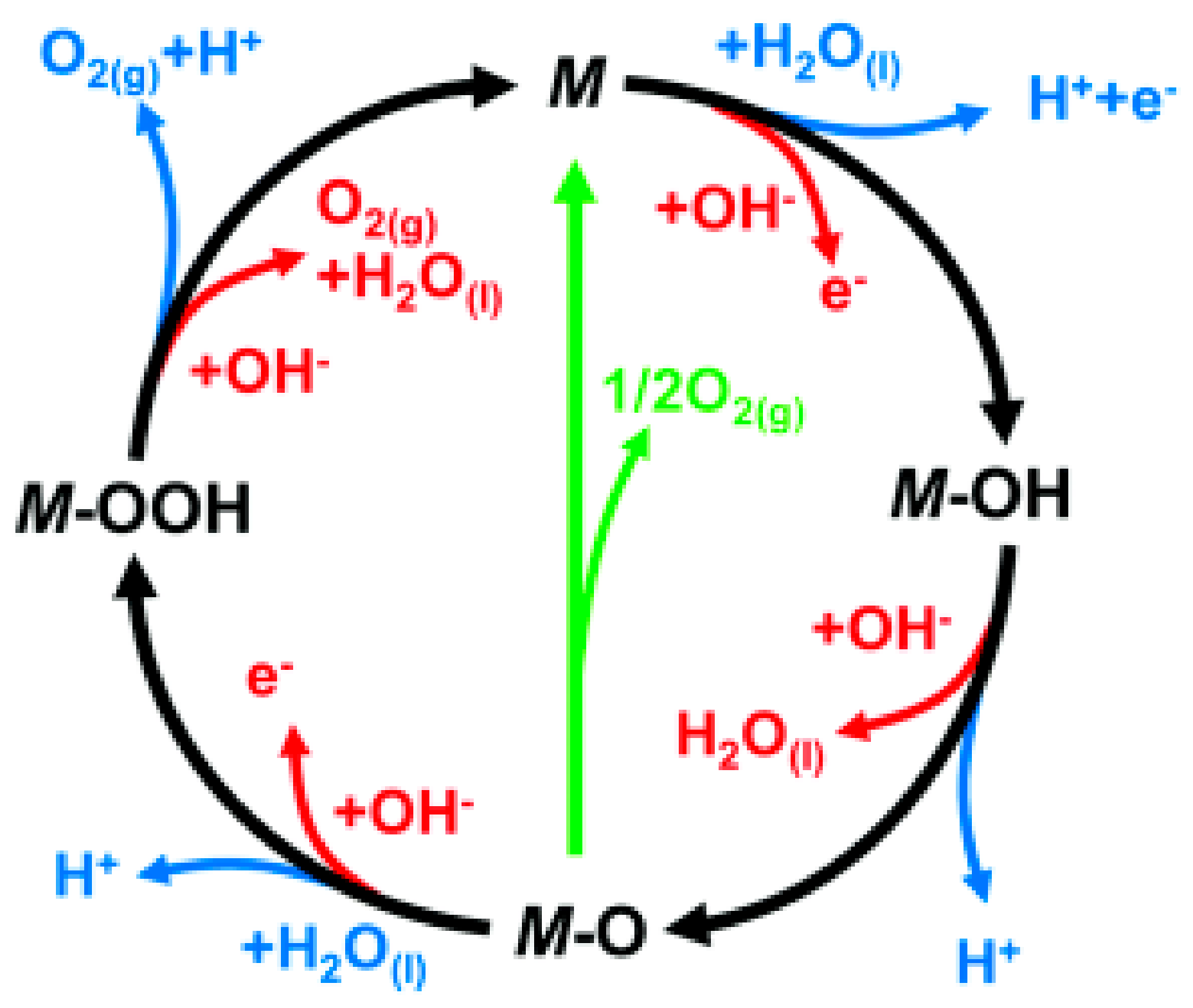
3.2. Oxygen Evolution Reaction
| Catalyst Name | Type | Electrolyte | η [mV] | Tafel Slope [mV dec−1] | Ref. |
|---|---|---|---|---|---|
| [Cu(2,5-pydc)(H2O)]n·2H2O NSs | 2D MOFs | 1.0 M KOH | 340 | 70 | [132] |
| NiFe-MOF-74 | 2D MOFs | 1.0 M KOH | 195 | 136 | [156] |
| Ni3S2@2D Co-MOF/CP | 2D MOFs | 1.0 M KOH | 140 | 90.3 | [133] |
| 2D CoP/Co-MOF | 2D MOFs derivatives | 0.5 M H2SO4 | 52 | 49 | [157] |
| 1.0 M KOH | 26 | 53 | |||
| 1.0 M PBS | 106 | 89 | |||
| NiCoSe | 2D MOFs derivatives | 1.0 M KOH | 170 | 82.3 | [134] |
| FeS2-MoS2@CoS2-MOF | 2D MOFs derivatives | 1.0 M KOH | 92 | 70.4 | [158] |
| FeNi3–Fe3O4 NPs/MOF-CNT | 2D MOFs derivatives | 1.0 M KOH | 108 | 96.7 | [182] |
| CCS-NiFeP | 2D MOFs derivatives | 1.0 M KOH | 56 | 38 | [184] |
| Ni/Ni3S2@CN | 2D MOFs derivatives | 1.0 M KOH | 141 | 91 | [185] |
| 0.5 M H2SO4 | 187 | 83 | |||
| Ce-MOF@Pt | 2D MOFs | 1.0 M KOH | 208 | 188.1 | [186] |
| Pt@CuFe-LDHm | 2D MOFs derivatives | 1.0 M KOH | 33 | 34 | [187] |
| 0.1 M KOH | 47 | 50.2 | |||
| 1.0 M PBS | 120 | 85.6 | |||
| Fe-NiS/MoS2 | 2D MOFs derivatives | 1.0 M KOH | 120 | 69 | [188] |
| Ni@CoO@CoMOFC | 2D MOFs derivatives | 1.0 M KOH | 138 | 59 | [189] |
| CoNiP/NF | 2D MOFs derivatives | 1.0 M KOH | 147 | 51 | [190] |
| TS-Co3O4@VS2 | 2D MOFs derivatives | 0.5 M H2SO4 | 175.2 | 57 | [191] |
| Catalyst Name | Type | Electrolyte | η [mV] | Tafel Slope [mV dec−1] | Ref. |
|---|---|---|---|---|---|
| NiFe-MOF-74 | 2D MOFs | 1.0 m KOH | 208 | 54 | [156] |
| NiCoSe | 2D MOFs derivatives | 1.0 M KOH | 278 [20 mA cm−2] | 92 | [134] |
| FeS2-MoS2@CoS2-MOF | 2D MOFs derivatives | 1.0 M KOH | 211 [20 mA cm−2] | 64.5 | [158] |
| NiCo-MOF | 2D MOFs | 1.0 M KOH | 310 | 106.3 | [178] |
| 2D MOF-Fe/Co | 2D MOFs | 1.0 M KOH | 238 | 52 | [179] |
| NiFe MOF/NF | 2D MOFs | 1.0 M KOH | 221 | 40 | [180] |
| Te,Cl-NiFe MOF | 2D MOFs derivatives | 1.0 M KOH | 224 [30 mA cm−2] | 37.6 | [181] |
| FeNi3–Fe3O4 NPs/MOF-CNT | 2D MOFs derivatives | 1.0 M KOH | 234 | 37 | [182] |
| Fe-CoP/C | 2D MOFs derivatives | 1.0 M KOH | 290 | 50 | [183] |
| Ce-MOF@Pt | 2D MOFs | 1.0 M KOH | 340 | 47.9 | [186] |
| Fe-NiS/MoS2 | 2D MOFs derivatives | 1.0 M KOH | 297 | 54.7 | [188] |
| NiFe-BTC/CCHH/NF | 2D MOFs derivatives | 1.0 M KOH | 270 [50 mA cm−2] | / | [192] |
| Ni@CoO@CoMOFC | 2D MOFs derivatives | 1.0 M KOH | 247 | 51 | [189] |
| CoNiP/NF | 2D MOFs derivatives | 1.0 M KOH | 234 | 47 | [190] |
| Ru-NiFeP/NF | 2D MOFs derivatives | 1.0 M KOH | 179 | 44.9 | [193] |
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Egeland-Eriksen, T.; Hajizadeh, A.; Sartori, S. Hydrogen-based systems for integration of renewable energy in power systems: Achievements and perspectives. Int. J. Hydrogen Energy 2021, 46, 31963–31983. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Ahmed, A.; Al-Amin, A.Q.; Ambrose, A.F.; Saidur, R. Hydrogen fuel and transport system: A sustainable and environmental future. Int. J. Hydrogen Energy 2016, 41, 1369–1380. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-performance perovskite composite electrocatalysts enabled by controllable interface engineering. Small 2021, 17, e2101573. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Amorphous Catalysts and Electrochemical Water Splitting: An Untold Story of Harmony. Small 2020, 16, e1905779. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, Y.; He, G.; Chen, H. Race on engineering noble metal single-atom electrocatalysts for water splitting. Int. J. Hydrogen Energy 2022, 47, 14257–14279. [Google Scholar] [CrossRef]
- Guo, C.; Shi, Y.; Lu, S.; Yu, Y.; Zhang, B. Amorphous nanomaterials in electrocatalytic water splitting. Chin. J. Catal. 2021, 42, 1287–1296. [Google Scholar] [CrossRef]
- You, B.; Tang, M.T.; Tsai, C.; Abild-Pedersen, F.; Zheng, X.; Li, H. Enhancing Electrocatalytic Water Splitting by Strain Engineering. Adv. Mater. 2019, 31, e1807001. [Google Scholar] [CrossRef]
- Yang, X.; Guo, R.; Cai, R.; Ouyang, Y.; Yang, P.; Xiao, J. Engineering high-entropy materials for electrocatalytic water splitting. Int. J. Hydrogen Energy 2022, 47, 13561–13578. [Google Scholar] [CrossRef]
- Verma, J.; Goel, S. Cost-effective electrocatalysts for Hydrogen Evolution Reactions (HER): Challenges and Prospects. Int. J. Hydrogen Energy 2022, 47, 38964–38982. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, C.; Lin, Y.; Liu, Z.; Wang, M.; Chen, C. Electrocatalyst engineering and structure-activity relationship in hydrogen evolution reaction: From nanostructures to single atoms. Sci. China Mater. 2020, 63, 921–948. [Google Scholar] [CrossRef]
- Baek, D.S.; Lee, J.; Lim, J.S.; Joo, S.H. Nanoscale electrocatalyst design for alkaline hydrogen evolution reaction through activity descriptor identification. Mater. Chem. Front. 2021, 5, 4042–4058. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Liao, L.; Beine, A.K.; Palkovits, R. Stability and deactivation of OER electrocatalysts: A review. J. Energy Chem. 2022, 69, 301–329. [Google Scholar] [CrossRef]
- Zubair, M.; Hassan, M.M.U.; Mehran, M.T.; Baig, M.M.; Hussain, S.; Shahzad, F. 2D MXenes and their heterostructures for HER, OER and overall water splitting: A review. Int. J. Hydrogen Energy 2022, 47, 2794–2818. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, R. Advanced Transition Metal-Based OER Electrocatalysts: Current Status, Opportunities, and Challenges. Small 2021, 17, e2100129. [Google Scholar]
- Raeisi-Kheirabadi, N.; Nezamzadeh-Ejhieh, A.; Aghaei, H. Electrochemical amperometric sensing of loratadine using NiO modified paste electrode as an amplified sensor. Iran. J. Catal. 2021, 11, 181–189. [Google Scholar]
- Tamiji, T.; Nezamzadeh-Ejhieh, A. Electrocatalytic behavior of AgBr NPs as modifier of carbon past electrode in the presence of methanol and ethanol in aqueous solution: A kinetic study. J. Taiwan Inst. Chem. Eng. 2019, 104, 130–138. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, J.; Lyu, Z.; Zhang, Q.; Lee, T.H.; Pang, H.; Kang, D.J. Durable hierarchical phosphorus-doped biphase MoS2 electrocatalysts with enhanced H* adsorption. Carbon Energy 2023, e376. [Google Scholar] [CrossRef]
- Qian, Y.; Du, J.; Kang, D.J. Enhanced electrochemical performance of porous Co-doped TiO2 nanomaterials prepared by a solvothermal method. Microporous Mesoporous Mater. 2019, 273, 148–155. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, C.; Han, A.; Wang, L.; Sun, Y.; Zhu, C.; Li, R.; Ye, S. Modulation of morphology and electronic structure on MoS2-based electrocatalysts for water splitting. Nano Res. 2022, 15, 6862–6887. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Pan, J.; Huang, M.; Zhu, H. A highly efficient Fe-doped Ni3S2 electrocatalyst for overall water splitting. Nano Res. 2021, 14, 4740–4747. [Google Scholar] [CrossRef]
- Pang, Y.; Xu, W.; Zhu, S.; Cui, Z.; Liang, Y.; Li, Z.; Wu, S.; Chang, C.; Luo, S. Self-supporting amorphous nanoporous NiFeCoP electrocatalyst for efficient overall water splitting. J. Mater. Sci. Technol. 2021, 82, 96–104. [Google Scholar] [CrossRef]
- Huang, L.; Yao, R.; Wang, X.; Sun, S.; Zhu, X.; Liu, X.; Kim, M.G.; Lian, J.; Liu, F.; Li, Y.; et al. In situ phosphating of Zn-doped bimetallic skeletons as a versatile electrocatalyst for water splitting. Energy Environ. Sci. 2022, 15, 2425–2434. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Yan, F.; Zhu, C.; Gao, P.; Zhang, X.; Chen, Y. Self-supported NiMo-based nanowire arrays as bifunctional electrocatalysts for full water splitting. J. Mater. Chem. A 2018, 6, 8479–8487. [Google Scholar]
- Yu, X.; Guo, J.; Li, B.; Xu, J.; Gao, P.; Hui, K.S.; Hui, K.N.; Shao, H. Sub-Nanometer Pt Clusters on Defective NiFe LDH Nanosheets as Trifunctional Electrocatalysts for Water Splitting and Rechargeable Hybrid Sodium–Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 26891–26903. [Google Scholar] [CrossRef]
- Peng, J.; Tao, P.; Song, C.; Shang, W.; Deng, T.; Wu, J. Structural evolution of Pt-based oxygen reduction reaction electrocatalysts. Chin. J. Catal. 2022, 43, 47–58. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, J.; Zhang, Y.; Zhang, F.; Kang, Y.; Su, C.; Shi, H.; Kang, D.J.; Pang, H. Interfacial microenvironment modulation enhancing catalytic kinetics of binary metal sulfides heterostructures for advanced water splitting electrocatalysts. Small Methods 2022, 6, 2101186. [Google Scholar] [CrossRef]
- Liu, H.; Xi, C.; Xin, J.; Zhang, G.; Zhang, S.; Zhang, Z.; Huang, Q.; Li, J.; Liu, H.; Kang, J. Free-standing nanoporous NiMnFeMo alloy: An efficient non-precious metal electrocatalyst for water splitting. Chem. Eng. J. 2021, 404, 126530. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Lou, F.; Guo, K.; Yu, Z. Non-precious metal activated MoSi2N4 monolayers for high-performance OER and ORR electrocatalysts: A first-principles study. Appl. Surf. Sci. 2022, 579, 152234. [Google Scholar] [CrossRef]
- Rojas, S.; Rodríguez-Diéguez, A.; Horcajada, P. Metal–Organic Frameworks in Agriculture. ACS Appl. Mater. Interfaces 2022, 14, 16983–17007. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lu, L.; Liu, D.; Wang, J.; Sakiyama, H.; Muddassir, M.; Nezamzadeh-Ejhieh, A.; Liu, J. Series of highly stable Cd(ii)-based MOFs as sensitive and selective sensors for detection of nitrofuran antibiotic. CrystEngComm 2021, 23, 8043–8052. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Kang, D.J.; Pang, H. A review of metal–organic framework-based compounds for environmental applications. Energy Environ. Mater. 2023, 6, 12414. [Google Scholar] [CrossRef]
- Rao, C.; Liao, D.; Pan, Y.; Zhong, Y.; Zhang, W.; Ouyang, Q.; Nezamzadeh-Ejhieh, A.; Liu, J. Novel formulations of metal-organic frameworks for controlled drug delivery. Expert Opin. Drug Deliv. 2022, 19, 1183–1202. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, F.; Luo, D.; Huang, J.; Ouyang, J.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Peng, Y. Recent advances in Ti-based MOFs in biomedical applications. Dalton Trans. 2022, 51, 14817–14832. [Google Scholar] [CrossRef]
- Nicks, J.; Sasitharan, K.; Prasad, R.R.R.; Ashworth, D.J.; Foster, J.A. Metal–Organic Framework Nanosheets: Programmable 2D Materials for Catalysis, Sensing, Electronics, and Separation Applications. Adv. Funct. Mater. 2021, 31, 2103723. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating metal–organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. Susmat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Zhao, S.; Bian, C.; Mao, H.; Kang, D.J.; Pang, H. Recent progress of metal-organic framework-derived composites: Synthesis and their energy conversion applications. Nano Energy 2023, 111, 108415. [Google Scholar] [CrossRef]
- Liu, J.; Song, X.; Zhang, T.; Liu, S.; Wen, H.; Chen, L. 2D Conductive Metal–Organic Frameworks: An Emerging Platform for Electrochemical Energy Storage. Angew. Chem. Int. Ed. 2021, 60, 5612–5624. [Google Scholar] [CrossRef]
- Qian, Y.; Lyu, Z.; Zhang, Q.; Lee, T.H.; Kang, T.K.; Sohn, M.; Shen, L.; Kim, D.H.; Kang, D.J. High-Performance Flexible Energy Storage Devices Based on Graphene Decorated with Flower-Shaped MoS2 Heterostructures. Micromachines 2023, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Yin, J.; Shekhah, O.; Bakr, O.M.; Eddaoudi, M.; Mohammed, O.F. Energy Transfer in Metal–Organic Frameworks for Fluorescence Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9970–9986. [Google Scholar] [CrossRef] [PubMed]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal–organic frameworks for chemical sensing devices. Mater. Horizons 2021, 8, 2387–2419. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, K.; Min, H.; Xu, J.; Shi, W.; Cheng, P. Bifunctionalized Metal–Organic Frameworks for Pore-Size-Dependent Enantioselective Sensing. Angew. Chem. Int. Edit. 2022, 61, e202204066. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.A.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Rare earth metal–organic frameworks (RE-MOFs): Synthesis, properties, and biomedical applications. Coordin. Chem. Rev. 2021, 429, 213620. [Google Scholar] [CrossRef]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef]
- Cun, J.-E.; Fan, X.; Pan, Q.; Gao, W.; Luo, K.; He, B.; Pu, Y. Copper-based metal–organic frameworks for biomedical applications. Adv. Colloid Interface Sci. 2022, 305, 102686. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, X.; Kang, Z.; Liu, X.; Sun, D. Isoreticular chemistry within metal–organic frameworks for gas storage and separation. Coord. Chem. Rev. 2021, 443, 213968. [Google Scholar] [CrossRef]
- Amooghin, A.E.; Sanaeepur, H.; Luque, R.; Garcia, H.; Chen, B. Fluorinated metal–organic frameworks for gas separation. Chem. Soc. Rev. 2022, 51, 7427–7508. [Google Scholar] [CrossRef]
- Shi, Y.; Liang, B.; Lin, R.-B.; Zhang, C.; Chen, B. Gas Separation via Hybrid Metal–Organic Framework/Polymer Membranes. Trends Chem. 2020, 2, 254–269. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fan, W.; Sun, D. Flexible metal–organic frameworks for gas storage and separation. Dalton Trans. 2022, 51, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mian, M.R.; Lee, S.-J.; Chen, H.; Zhang, X.; Kirlikovali, K.O.; Shulda, S.; Melix, P.; Rosen, A.S.; Parilla, P.A.; et al. Fine-Tuning a Robust Metal–Organic Framework toward Enhanced Clean Energy Gas Storage. J. Am. Chem. Soc. 2021, 143, 18838–18843. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.B.; Gibbons, B.; Morris, A.J.; Morris, J.R.; Troya, D. Reversible Dissociation for Effective Storage of Diborane Gas within the UiO-66-NH2 Metal–Organic Framework. ACS Appl. Mater. Interfaces 2022, 14, 8322–8332. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, J.H.; Lee, G.; Lee, J.; Song, H.; Jho, J.Y.; Lee, H.H.; Kim, Y.H. Synthesis of a Carbonaceous Two-Dimensional Material. ACS Appl. Mater. Interfaces 2019, 11, 21308–21313. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.K.; Hersam, M.C. Electronic Transport in Two-Dimensional Materials. Annu. Rev. Phys. Chem. 2018, 69, 299–325. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Seo, H.; Galli, G. Spin coherence in two-dimensional materials. NPJ Comput. Mater. 2019, 5, 44. [Google Scholar] [CrossRef]
- Liu, X.; Sachan, A.K.; Howell, S.T.; Conde-Rubio, A.; Knoll, A.W.; Boero, G.; Zenobi, R.; Brugger, J. Thermomechanical Nanostraining of Two-Dimensional Materials. Nano Lett. 2020, 20, 8250–8257. [Google Scholar] [CrossRef]
- Sohier, T.D.P.; Gibertini, M.; Campi, D.; Pizzi, G.; Marzari, N. Valley-Engineering Mobilities in Two-Dimensional Materials. Nano Lett. 2019, 19, 3723–3729. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, X.; Zhang, Z.; Sun, Y.; Zhao, Y.; Zhang, Y.; Zhang, G. Thermo-mechanical correlation in two-dimensional materials. Nanoscale 2021, 13, 1425–1442. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, J.; Yan, C.; Liao, T.; Bell, J.; Sun, Z. Bioinspired 2D Nanomaterials: Bioinspired 2D Nanomaterials for Sustainable Applications (Adv. Mater. 18/2020). Adv. Mater. 2020, 32, 2070144. [Google Scholar] [CrossRef]
- Szunerits, S. 2D nanomaterials for electroanalysis. Anal. Bioanal. Chem. 2021, 413, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Lokhande, G.; Deo, K.A.; Brokesh, A.; Gaharwar, A.K. Emerging 2D nanomaterials for biomedical applications. Mater. Today 2021, 50, 276–302. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.M.; Wang, Y.; Bowen, C.R.; Yang, Y. 2D Nanomaterials for Effective Energy Scavenging. Nano-Micro Lett. 2021, 13, 82. [Google Scholar] [CrossRef]
- Huang, T.; Jiang, Y.; Shen, G.; Chen, D. Recent Advances of Two-Dimensional Nanomaterials for Electrochemical Capacitors. Chemsuschem 2020, 13, 1093–1113. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhu, Y.; Ma, B.; Xu, X.; Huang, R.; Cheng, L.; Zhu, R. Bioactive 2D nanomaterials for neural repair and regeneration. Adv. Drug Deliv. Rev. 2022, 187, 114379. [Google Scholar] [CrossRef] [PubMed]
- Pimpilova, M.; Ivanova-Kolcheva, V.; Stoyanova, M.; Dimcheva, N. 2D Nanomaterial—Based Electrocatalyst for Water Soluble Hydroperoxide Reduction. Catalysts 2022, 12, 807. [Google Scholar] [CrossRef]
- Shi, L.-N.; Cui, L.-T.; Ji, Y.-R.; Xie, Y.; Zhu, Y.-R.; Yi, T.-F. Towards high-performance electrocatalysts: Activity optimization strategy of 2D MXenes-based nanomaterials for water-splitting. Coord. Chem. Rev. 2022, 469, 214668. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Ma, T.; Jiang, Y.; Chen, J.; Pan, H.; Sun, W. 2D Metal-Free Nanomaterials Beyond Graphene and Its Analogues toward Electrocatalysis Applications. Adv. Energy Mater. 2021, 11, 2101202. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, W.; Zhang, X.; Zhang, Y.; Chen, W. Sub-2 nm Ultrathin and Robust 2D FeNi Layered Double Hydroxide Nanosheets Packed with 1D FeNi-MOFs for Enhanced Oxygen Evolution Electrocatalysis. Adv. Funct. Mater. 2021, 31, 2103318. [Google Scholar] [CrossRef]
- Pang, Y.; Su, C.; Jia, G.; Xu, L.; Shao, Z. Emerging two-dimensional nanomaterials for electrochemical nitrogen reduction. Chem. Soc. Rev. 2021, 50, 12744–12787. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, W.; Yuan, F.; Liu, J. Two host-guest 2D MOFs based on hexyl viologen cations: Photochromism. Dye. Pigment. 2021, 188, 109196. [Google Scholar] [CrossRef]
- Chang, H.; Zhou, Y.; Zheng, X.; Liu, W.; Xu, Q. Single-Layer 2D Ni−BDC MOF Obtained in Supercritical CO2-Assisted Aqueous Solution. Chem.-Eur. J. 2022, 28, e202201811. [Google Scholar] [CrossRef] [PubMed]
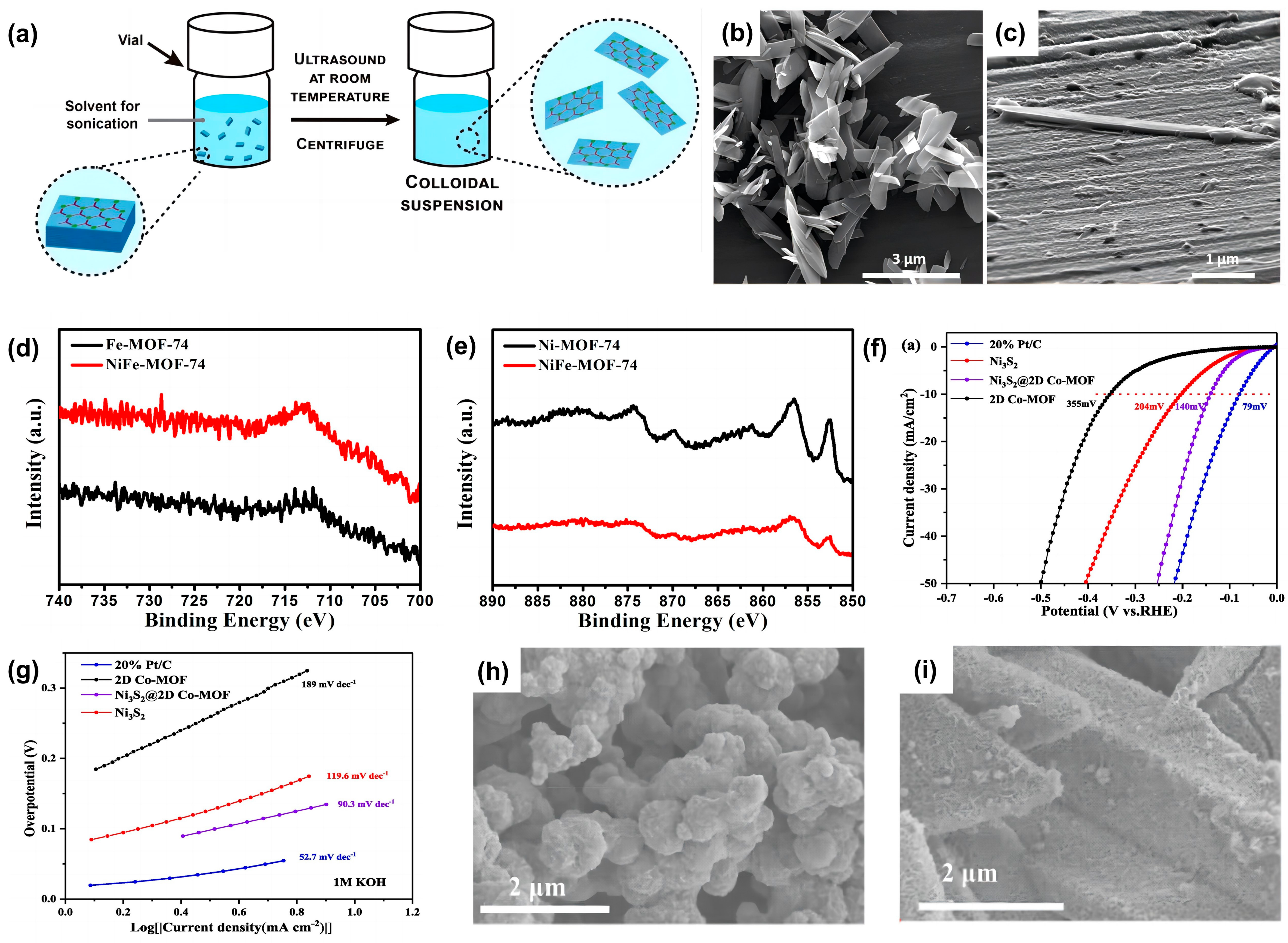
- Choi, J.Y.; Flood, J.; Stodolka, M.; Pham, H.T.B.; Park, J. From 2D to 3D: Postsynthetic Pillar Insertion in Electrically Conductive MOF. ACS Nano 2022, 16, 3145–3151. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Saji, S.E.; Wu, L.; Wang, Z.; Chen, Z.; Du, Y.; Yu, X.; Zhao, H.; Yin, Z. Emerging Synthesis Strategies of 2D MOFs for Electrical Devices and Integrated Circuits. Small 2022, 18, e2201642. [Google Scholar] [CrossRef]
- Dong, R.; Feng, X. Making large single crystals of 2D MOFs. Nat. Mater. 2021, 20, 122–123. [Google Scholar] [CrossRef]
- Wei, H.; Tian, Y.; Chen, Q.; Estevez, D.; Xu, P.; Peng, H.-X.; Qin, F. Microwave absorption performance of 2D Iron-Quinoid MOF. Chem. Eng. J. 2021, 405, 126637. [Google Scholar] [CrossRef]
- Majidi, L.; Ahmadiparidari, A.; Shan, N.; Misal, S.N.; Kumar, K.; Huang, Z.; Rastegar, S.; Hemmat, Z.; Zou, X.; Zapol, P.; et al. 2D Copper Tetrahydroxyquinone Conductive Metal–Organic Framework for Selective CO2 Electrocatalysis at Low Overpotentials. Adv. Mater. 2021, 33, e2004393. [Google Scholar] [CrossRef]
- Yang, D.; Zuo, S.; Yang, H.; Zhou, Y.; Lu, Q.; Wang, X. Tailoring Layer Number of 2D Porphyrin-Based MOFs Towards Photocoupled Electroreduction of CO2. Adv. Mater. 2022, 34, e2107293. [Google Scholar] [CrossRef]
- Tang, D.; Yang, X.; Wang, B.; Ding, Y.; Xu, S.; Liu, J.; Peng, Y.; Yu, X.; Su, Z.; Qin, X. One-Step Electrochemical Growth of 2D/3D Zn(II)-MOF Hybrid Nanocomposites on an Electrode and Utilization of a PtNPs@2D MOF Nanocatalyst for Electrochemical Immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 46225–46232. [Google Scholar] [CrossRef]
- Lee, M.K.; Shokouhimehr, M.; Kim, S.Y.; Jang, H.W. Two-Dimensional Metal–Organic Frameworks and Covalent–Organic Frameworks for Electrocatalysis: Distinct Merits by the Reduced Dimension. Adv. Energy Mater. 2021, 12, 2003990. [Google Scholar] [CrossRef]
- Meng, R.; Du, Q.; Zhong, N.; Zhou, X.; Liu, S.; Yin, S.; Liang, X. A Tandem Electrocatalysis of Sulfur Reduction by Bimetal 2D MOFs. Adv. Energy Mater. 2021, 11, 2102819. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Sun, X.-B.; Wang, G.-S. A MXene-modulated 3D crosslinking network of hierarchical flower-like MOF derivatives towards ultra-efficient microwave absorption properties. J. Mater. Chem. A 2021, 9, 24571–24581. [Google Scholar] [CrossRef]
- Song, Y.; Song, X.; Wang, X.; Bai, J.; Cheng, F.; Lin, C.; Wang, X.; Zhang, H.; Sun, J.; Zhao, T.; et al. Two-Dimensional Metal–Organic Framework Superstructures from Ice-Templated Self-Assembly. J. Am. Chem. Soc. 2022, 144, 17457–17467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-D.; Zhou, L.; Wang, H.-R.; Xu, H.; Zhu, H.; Jiang, Y.; Yan, X.; Gu, Z.-G. A Hexagonal Nut-Like Metal–Organic Framework and Its Conformal Transformation. Small 2022, 18, 2203356. [Google Scholar] [CrossRef]
- Wei, Y.; Zou, L.; Wang, H.; Wang, Y.; Xu, Q. Micro/Nano-Scaled Metal-Organic Frameworks and Their Derivatives for Energy Applications. Adv. Energy Mater. 2022, 12, 2003970. [Google Scholar] [CrossRef]
- Huang, K.; Guo, S.; Wang, R.; Lin, S.; Hussain, N.; Wei, H.; Deng, B.; Long, Y.; Lei, M.; Tang, H.; et al. Two-dimensional MOF/MOF derivative arrays on nickel foam as efficient bifunctional coupled oxygen electrodes. Chin. J. Catal. 2020, 41, 1754–1760. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, H.; Yuan, S.; Ren, H.; Ge, Z.; Zhu, H.; Guo, W.; Ding, F.; Zhao, W. Benzenehexol-based 2D MOF as high-performance electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2022, 601, 154187. [Google Scholar] [CrossRef]
- Huo, M.; Sun, T.; Wang, Y.; Sun, P.; Dang, J.; Wang, B.; Dharanipragada, N.V.R.A.; Inge, A.K.; Zhang, W.; Cao, R.; et al. A heteroepitaxially grown two-dimensional metal–organic framework and its derivative for the electrocatalytic oxygen reduction reaction. J. Mater. Chem. A 2022, 10, 10408–10416. [Google Scholar] [CrossRef]
- Pang, W.; Shao, B.; Tan, X.-Q.; Tang, C.; Zhang, Z.; Huang, J. Exfoliation of metal–organic frameworks into efficient single-layer metal–organic nanosheet electrocatalysts by the synergistic action of host–guest interactions and sonication. Nanoscale 2020, 12, 3623–3629. [Google Scholar] [CrossRef]
- Villalpando, G.; Ferrenti, A.M.; Singha, R.; Song, X.; Cheng, G.; Yao, N.; Schoop, L.M. Chemical Exfoliation toward Magnetic 2D VOCl Monolayers. ACS Nano 2022, 16, 13814–13820. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.; Cheng, Z. Ultrasonic-assisted exfoliation for 2D Zn(Bim)(OAc) nanosheets used as an oil-soluble additive in lubricants. Appl. Organomet. Chem. 2020, 34, e5950. [Google Scholar] [CrossRef]
- Nicks, J.; Foster, J.A. Post-exfoliation functionalisation of metal–organic framework nanosheets via click chemistry. Nanoscale 2022, 14, 6220–6227. [Google Scholar] [CrossRef]
- Nian, P.; Liu, H.; Zhang, X. Bottom-up synthesis of 2D Co-based metal–organic framework nanosheets by an ammonia-assisted strategy for tuning the crystal morphology. CrystEngComm 2019, 21, 3199–3208. [Google Scholar] [CrossRef]
- Jia, J.; Wei, L.; Li, F.; Yu, C.; Yang, K.; Liang, T. In situ growth of NiFe MOF/NF by controlling solvent mixtures as efficient electrocatalyst in oxygen evolution. Inorg. Chem. Commun. 2021, 128, 108605. [Google Scholar] [CrossRef]
- Song, D.; Guo, H.; Huang, K.; Zhang, H.; Chen, J.; Wang, L.; Lian, C.; Wang, Y. Carboxylated carbon quantum dot-induced binary metal–organic framework nanosheet synthesis to boost the electrocatalytic performance. Mater. Today 2022, 54, 42–51. [Google Scholar] [CrossRef]
- Zeng, R.; He, T.; Lu, L.; Li, K.; Luo, Z.; Cai, K. Ultra-thin metal–organic framework nanosheets for chemo-photodynamic synergistic therapy. J. Mater. Chem. B 2021, 9, 4143–4153. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Weng, B.; Xiao, L.; Tian, Z.; Yang, J.; Liu, T.; Lai, F. Edge engineering of platinum nanoparticles via porphyrin-based ultrathin 2D metal–organic frameworks for enhanced photocatalytic hydrogen generation. Chem. Eng. J. 2022, 442, 136144. [Google Scholar] [CrossRef]
- Yaghi, O.M. Evolution of MOF single crystals. Chem 2022, 8, 1541–1543. [Google Scholar] [CrossRef]
- Suresh, K.; Aulakh, D.; Purewal, J.; Siegel, D.J.; Veenstra, M.; Matzger, A.J. Optimizing Hydrogen Storage in MOFs through Engineering of Crystal Morphology and Control of Crystal Size. J. Am. Chem. Soc. 2021, 143, 10727–10734. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Li, Y.; Zhang, X.; Huang, Y. 2D bimetallic Ni/Fe MOF nanosheet composites as a peroxidase-like nanozyme for colorimetric assay of multiple targets. Anal. Methods 2021, 13, 2066–2074. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Ma, S.-H.; Dong, H.; Zou, Y.-C.; Xu, K.-X.; Su, S.; Jin, X.-W.; Zhang, L.; Fang, W.-X. Two-dimensional nanosheets of metal–organic frameworks with tailorable morphologies. Mater. Today Chem. 2021, 22, 100517. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Yang, Z.; Zhang, C.; Liu, S. Ultrasound-treated metal-organic framework with efficient electrocatalytic oxygen evolution activity. Ultrason. Sonochem. 2019, 59, 104714. [Google Scholar] [CrossRef] [PubMed]
- Amo-Ochoa, P.; Welte, L.; González-Prieto, R.; Miguel, P.J.S.; Gómez-García, C.J.; Mateo-Martí, E.; Delgado, S.; Gómez-Herrero, J.; Zamora, F. Single layers of a multifunctional laminar Cu(I,II) coordination polymer. Chem. Commun. 2010, 46, 3262–3264. [Google Scholar] [CrossRef] [PubMed]
- Tamuly, P.; Sama, F.; Moorthy, J.N. Metal–Organic Nanosheets (MONs): Exfoliation by Mechanical Grinding and Iodine Capture. Adv. Mater. Interfaces 2022, 9, 2200337. [Google Scholar] [CrossRef]
- Wang, M.; Shi, H.; Zhang, P.; Liao, Z.; Wang, M.; Zhong, H.; Schwotzer, F.; Nia, A.S.; Zschech, E.; Zhou, S.; et al. Phthalocyanine-Based 2D Conjugated Metal-Organic Framework Nanosheets for High-Performance Micro-Supercapacitors. Adv. Funct. Mater. 2020, 30, 2002664. [Google Scholar] [CrossRef]
- Han, L.-J.; Zheng, D.; Chen, S.-G.; Zheng, H.-G.; Ma, J. A Highly Solvent-Stable Metal–Organic Framework Nanosheet: Morphology Control, Exfoliation, and Luminescent Property. Small 2018, 14, 1703873. [Google Scholar] [CrossRef]
- Shen, Y.; Shan, B.; Cai, H.; Qin, Y.; Agarwal, A.; Trivedi, D.B.; Chen, B.; Liu, L.; Zhuang, H.; Mu, B.; et al. Ultimate Control over Hydrogen Bond Formation and Reaction Rates for Scalable Synthesis of Highly Crystalline vdW MOF Nanosheets with Large Aspect Ratio. Adv. Mater. 2018, 30, e1802497. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Ban, Y.; Yang, W. Two-Dimensional Metal–Organic Framework Nanosheets for Membrane-Based Gas Separation. Angew. Chem. Int. Edit. 2017, 56, 9757–9761. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.-P.; Zhang, X.; Chen, L.; Dong, Z.; Jiang, H.-L.; Xu, H.; Zhou, H.-C. Controlled Intercalation and Chemical Exfoliation of Layered Metal–Organic Frameworks Using a Chemically Labile Intercalating Agent. J. Am. Chem. Soc. 2017, 139, 9136–9139. [Google Scholar] [CrossRef]
- Qin, M.-F.; Mu, Q.-Q.; Bao, S.-S.; Liang, X.; Peng, Y.; Zheng, L.-M. Chemically Exfoliated Semiconducting Bimetallic Porphyrinylphosphonate Metal–Organic Layers for Photocatalytic CO2 Reduction under Visible Light. ACS Appl. Energy Mater. 2021, 4, 4319–4326. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Huang, R.; He, C.; Gong, L.; Hu, Q.; Wang, L.; Xu, Y.; Tian, X.; Liu, S.; et al. Electrochemical Exfoliation of Pillared-Layer Metal–Organic Framework to Boost the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2018, 57, 4632–4636. [Google Scholar] [CrossRef]
- Li, H.; Hou, J.; Bennett, T.D.; Liu, J.; Zhang, Y. Templated growth of vertically aligned 2D metal–organic framework nanosheets. J. Mater. Chem. A 2019, 7, 5811–5818. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Zheng, H.; Fan, F.; Gu, Z.; He, W.; Zhang, B.; Shao, L.; Chen, H.; Li, Y.; et al. Fabrication of mesoporous MOF nanosheets via surfactant-template method for C–S coupling reactions. Microporous Mesoporous Mater. 2020, 303, 110254. [Google Scholar] [CrossRef]
- Han, M.; Zhang, X.; Gao, H.; Chen, S.; Cheng, P.; Wang, P.; Zhao, Z.; Dang, R.; Wang, G. In situ semi-sacrificial template-assisted growth of ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Chem. Eng. J. 2021, 426, 131348. [Google Scholar] [CrossRef]
- Chen, I.-F.; Lu, C.-F.; Su, W.-F. Highly Conductive 2D Metal–Organic Framework Thin Film Fabricated by Liquid–Liquid Interfacial Reaction Using One-Pot-Synthesized Benzenehexathiol. Langmuir 2018, 34, 15754–15762. [Google Scholar] [CrossRef]
- Wang, M.; Dong, X.; Meng, Z.; Hu, Z.; Lin, Y.; Peng, C.; Wang, H.; Pao, C.; Ding, S.; Li, Y.; et al. An Efficient Interfacial Synthesis of Two-Dimensional Metal–Organic Framework Nanosheets for Electrochemical Hydrogen Peroxide Production. Angew. Chem. Int. Ed. 2021, 60, 11190–11195. [Google Scholar] [CrossRef]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés, I.; Xamena, F.X.; Gascon, J. Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2014, 14, 48–55. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Ma, Q.; Huang, Y.; Zhang, X.; Ping, J.; Zhang, Z.; Lu, Q.; Yu, Y.; Xu, H.; et al. Ultrathin 2D Metal-Organic Framework Nanosheets. Adv. Mater. 2015, 27, 7372–7378. [Google Scholar] [CrossRef]
- Ma, J.; Bai, W.; Liu, X.; Zheng, J. Electrochemical dopamine sensor based on bi-metallic Co/Zn porphyrin metal–organic framework. Microchim. Acta 2021, 189, 20. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Surfactant assisted synthesis of hierarchical porous metal-organic frameworks nanosheets. Nanotechnology 2019, 30, 435601. [Google Scholar] [CrossRef]
- Fan, H.; Yu, H.; Wu, X.; Zhang, Y.; Luo, Z.; Wang, H.; Guo, Y.; Madhavi, S.; Yan, Q. Controllable Preparation of Square Nickel Chalcogenide (NiS and NiSe2) Nanoplates for Superior Li/Na Ion Storage Properties. ACS Appl. Mater. Interfaces 2016, 8, 25261–25267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, P.; Chen, C.; Zhang, J.; Yang, G.; Zheng, L.; Zhang, J.; Han, B. Fabrication of 2D metal–organic framework nanosheets with tailorable thickness using bio-based surfactants and their application in catalysis. Green Chem. 2019, 21, 54–58. [Google Scholar] [CrossRef]
- Lin, Y.; Wan, H.; Wu, D.; Chen, G.; Zhang, N.; Liu, X.; Li, J.; Cao, Y.; Qiu, G.; Ma, R. Metal-Organic Framework Hexagonal Nanoplates: Bottom-up Synthesis, Topotactic Transformation, and Efficient Oxygen Evolution Reaction. J. Am. Chem. Soc. 2020, 142, 7317–7321. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, G.; Wan, H.; Chen, F.; Liu, X.; Ma, R. 2D Free-Standing Nitrogen-Doped Ni-Ni3S2@Carbon Nanoplates Derived from Metal–Organic Frameworks for Enhanced Oxygen Evolution Reaction. Small 2019, 15, 1900348. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chen, Y.; Zhang, Z.; Ni, B.; He, W.; Wang, X. Competitive coordination strategy for the synthesis of hierarchical-pore metal–organic framework nanostructures. Chem. Sci. 2016, 7, 7101–7105. [Google Scholar] [CrossRef]
- Cliffe, M.J.; Castillo-Martínez, E.; Wu, Y.; Lee, J.; Forse, A.C.; Firth, F.C.N.; Moghadam, P.Z.; Fairen-Jimenez, D.; Gaultois, M.W.; Hill, J.A.; et al. Metal–Organic Nanosheets Formed via Defect-Mediated Transformation of a Hafnium Metal–Organic Framework. J. Am. Chem. Soc. 2017, 139, 5397–5404. [Google Scholar] [CrossRef]
- Zheng, G.; Xing, Z.; Gao, X.; Nie, C.; Xu, Z.; Ju, Z. Fabrication of 2D Cu-BDC MOF and its derived porous carbon as anode material for high-performance Li/K-ion batteries. Appl. Surf. Sci. 2021, 559, 149701. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, H.; Hui, Z.; Sun, Y.; Yu, C.; Xue, J.; Zhou, R.; Wang, L.; Dai, H.; Zhao, Y.; et al. Electrostatically Assembling 2D Nanosheets of MXene and MOF-Derivatives into 3D Hollow Frameworks for Enhanced Lithium Storage. Small 2019, 15, e1904255. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, R.; Li, J.; Yang, Y.; Wu, Y.; Zhou, X.; Ma, G.; Ren, S. 2D DUT-8(Ni)-derived Ni@C nanosheets for efficient hydrogen evolution. J. Solid State Electrochem. 2020, 24, 2461–2467. [Google Scholar] [CrossRef]
- Ren, J.-T.; Zheng, Y.-L.; Yuan, K.; Zhou, L.; Wu, K.; Zhang, Y.-W. Self-templated synthesis of Co3O4 hierarchical nanosheets from a metal–organic framework for efficient visible-light photocatalytic CO2 reduction. Nanoscale 2020, 12, 755–762. [Google Scholar] [CrossRef]
- He, P.; Xie, Y.; Dou, Y.; Zhou, J.; Zhou, A.; Wei, X.; Li, J.-R. Partial Sulfurization of a 2D MOF Array for Highly Efficient Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 41595–41601. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Pereda, N.; Moghzi, F.; Baselga, J.; Zhong, H.; Janczak, J.; Soleimannejad, J.; Dong, R.; Ruiz-Molina, D. Ultrasound-assisted exfoliation of a layered 2D coordination polymer with HER electrocatalytic activity. Ultrason. Sonochem. 2021, 70, 105292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, X.; Yang, X.; Xia, R.; Xu, Y.; Sun, W.; Zhou, J. Hierarchical Ni3S2@2D Co MOF nanosheets as efficient hetero-electrocatalyst for hydrogen evolution reaction in alkaline solution. Fuel Process. Technol. 2022, 229, 107174. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Wei, M.; Fan, H.; Liu, X.; Liu, Q.; Liu, Y.; Cao, J.; Yang, L. 2D MOF-derived porous NiCoSe nanosheet arrays on Ni foam for overall water splitting. CrystEngComm 2021, 23, 69–81. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Rezazadeh, A. Hydrogen fuel and electricity generation from a new hybrid energy system based on wind and solar energies and alkaline fuel cell. Energy Rep. 2021, 7, 2594–2604. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Xu, L.; Tang, Y.; Wu, X.; Liu, J. Thermodynamic evaluation of electricity and hydrogen cogeneration from solar energy and fossil fuels. Energy Convers. Manag. 2022, 256, 115344. [Google Scholar] [CrossRef]
- Sekar, S.; Devi, S.B.; Maruthasalamoorthy, S.; Maiyalagan, T.; Kim, D.Y.; Lee, S.; Navamathavan, R. One-step facile hydrothermal synthesis of rGO-CoS2 nanocomposites for high performance HER electrocatalysts. Int. J. Hydrogen Energy 2022, 47, 40359–40367. [Google Scholar] [CrossRef]
- Habib, A.; Mandavkar, R.; Burse, S.; Lin, S.; Kulkarni, R.; Patil, C.S.; Jeong, J.-H.; Lee, J. Design of boron-based ternary W3CoB3 electrocatalyst for the improved HER and OER performances. Mater. Today Energy 2022, 26, 101021. [Google Scholar] [CrossRef]
- Liang, T.; Xie, J.; Wang, A.; Ma, D.; Mao, Z.; Wang, J.; Li, H. Prediction of HER electrocatalyst with enhanced performance based on atoms-doped black phosphorene: A first-principles study. Appl. Surf. Sci. 2022, 604, 154508. [Google Scholar] [CrossRef]
- Prats, H.; Chan, K. The determination of the HOR/HER reaction mechanism from experimental kinetic data. Phys. Chem. Chem. Phys. 2021, 23, 27150–27158. [Google Scholar] [CrossRef]
- Ma, R.; Hong, K. Theoretical Investigation of HER Mechanism Using Density Functional and Ab Initio Calculations. Bull. Korean Chem. Soc. 2021, 42, 1289–1292. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, T.; Zhang, J.; Li, Y. The Mechanism of One-Step Synthesis of Oxides from Metal Chlorides and Their Electrocatalytic HER Study. Cryst. Res. Technol. 2022, 57, 2200008. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, N.; Dai, C.; Xu, R.; Wu, B.; Yu, G.; Chen, B.; Du, Y. H2-Directing Strategy on In Situ Synthesis of Co-MoS2 with Highly Expanded Interlayer for Elegant HER Activity and its Mechanism. Adv. Energy Mater. 2020, 10, 2000291. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, S.; Rao, D. Theoretical study the component and facet dependence of HER performance on nickel phosphides surfaces. Int. J. Hydrogen Energy 2022, 47, 2992–3000. [Google Scholar] [CrossRef]
- Wu, T.; Melander, M.M.; Honkala, K. Coadsorption of NRR and HER Intermediates Determines the Performance of Ru-N4 toward Electrocatalytic N2 Reduction. ACS Catal. 2022, 12, 2505–2512. [Google Scholar] [CrossRef]
- Karthick, K.; Basha, A.B.M.; Sivakumaran, A.; Kundu, S. Enhancement of HER kinetics with RhNiFe for high-rate water electrolysis. Catal. Sci. Technol. 2020, 10, 3681–3693. [Google Scholar] [CrossRef]
- Zhu, J.; Tu, Y.; Cai, L.; Ma, H.; Chai, Y.; Zhang, L.; Zhang, W. Defect-Assisted Anchoring of Pt Single Atoms on MoS2 Nanosheets Produces High-Performance Catalyst for Industrial Hydrogen Evolution Reaction. Small 2021, 18, 2104824. [Google Scholar] [CrossRef]
- Yasin, G.; Ibraheem, S.; Ali, S.; Arif, M.; Ibrahim, S.; Iqbal, R.; Kumar, A.; Tabish, M.; Mushtaq, M.; Saad, A.; et al. Defects-engineered tailoring of tri-doped interlinked metal-free bifunctional catalyst with lower gibbs free energy of OER/HER intermediates for overall water splitting. Mater. Today Chem. 2022, 23, 100634. [Google Scholar] [CrossRef]
- Ohm, D.; Domke, K.F. Controlled deposition of 2D-confined Pd or Ir nano-islands on Au(111) following Cu UPD, and their HER activity. Electroanal. Chem. 2021, 896, 115285. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, X.; Xu, R.; Dai, C.; Wu, B.; Yu, G.; Chen, B.; Wang, X.; Liu, N. H2 In Situ Inducing Strategy on Pt Surface Segregation Over Low Pt Doped PtNi5 Nanoalloy with Superhigh Alkaline HER Activity. Adv. Funct. Mater. 2021, 31, 2008298. [Google Scholar] [CrossRef]
- Sun, C.; Duan, X.; Song, J.; Zhang, M.; Jin, Y.; Zhang, M.; Song, L.; Cao, H. Rh particles in N-doped porous carbon materials derived from ZIF-8 as an efficient bifunctional electrocatalyst for the ORR and HER. RSC Adv. 2021, 11, 13906–13911. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pan, G.; Liang, N.; Hong, S.; Ma, J.; Liu, Y. Ir Single Atom Catalyst Loaded on Amorphous Carbon Materials with High HER Activity. Adv. Sci. 2022, 9, e2105392. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An overview on Pd-based electrocatalysts for the hydrogen evolution reaction. Inorg. Chem. Front. 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Liang, L.; Jin, H.; Zhou, H.; Liu, B.; Hu, C.; Chen, D.; Wang, Z.; Hu, Z.; Zhao, Y.; Li, H.-W.; et al. Cobalt single atom site isolated Pt nanoparticles for efficient ORR and HER in acid media. Nano Energy 2021, 88, 106221. [Google Scholar] [CrossRef]
- Li, D.; Chen, X.; Lv, Y.; Zhang, G.; Huang, Y.; Liu, W.; Li, Y.; Chen, R.; Nuckolls, C.; Ni, H. An effective hybrid electrocatalyst for the alkaline HER: Highly dispersed Pt sites immobilized by a functionalized NiRu-hydroxide. Appl. Catal. B-Environ. 2020, 269, 118824. [Google Scholar] [CrossRef]
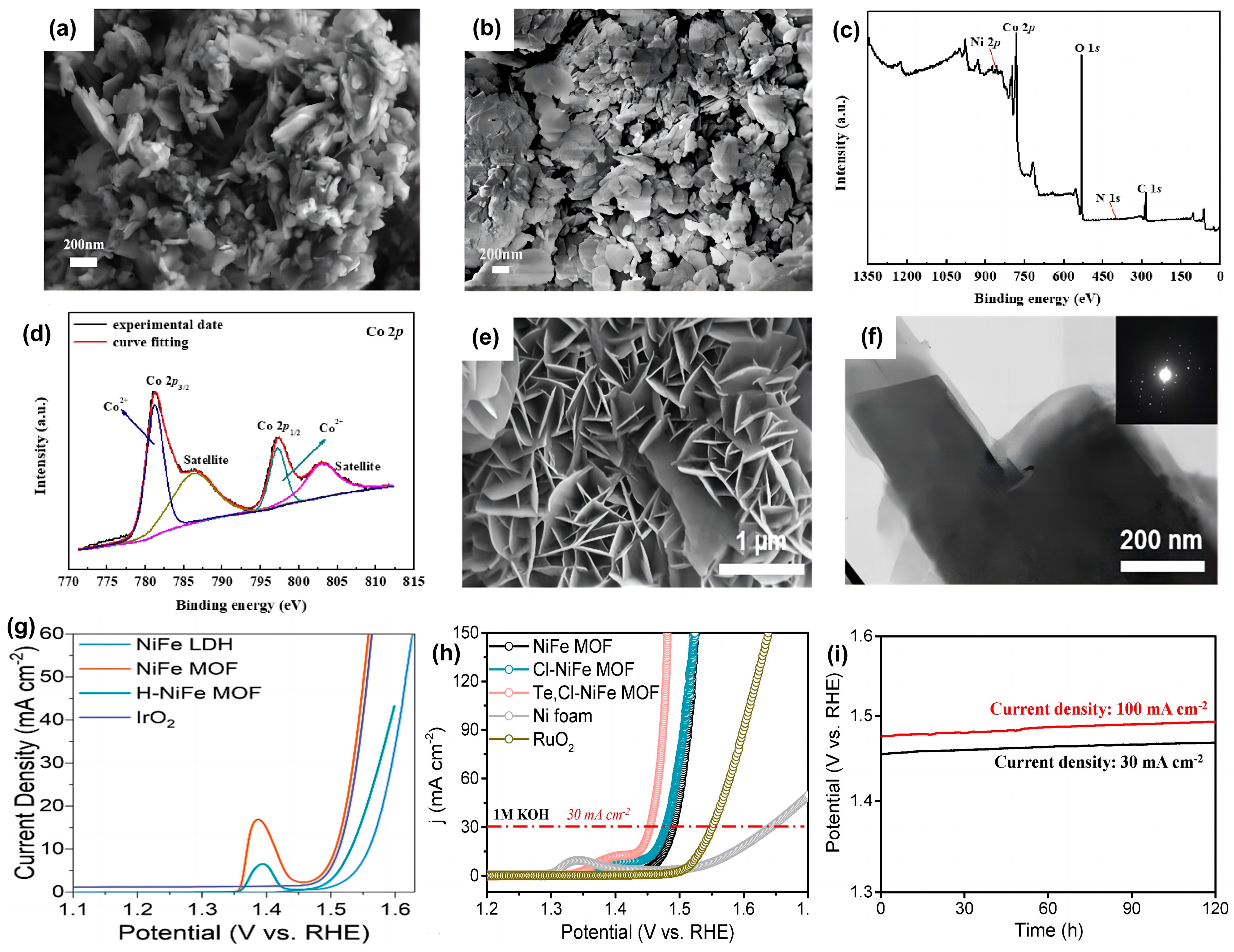
- Chen, C.; Suo, N.; Han, X.; He, X.; Dou, Z.; Lin, Z.; Cui, L. Tuning the morphology and electron structure of metal-organic framework-74 as bifunctional electrocatalyst for OER and HER using bimetallic collaboration strategy. J. Alloys Compd. 2021, 865, 158795. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y.; Chen, C.; Liu, Q.; Huo, J.; Yi, H. Building CoP/Co-MOF heterostructure in 2D nanosheets for improving electrocatalytic hydrogen evolution over a wide pH range. Electroanal. Chem. 2021, 895, 115514. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.-H.; Ko, T.; Choi, Y.; Kim, H. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, Z.; Liao, J.; Liang, H.; Wei, B.; Xu, X.; Xu, H.; Zheng, J.; Zhu, W.; Cavallo, L.; et al. Construction of hydroxide pn junction for water splitting electrocatalysis. Appl. Catal. B Environ. 2021, 292, 120160. [Google Scholar] [CrossRef]
- Martinez, J.; Mazarío, J.; Olloqui-Sariego, J.L.; Calvente, J.J.; Darawsheh, M.D.; Mínguez-Espallargas, G.; Domine, M.E.; Oña-Burgos, P. Bimetallic Intersection in PdFe@FeOx-C Nanomaterial for Enhanced Water Splitting Electrocatalysis. Adv. Sustain. Syst. 2022, 6, 2200096. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, P.; Zhang, H.; Tian, W.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Construction of hierarchical IrTe nanotubes with assembled nanosheets for overall water splitting electrocatalysis. J. Mater. Chem. A 2021, 9, 18576–18581. [Google Scholar] [CrossRef]
- Ai, L.; Chen, M.; Wang, X.; Luo, Y.; Wei, W.; Jiang, J. Localized photothermal heating of phosphate-incorporated iron oxide nanosheets enables greatly enhanced water splitting electrocatalysis. J. Alloys Compd. 2022, 925, 166750. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, W.; Yu, H.; Zeng, Y.; Ming, F.; Liang, H.; Wang, Z. NiCo/NiCo–OH and NiFe/NiFe–OH core shell nanostructures for water splitting electrocatalysis at large currents. Appl. Catal. B Environ. 2020, 278, 119326. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Liao, J.; Wang, L.; Li, M.; Xu, R.; Yang, L. Surface engineering of superhydrophilic Ni2P@NiFe LDH heterostructure toward efficient water splitting electrocatalysis. Appl. Surf. Sci. 2022, 602, 154287. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, K.; Guo, W.; Zhang, H.; Xiao, X.; He, M.; Yu, T.; Zhao, H.; Zhang, D.; Yang, T. Vanadium-phosphorus incorporation induced interfacial modification on cobalt catalyst and its super electrocatalysis for water splitting in alkaline media. Appl. Catal. B Environ. 2022, 304, 120985. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Zhu, Z.; Xu, K.; Zhang, H.; Wei, W.; Xiao, X.; Liang, W.; He, M.; Yu, T.; et al. Interfacial boron modification on mesoporous octahedral rhodium shell and its enhanced electrocatalysis for water splitting and oxygen reduction. Chem. Eng. J. 2022, 435, 134982. [Google Scholar] [CrossRef]
- Liang, T.; Lenus, S.; Liu, Y.; Chen, Y.; Sakthivel, T.; Chen, F.; Ma, F.; Dai, Z. Interface and M3+/M2+ Valence Dual-Engineering on Nickel Cobalt Sulfoselenide/Black Phosphorus Heterostructure for Efficient Water Splitting Electrocatalysis. Energy Environ. Mater. 2021, 6, e12332. [Google Scholar]
- Yi, L.; Feng, B.; Chen, N.; Li, W.; Li, J.; Fang, C.; Yao, Y.; Hu, W. Electronic interaction boosted electrocatalysis of iridium nanoparticles on nitrogen-doped graphene for efficient overall water splitting in acidic and alkaline media. Chem. Eng. J. 2021, 415, 129034. [Google Scholar] [CrossRef]
- Huang, R.; Wen, Y.; Peng, H.; Zhang, B. Improved kinetics of OER on Ru-Pb binary electrocatalyst by decoupling proton-electron transfer. Chin. J. Catal. 2022, 43, 130–138. [Google Scholar] [CrossRef]
- Binninger, T.; Doublet, M.-L. The Ir–OOOO–Ir transition state and the mechanism of the oxygen evolution reaction on IrO2(110). Energy Environ. Sci. 2022, 15, 2519–2528. [Google Scholar] [CrossRef]
- Moysiadou, A.; Lee, S.; Hsu, C.-S.; Chen, H.M.; Hu, X. Mechanism of Oxygen Evolution Catalyzed by Cobalt Oxyhydroxide: Cobalt Superoxide Species as a Key Intermediate and Dioxygen Release as a Rate-Determining Step. J. Am. Chem. Soc. 2020, 142, 11901–11914. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, G.; Wang, L.; Fu, H. Structural Design Strategy and Active Site Regulation of High-Efficient Bifunctional Oxygen Reaction Electrocatalysts for Zn–Air Battery. Small 2021, 17, e2006766. [Google Scholar] [CrossRef] [PubMed]
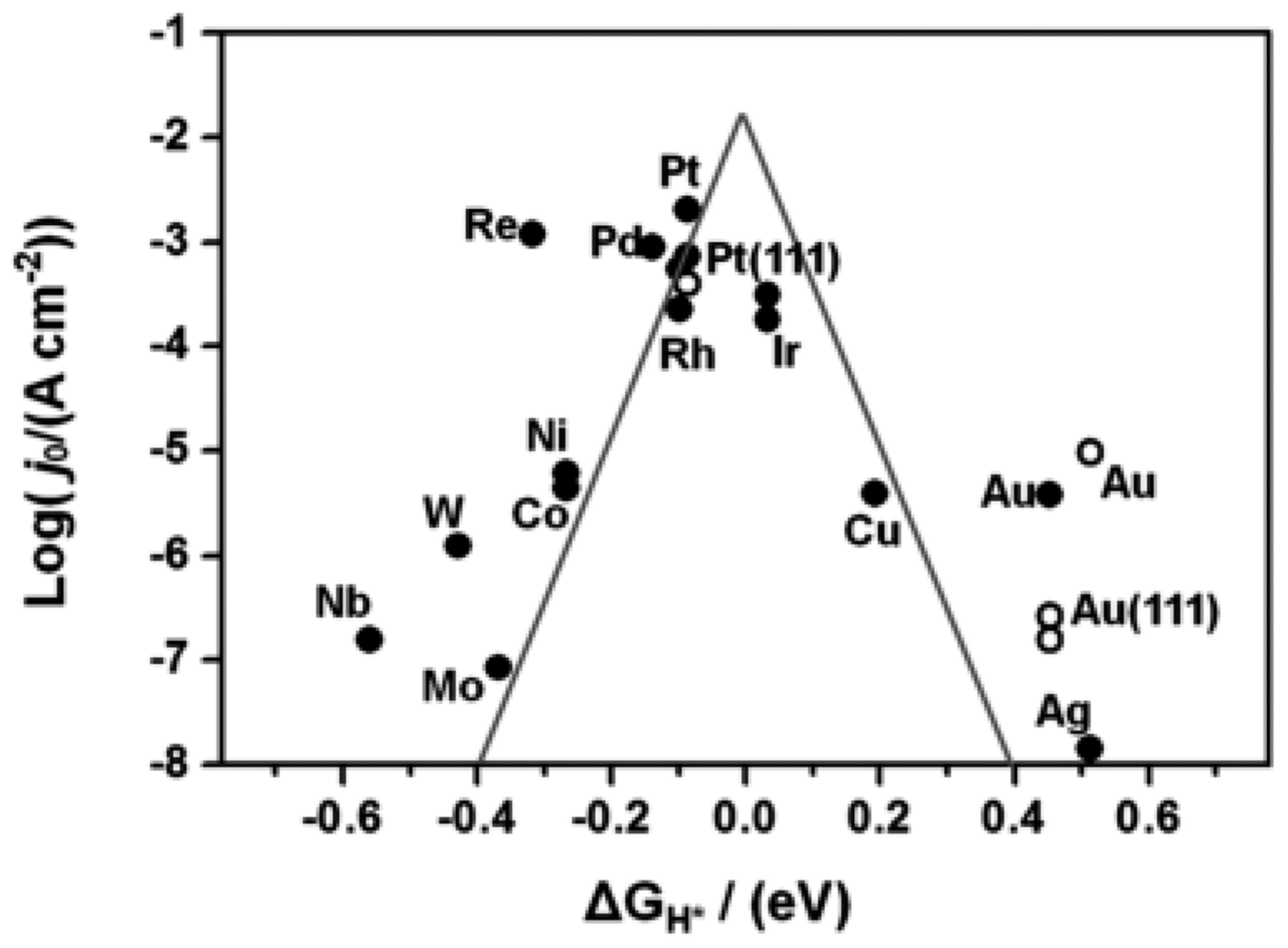
- Hai, G.; Gao, H.; Huang, X.; Tan, L.; Xue, X.; Feng, S.; Wang, G. An efficient factor for fast screening of high-performance two-dimensional metal–organic frameworks towards catalyzing the oxygen evolution reaction. Chem. Sci. 2022, 13, 4397–4405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-Y.; Sun, X.; Gu, H.; Niu, Z.; Braunstein, P.; Lang, J.-P. Engineering the Electronic Structures of Metal–Organic Framework Nanosheets via Synergistic Doping of Metal Ions and Counteranions for Efficient Water Oxidation. ACS Appl. Mater. Interfaces 2022, 14, 15133–15140. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Hu, Y.; Song, N.; Mu, T.; Bai, S.; Peng, Y.; Liu, L.-L.; Tang, Y. Two-dimensional heterostructures built from ultrathin CeO2 nanosheet surface-coordinated and confined metal–organic frameworks with enhanced stability and catalytic performance. Chem. Sci. 2022, 13, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Cao, S.; Xu, H.; Jiang, C.; Wang, Z.; Ouyang, Y.; Lu, X.; Dai, F.; Sun, D. Novel Two-Dimensional Metal Organic Frameworks: High-Performance Bifunctional Electrocatalysts for OER/ORR. ACS Mater. Lett. 2022, 4, 1991–1998. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J. Structural engineering of transition metal-based nanostructured electrocatalysts for efficient water splitting. Front. Chem. Sci. Eng. 2018, 12, 838–854. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Yang, P.; Yu, F.; Liu, Z.; Peng, B. Directly application of bimetallic 2D-MOF for advanced electrocatalytic oxygen evolution. Int. J. Hydrogen Energy 2021, 46, 416–424. [Google Scholar] [CrossRef]
- Ge, K.; Sun, S.; Zhao, Y.; Yang, K.; Wang, S.; Zhang, Z.; Cao, J.; Yang, Y.; Zhang, Y.; Pan, M.; et al. Facile Synthesis of Two-Dimensional Iron/Cobalt Metal–Organic Framework for Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2021, 60, 12097–12102. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Sun, Q.; Wang, Z.; Huang, W.; Guo, X.; Fan, Z.; Ye, R.; Zhu, Y.; Chueh, C.; et al. Freestanding 2D NiFe Metal–Organic Framework Nanosheets: Facilitating Proton Transfer via Organic Ligands for Efficient Oxygen Evolution Reaction. Small 2022, 18, e2201076. [Google Scholar] [CrossRef]
- Park, K.; Kwon, J.; Jo, S.; Choi, S.; Enkhtuvshin, E.; Kim, C.; Lee, D.; Kim, J.; Sun, S.; Han, H.; et al. Simultaneous electrical and defect engineering of nickel iron metal-organic-framework via co-doping of metalloid and non-metal elements for a highly efficient oxygen evolution reaction. Chem. Eng. J. 2022, 439, 135720. [Google Scholar] [CrossRef]
- Srinivas, K.; Lu, Y.; Chen, Y.; Zhang, W.; Yang, D. FeNi3–Fe3O4 Heterogeneous Nanoparticles Anchored on 2D MOF Nanosheets/1D CNT Matrix as Highly Efficient Bifunctional Electrocatalysts for Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 3820–3831. [Google Scholar] [CrossRef]
- Du, Y.-X.; Liu, L.; Li, Y.-K.; Liu, R.; Lu, W.-T.; Wang, J.-X.; Zhang, G.; Cao, F.-F. Fe-CoP/C composite nanoplate derived from 2D porphyrin MOF as an efficient catalyst for oxygen evolution reaction. New J. Chem. 2022, 46, 8271–8276. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Su, H.; Hong, A.N.; Wang, Y.; Yang, H.; Ge, L.; Song, W.; Liu, J.; Ma, T.; et al. Electron Redistributed S-Doped Nickel Iron Phosphides Derived from One-Step Phosphatization of MOFs for Significantly Boosting Electrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2200733. [Google Scholar] [CrossRef]
- Tian, J.; Xu, Y.; Li, J.; Chi, J.; Feng, L.; Pan, Q.; Li, X.; Su, Z. Post-decorated synthesis of metal-organic frameworks derived Ni/Ni3S2@CN electrocatalyst for efficient hydrogen evolution. J. Solid State Chem. 2022, 313, 123287. [Google Scholar] [CrossRef]
- Li, S.; Wang, R.; Xie, M.; Xu, Y.; Chen, J.; Jiao, Y. Construction of trifunctional electrode material based on Pt-Coordinated Ce-Based metal organic framework. J. Colloid Interface Sci. 2022, 622, 378–389. [Google Scholar] [CrossRef]
- Song, J.; Chen, J.-L.; Xu, Z.; Lin, R.Y.-Y. Metal–organic framework-derived 2D layered double hydroxide ultrathin nanosheets for efficient electrocatalytic hydrogen evolution reaction. Chem. Commun. 2022, 58, 10655–10658. [Google Scholar] [CrossRef]
- Liu, P.; Li, J.; Yan, J.; Song, W. Defect-rich Fe-doped NiS/MoS2 heterostructured ultrathin nanosheets for efficient overall water splitting. Phys. Chem. Chem. Phys. 2022, 24, 8344–8350. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, A.; Xue, Z.; Wang, L.; Li, X.; Wang, G. Ultrathin metal–organic framework nanosheet arrays and derived self-supported electrodes for overall water splitting. J. Mater. Chem. A 2021, 9, 22597–22602. [Google Scholar] [CrossRef]
- Liu, H.; Huang, R.; Chen, W.; Zhang, Y.; Wang, M.; Hu, Y.; Zhou, Y.; Song, Y. Porous 2D cobalt–nickel phosphide triangular nanowall architecture assembled by 3D microsphere for enhanced overall water splitting. Appl. Surf. Sci. 2021, 569, 150762. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, K.; Huang, R.; Zhu, Z. Porous Co3O4 stabilized VS2 nanosheets obtained with a MOF template for the efficient HER. CrystEngComm 2021, 23, 5097–5105. [Google Scholar] [CrossRef]
- Ma, N.; Fei, C.; Wang, J.; Wang, Y. Fabrication of NiFe-MOF/cobalt carbonate hydroxide hydrate heterostructure for a high-performance electrocatalyst of oxygen evolution reaction. J. Alloys Compd. 2022, 917, 165511. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, M.; Zhao, L.; Wang, L.; Cao, D.; Gong, Y. Ru doped bimetallic phosphide derived from 2D metal organic framework as active and robust electrocatalyst for water splitting. Appl. Surf. Sci. 2021, 536, 147952. [Google Scholar] [CrossRef]
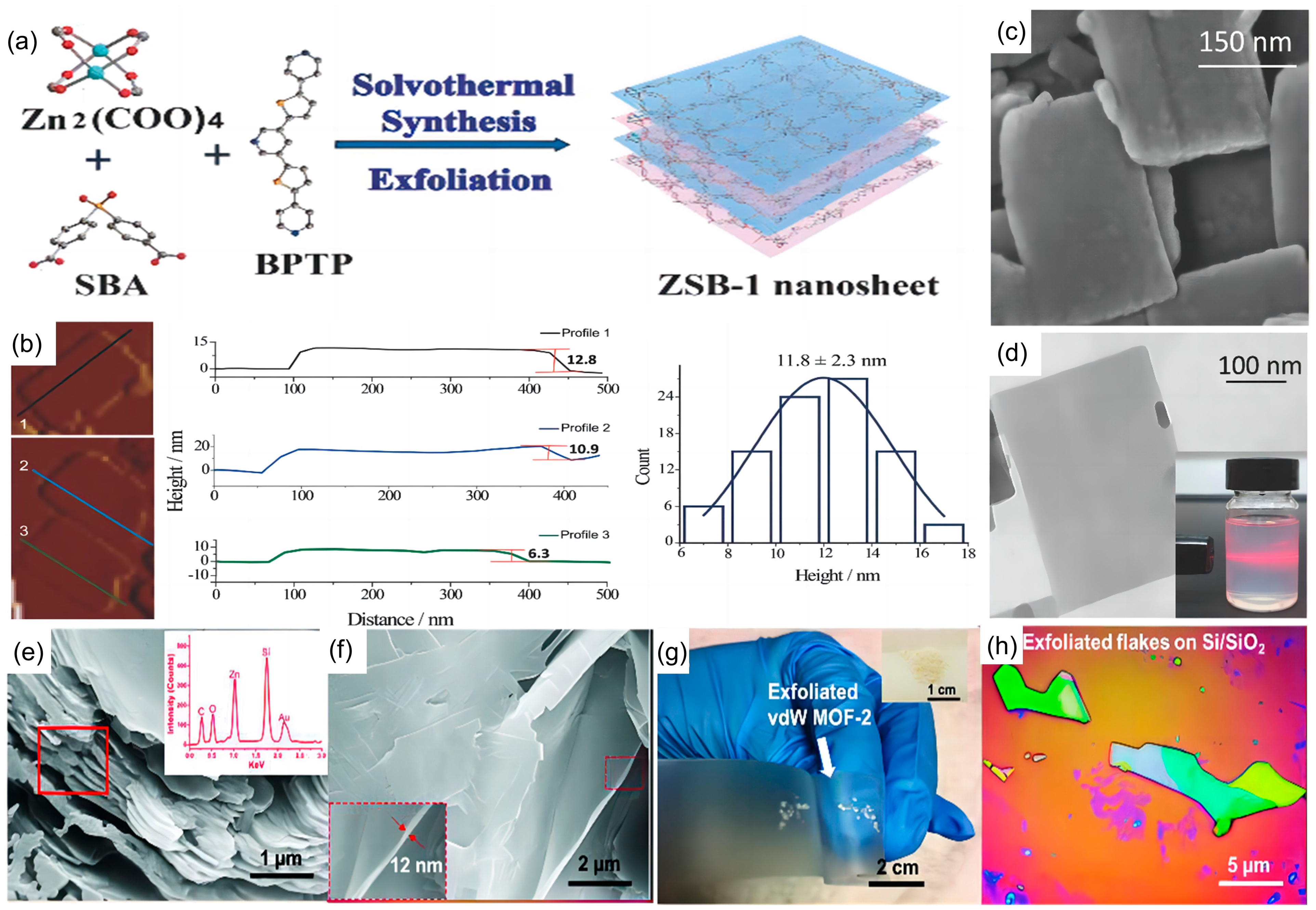

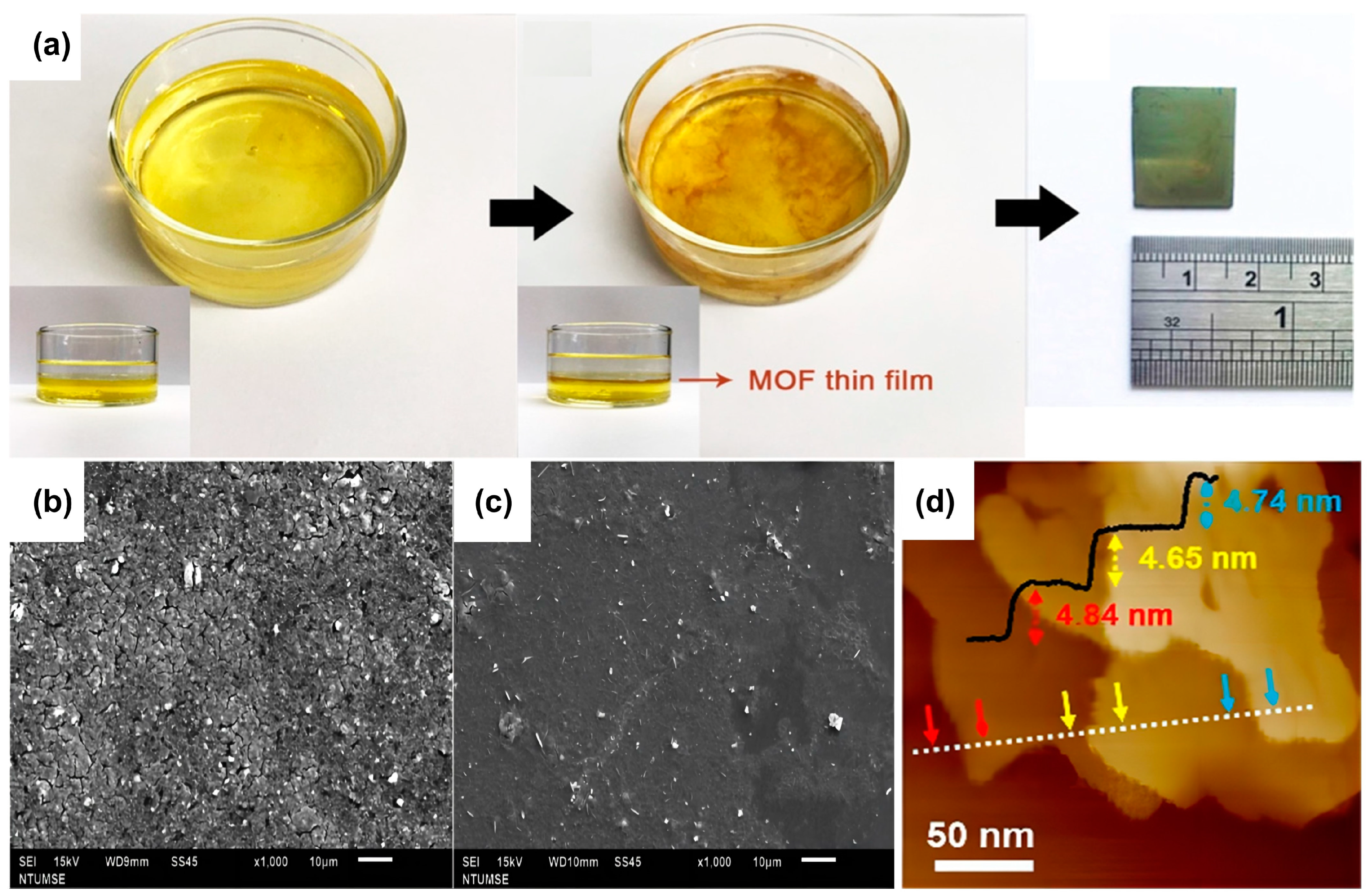
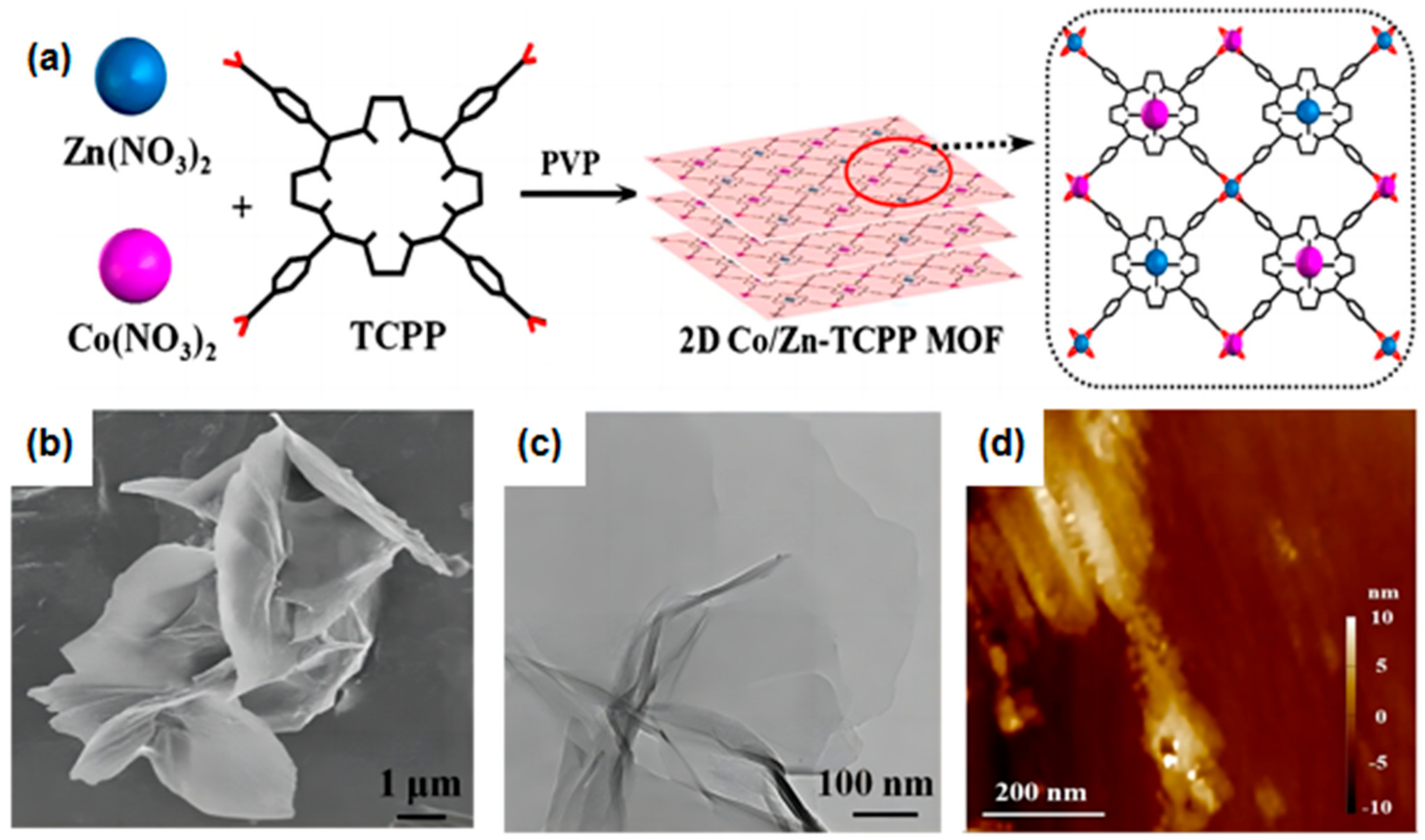
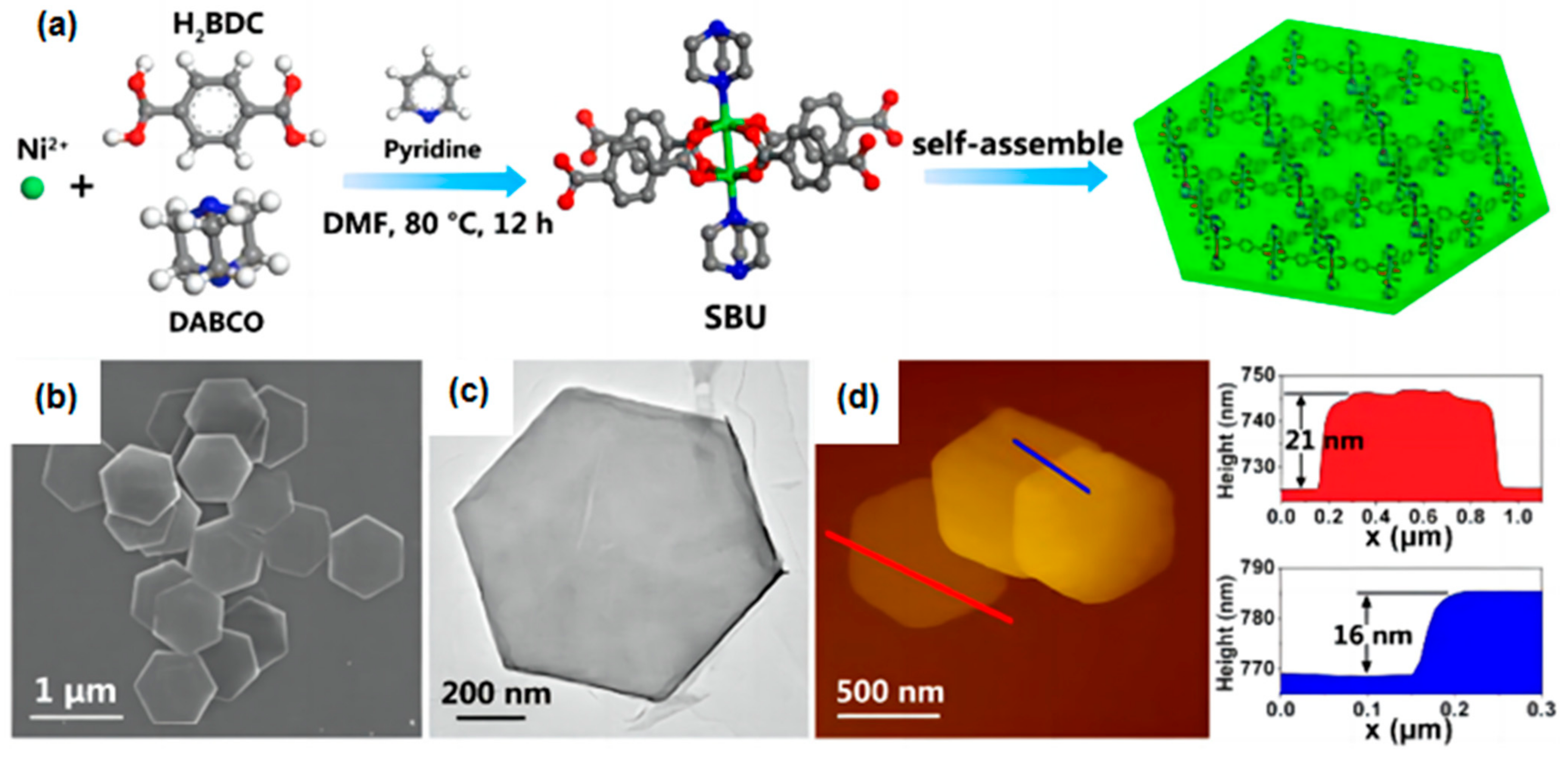
| 2D MOF | Synthesis Method | Synthesis Strategy | Topography | Thickness | Ref. |
|---|---|---|---|---|---|
| [Cu2Br(IN)2]n | Top-down Synthesis | Ultrasonic exfoliation | uniform | 0.5 ± 0.015 nm | [103] |
| ZSB-1 | Top-down Synthesis | Wet sphere milling/ultrasonic | uniform | 11.8 ± 2.3 nm | [104] |
| vdW MOF-2 | Top-down Synthesis | Micro-mechanical peel-off | uneven | 10.0 nm | [107] |
| Zn2(bim)3 | Top-down Synthesis | Wet sphere milling/ultrasonic | uneven | 1.6 nm | [108] |
| Zn2(PdTCPP) | Top-down Synthesis | Intercalation and chemical exfoliation | uneven | 1.0 nm | [109] |
| [Co(Ni-H7TPPP)2]·8H2O | Top-down Synthesis | Chemical exfoliation | uneven | 3.9 ± 0.2 nm | [110] |
| Co6O(dhbdc)2 | Top-down Synthesis | Electrochemical/chemical exfoliation | wrinkled | 2 nm | [111] |
| ZIF-L | Bottom-up Synthesis | Template assisted | uneven | 5.5 µm | [112] |
| Meso-CuBDC | Bottom-up Synthesis | Sacrificial templates | small square shape | 8.0 nm | [113] |
| Ag3BHT2 | Bottom-up Synthesis | liquid–liquid interfacial growth | disordered | 200 nm | [115] |
| Ni-MOF | Bottom-up Synthesis | liquid–liquid interfacial growth | uniform | 5 nm | [116] |
| CuBDC | Bottom-up Synthesis | Interfacial synthesis | uniform | 5–25 nm | [117] |
| Zn-TCPP | Bottom-up Synthesis | Surface-active agent-assisted | uniform | 7.6 ± 2.6 nm | [118] |
| Co/Zn-porphyrin | Bottom-up Synthesis | Surfactant-assisted | wrinkled | 4–5 nm | [119] |
| HXP | Bottom-up Synthesis | Molecular modulation assisted | uniform | 20 nm | [123] |
| [Cu(2,5-pydc)(H2O)]n·2H2O | Bottom-up Synthesis | Ultrasonic exfoliation | uniform | 126 nm | [132] |
| Ni3S2@2D Co-MOF | Bottom-up Synthesis | Self-sacrificial template | rough | 60 nm | [133] |
| NiCoSe | Bottom-up Synthesis | ion-exchange | uniform | 230 nm | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Qian, Y.; Lyu, Z.; Kim, D.-H.; Kang, D.J. Two-Dimensional Metal–Organic Frameworks and Their Derivative Electrocatalysts for Water Splitting. Appl. Sci. 2023, 13, 9343. https://doi.org/10.3390/app13169343
Shen L, Qian Y, Lyu Z, Kim D-H, Kang DJ. Two-Dimensional Metal–Organic Frameworks and Their Derivative Electrocatalysts for Water Splitting. Applied Sciences. 2023; 13(16):9343. https://doi.org/10.3390/app13169343
Chicago/Turabian StyleShen, Lin, Yongteng Qian, Zhiyi Lyu, Dong-Hwan Kim, and Dae Joon Kang. 2023. "Two-Dimensional Metal–Organic Frameworks and Their Derivative Electrocatalysts for Water Splitting" Applied Sciences 13, no. 16: 9343. https://doi.org/10.3390/app13169343







