Fabrication of Spiny-like Spherical Copper Metal–Organic Frameworks for the Microextraction of Arsenic(III) from Water and Food Samples before ICP-MS Detection
Abstract
:1. Introduction
2. Experiment
2.1. Chemicals and Instruments
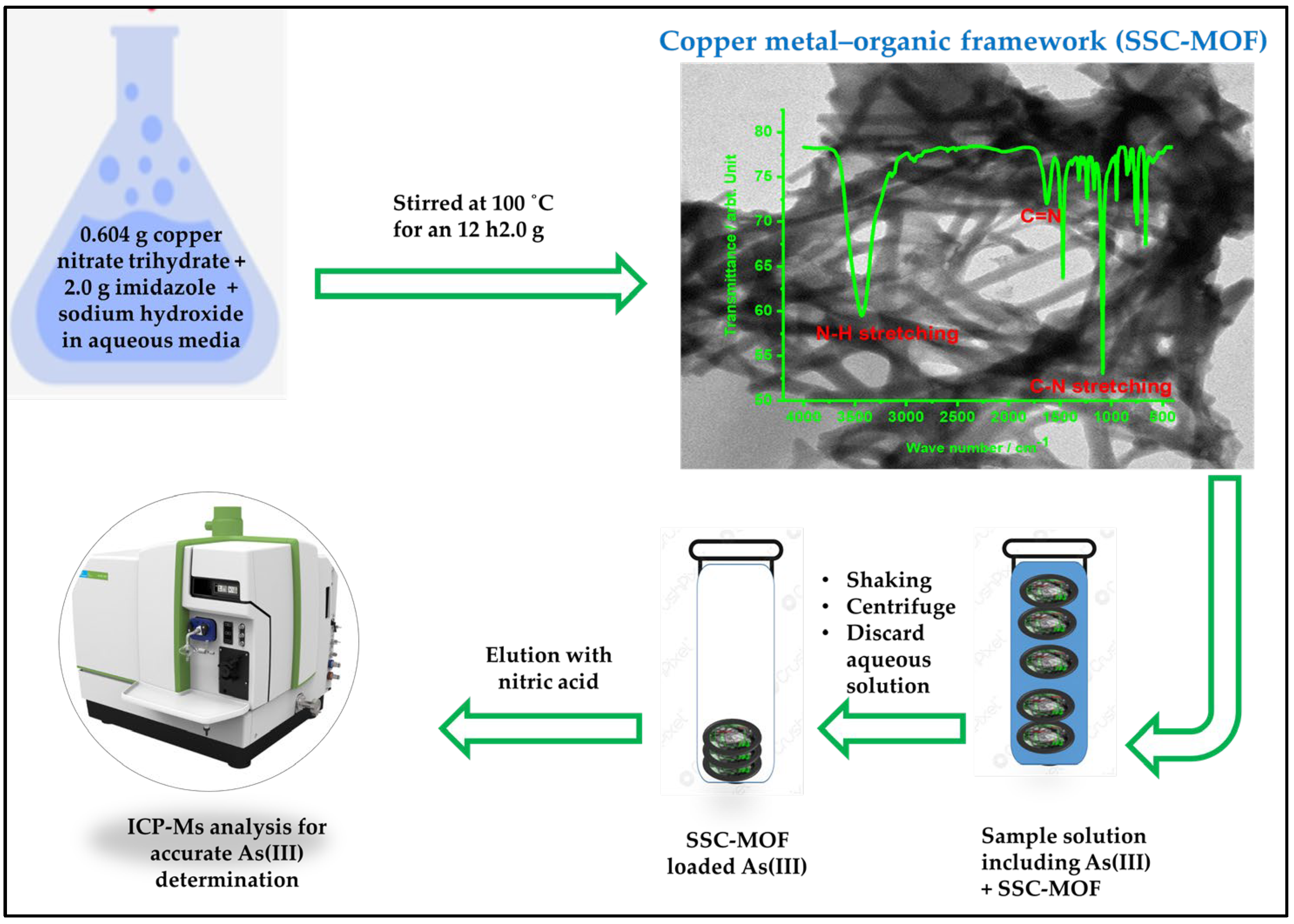
2.2. The Preparation of Spiny-like Spherical Copper Metal–Organic Frameworks
2.3. The Development of Solid-Phase Microextraction for Trace Arsenic(III) Preconcentration
2.4. Analyses of Real Water and Food Samples
3. Results and Discussion
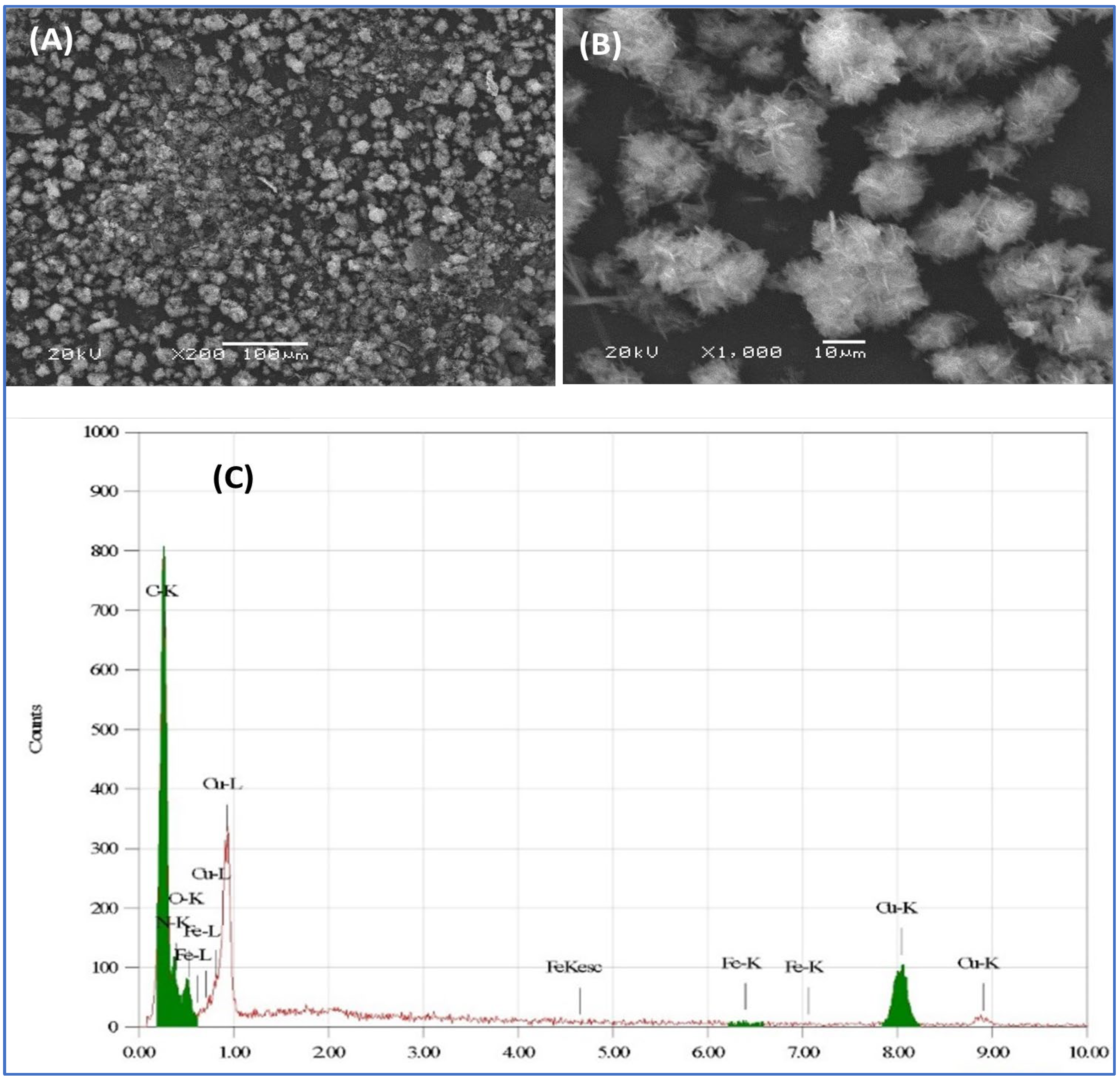
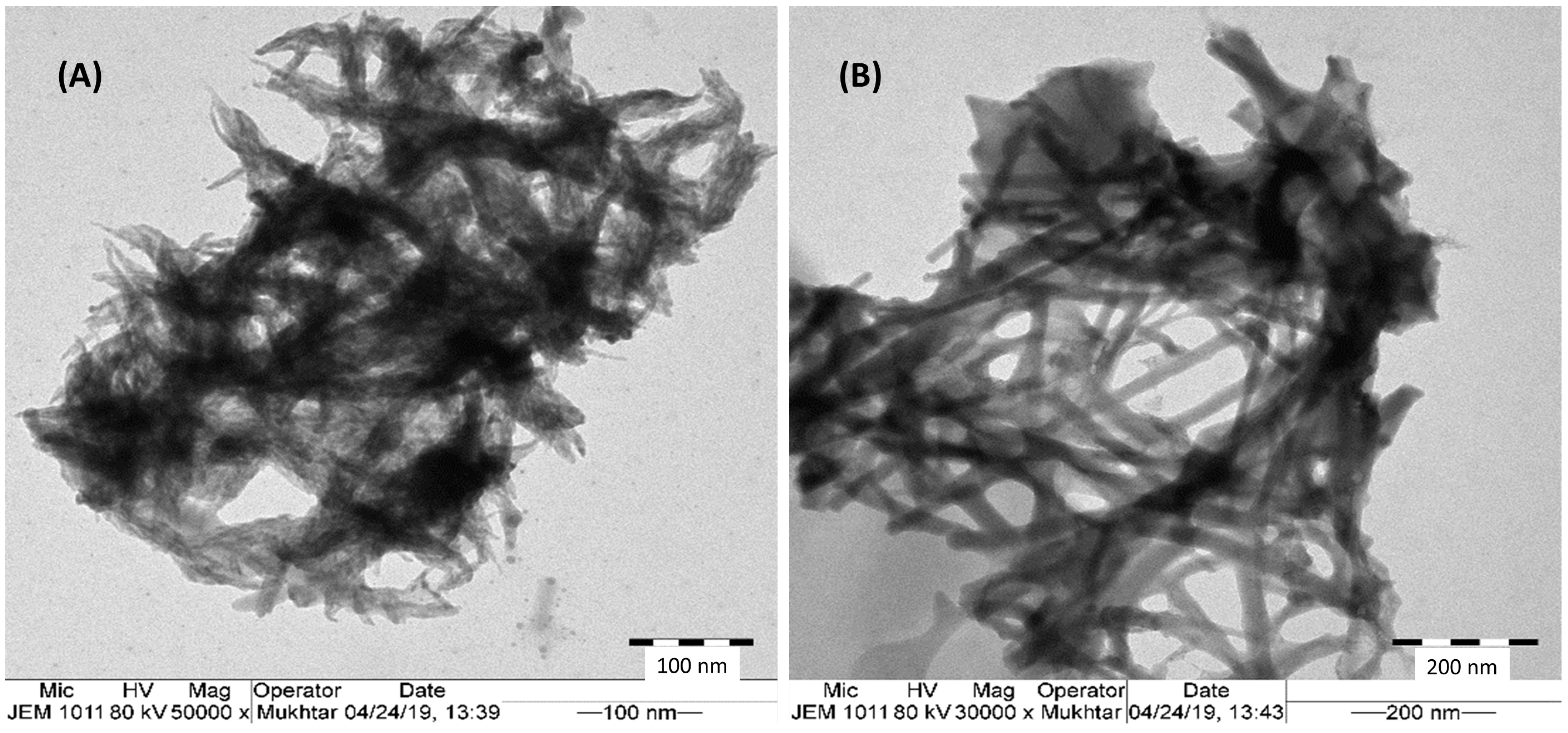
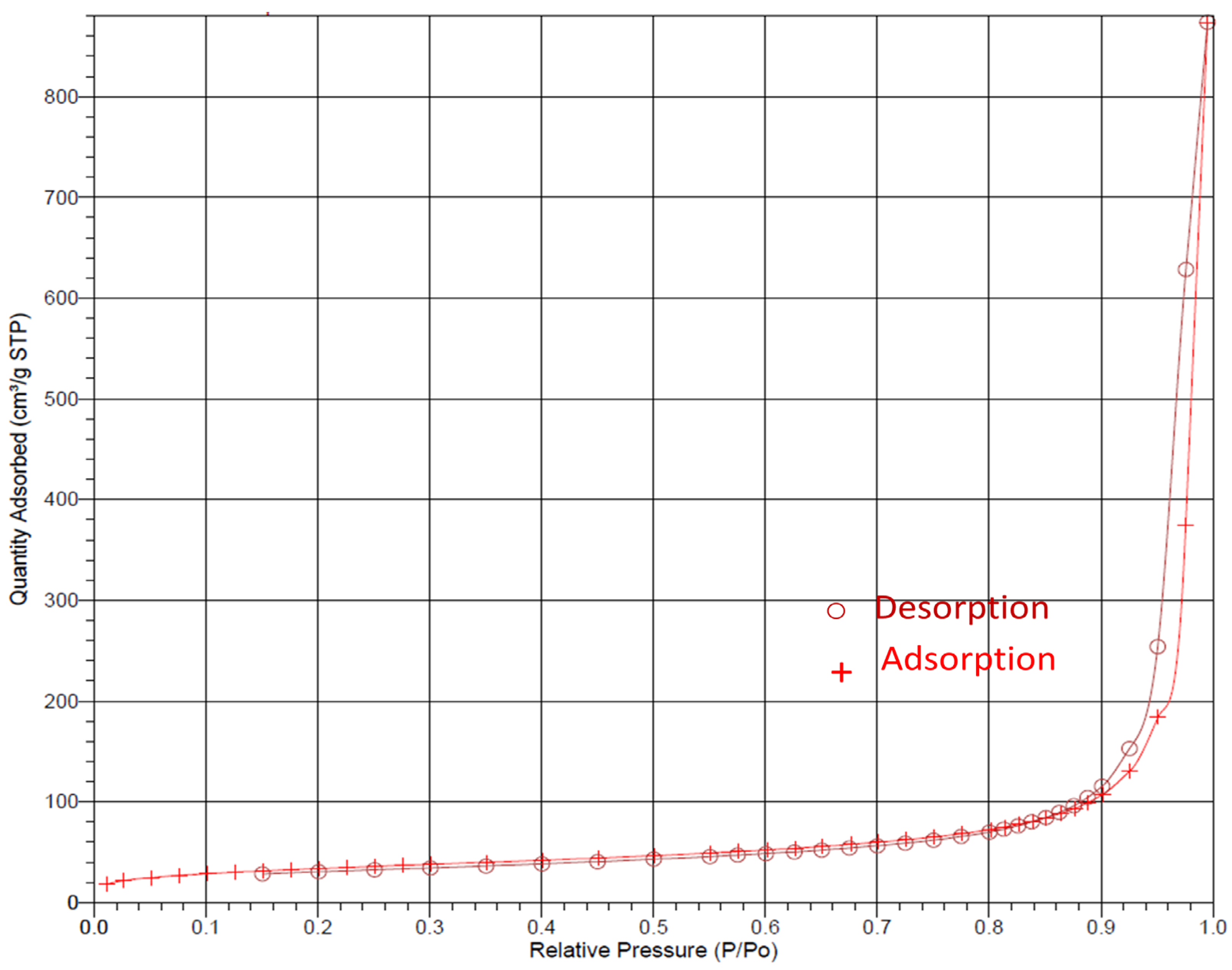
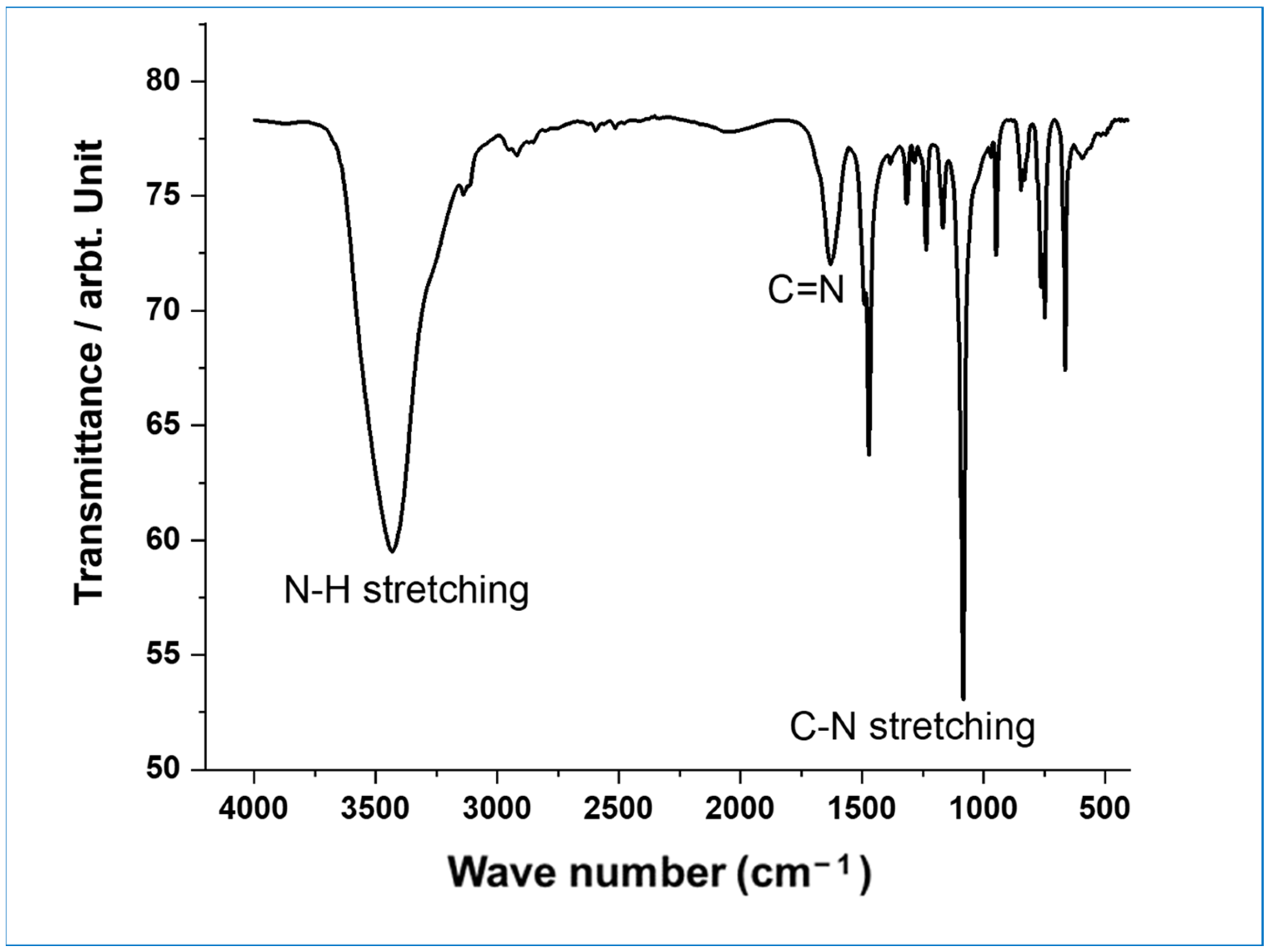
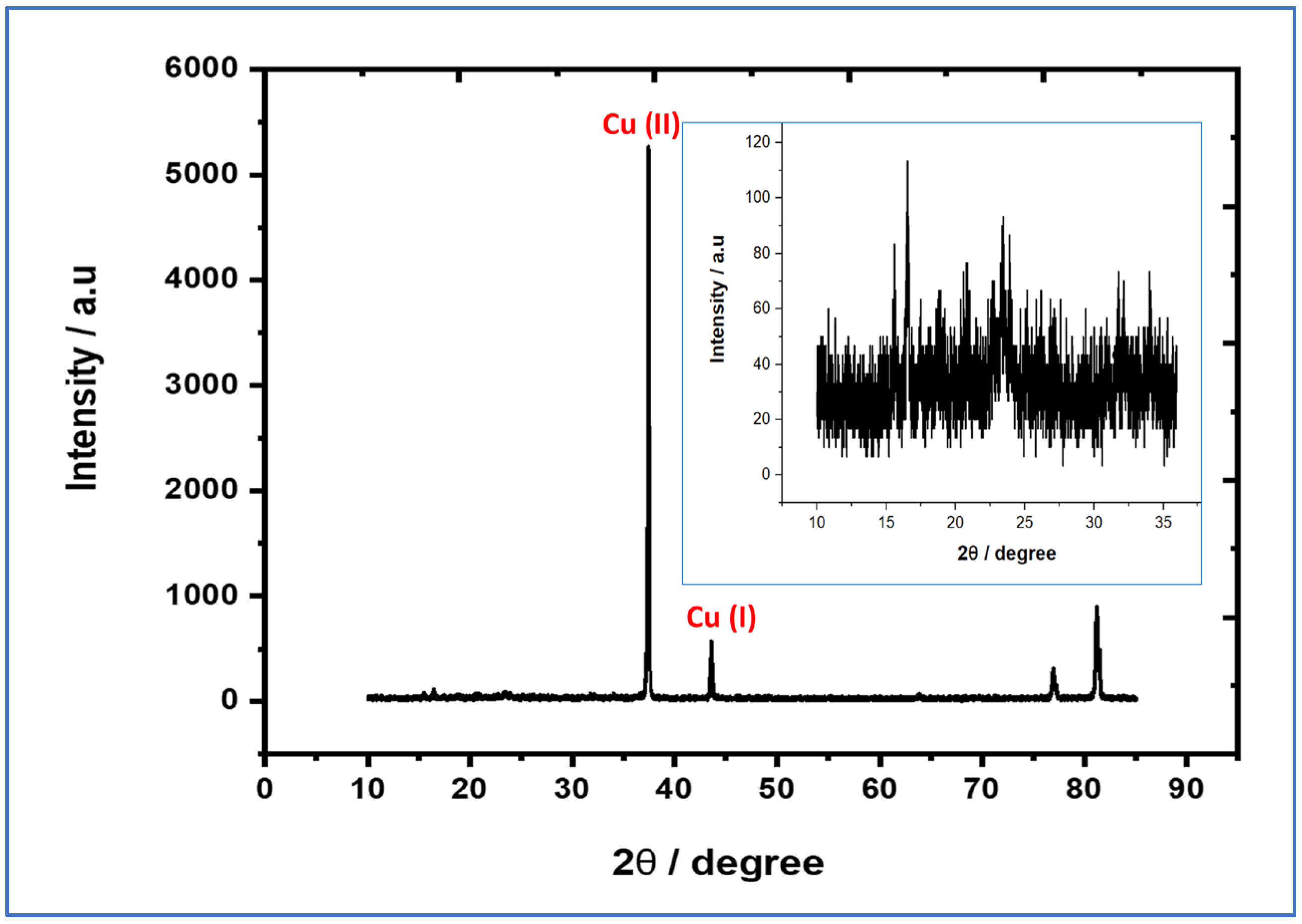
3.1. Morphological and Structural Investigations
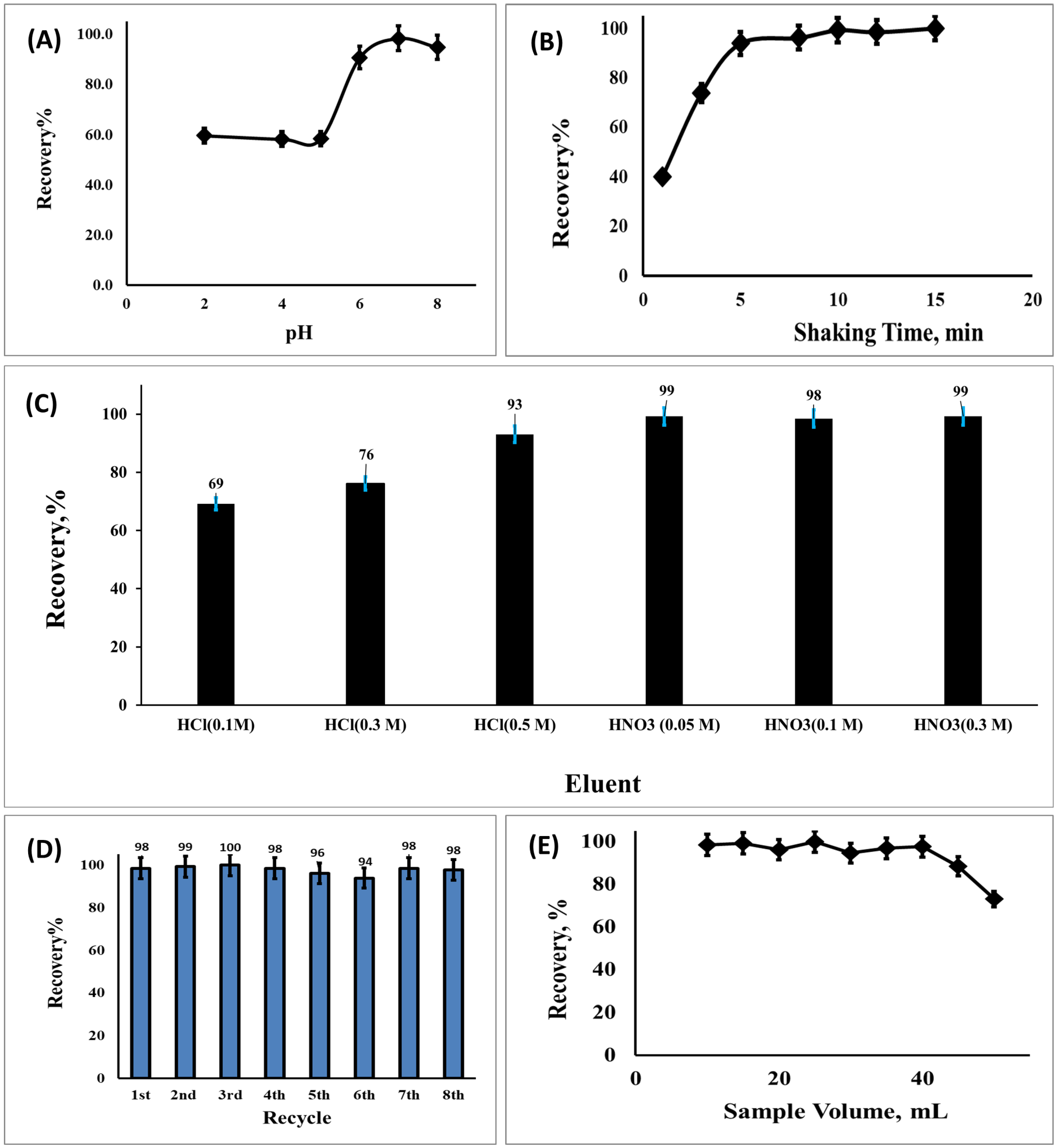
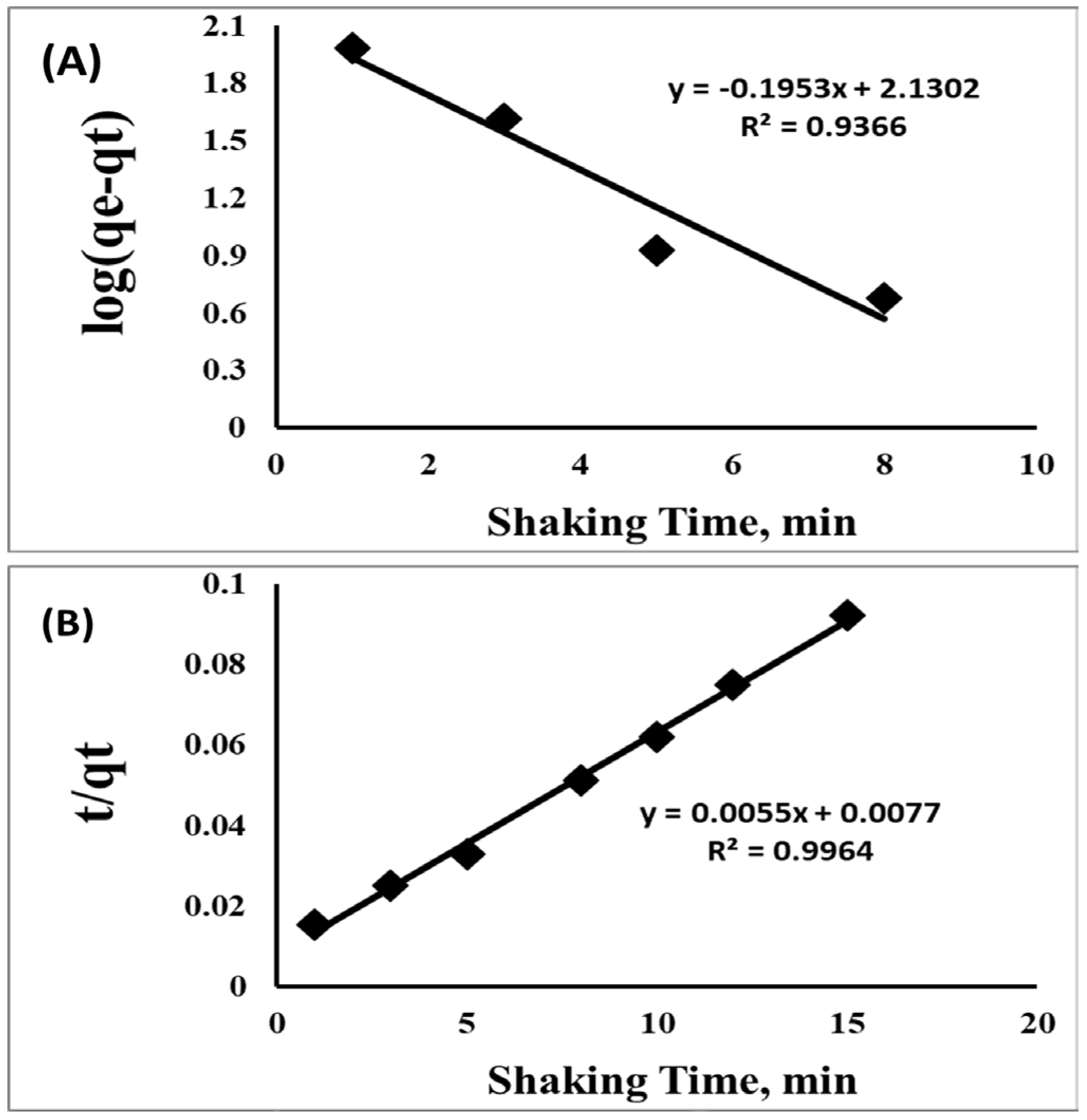
3.2. Optimizing the Developed Solid-Phase Microextraction onto SSC-MOFs
3.3. Interference Investigations and Analytical Merits for the Application of the Fabricated SSC-MOFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, B.A.; Cassano, V.A.; Murray, C. Arsenic Exposure, Assessment, Toxicity, Diagnosis, and Management. J. Occup. Environ. Med. 2018, 60, e634–e639. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Poynton, C.Y.; Kochian, L.V.; Elless, M.P. Phytofiltration of Arsenic from Drinking Water Using Arsenic- Hyperaccumulating Ferns. Environ. Sci. Technol. 2004, 38, 3412–3417. [Google Scholar] [CrossRef]
- Chen, C.; Wang, G.; Li, M.; Chen, X.; Chen, D.; Feng, Z.; Jiang, Y.; Yu, H.; Chao, Y.; Tang, Y.; et al. Mechanism of Arsenic Immobilization and Biotransformation in the Biological Aqua Crust of Mine Drainage. Sci. Total Environ. 2023, 892, 164230. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. A Review on Sources, Identification and Treatment Strategies for the Removal of Toxic Arsenic from Water System. J. Hazard. Mater. 2021, 418, 126299. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Wai, C.M. Arsenic in Drinking Water—A Global Environmental Problem. J. Chem. Educ. 2004, 81, 207–213. [Google Scholar] [CrossRef]
- Gulens, J.; Champ, D.R.; Jackson, R.E. Influence of Redox Environments on the Mobility of Arsenic in Ground Water. Hydrol. Geochem. Stud. Perch Lake Basin 1979, 25, 81–95. [Google Scholar]
- Rahidul Hassan, H. A Review on Different Arsenic Removal Techniques Used for Decontamination of Drinking Water. Environ. Pollut. Bioavailab. 2023, 35, 2165964. [Google Scholar] [CrossRef]
- Hamza, M.; Alam, S.; Rizwan, M.; Naz, A. Health Risks Associated with Arsenic Contamination and Its Biotransformation Mechanisms in Environment: A Review; Springer: Cham, Switzerland, 2022; pp. 241–288. [Google Scholar] [CrossRef]
- Hettick, B.E.; Cañas-Carrell, J.E.; French, A.D.; Klein, D.M. Arsenic: A Review of the Element’s Toxicity, Plant Interactions, and Potential Methods of Remediation. J. Agric. Food Chem. 2015, 63, 7097–7107. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Saha, D.; Saha, R.; Ghosh, T.; Saha, B. A Review on Sources, Toxicity and Remediation Technologies for Removing Arsenic from Drinking Water. Res. Chem. Intermed. 2014, 40, 447–485. [Google Scholar] [CrossRef]
- Singh, K.P.; Kumari, R.; Treas, J.; Dumond, J.W. Chronic Exposure to Arsenic Causes Increased Cell Survival, DNA Damage, and Increased Expression of Mitochondrial Transcription Factor A (MtTFA) in Human Prostate Epithelial Cells. Chem. Res. Toxicol. 2011, 24, 340–349. [Google Scholar] [CrossRef]
- Hong, Y.S.; Song, K.H.; Chung, J.Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public. Health 2014, 47, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.R.; Allan, A.M. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr. Environ. Health Rep. 2014, 1, 132–147. [Google Scholar] [CrossRef]
- Clancy, T.M.; Hayes, K.F.; Raskin, L. Arsenic Waste Management: A Critical Review of Testing and Disposal of Arsenic-Bearing Solid Wastes Generated during Arsenic Removal from Drinking Water. Environ. Sci. Technol. 2013, 47, 10799–10812. [Google Scholar] [CrossRef]
- Lewchalermvong, K.; Rangkadilok, N.; Nookabkaew, S.; Suriyo, T.; Satayavivad, J. Arsenic Speciation and Accumulation in Selected Organs after Oral Administration of Rice Extracts in Wistar Rats. J. Agric. Food Chem. 2018, 66, 3199–3209. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Mitra, K.; Sarkhel, S.; Hobbes, M.; Burló, F.; De Groot, W.T.; Carbonell-Barrachina, A.A. Arsenic Speciation in Food and Estimation of the Dietary Intake of Inorganic Arsenic in a Rural Village of West Bengal, India. J. Agric. Food Chem. 2008, 56, 9469–9474. [Google Scholar] [CrossRef]
- Ahmad, H.; Zhao, L.; Liu, C.; Cai, C.; Ma, F. Ultrasound Assisted Dispersive Solid Phase Microextraction of Inorganic Arsenic from Food and Water Samples Using CdS Nanoflowers Combined with ICP-OES Determination. Food Chem. 2021, 338, 128028. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, B.; He, M.; Hu, B. Magnetic Metal-Organic Framework Composites for Dual-Column Solid-Phase Microextraction Combined with ICP-MS for Speciation of Trace Levels of Arsenic. Microchim. Acta 2020, 187, 48. [Google Scholar] [CrossRef]
- Reid, M.S.; Hoy, K.S.; Schofield, J.R.M.; Uppal, J.S.; Lin, Y.; Lu, X.; Peng, H.; Le, X.C. Arsenic Speciation Analysis: A Review with an Emphasis on Chromatographic Separations. TrAC—Trends Anal. Chem. 2020, 123, 115770. [Google Scholar] [CrossRef]
- Borahan, T.; Unutkan, T.; Turan, N.B.; Turak, F.; Bakırdere, S. Determination of Lead in Milk Samples Using Vortex Assisted Deep Eutectic Solvent Based Liquid Phase Microextraction-Slotted Quartz Tube-Flame Atomic Absorption Spectrometry System. Food Chem. 2019, 299, 125065. [Google Scholar] [CrossRef]
- Jalili, V.; Barkhordari, A.; Norouzian Baghani, A. The Role of Microextraction Techniques in Occupational Exposure Assessment. A Review. Microchem. J. 2019, 150, 104086. [Google Scholar] [CrossRef]
- Najafi, A.; Hashemi, M. Feasibility of Liquid Phase Microextraction Based on a New Supramolecular Solvent for Spectrophotometric Determination of Orthophosphate Using Response Surface Methodology Optimization. J. Mol. Liq. 2020, 297, 111768. [Google Scholar] [CrossRef]
- Fiorentini, E.F.; Canizo, B.V.; Wuilloud, R.G. Determination of As in Honey Samples by Magnetic Ionic Liquid-Based Dispersive Liquid-Liquid Microextraction and Electrothermal Atomic Absorption Spectrometry. Talanta 2019, 198, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. New Extraction Media in Microextraction Techniques. A Review of Reviews. Microchem. J. 2020, 153, 104386. [Google Scholar] [CrossRef]
- dos Santos, Q.O.; Silva Junior, M.M.; Lemos, V.A.; Ferreira, S.L.C.; de Andrade, J.B. An Online Preconcentration System for Speciation Analysis of Arsenic in Seawater by Hydride Generation Flame Atomic Absorption Spectrometry. Microchem. J. 2018, 143, 175–180. [Google Scholar] [CrossRef]
- Wan, X.; Dong, H.; Feng, L.; Lin, Z.; Luo, Q. Comparison of Three Sequential Extraction Procedures for Arsenic Fractionation in Highly Polluted Sites. Chemosphere 2017, 178, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, S.L.; Erickson, M.L.; Woodruff, L.G.; Knaeble, A.R.; Marcus, M.A.; Lynch, J.K.; Toner, B.M. Solid-Phase Arsenic Speciation in Aquifer Sediments: A Micro-X-Ray Absorption Spectroscopy Approach for Quantifying Trace-Level Speciation. Geochim. Cosmochim. Acta 2017, 211, 228–255. [Google Scholar] [CrossRef]
- López-García, I.; Marín-Hernández, J.J.; Hernández-Córdoba, M. Magnetic Ferrite Particles Combined with Electrothermal Atomic Absorption Spectrometry for the Speciation of Low Concentrations of Arsenic. Talanta 2018, 181, 6–12. [Google Scholar] [CrossRef]
- Lukojko, E.; Talik, E.; Gagor, A.; Sitko, R. Highly Selective Determination of Ultratrace Inorganic Arsenic Species Using Novel Functionalized Miniaturized Membranes. Anal. Chim. Acta 2018, 1008, 57–65. [Google Scholar] [CrossRef]
- Anuradha Jabasingh, S.; Ravi, T.; Abubeker, Y. Magnetic Hetero-Structures as Prospective Sorbents to Aid Arsenic Elimination from Life Water Streams. Water Sci. 2018, 32, 151–170. [Google Scholar] [CrossRef]
- Wen, S.; Zhu, X. Speciation of Inorganic Arsenic(III) and Arsenic(V) by a Facile Dual-Cloud Point Extraction Coupled with Inductively Plasma-Optical Emission Spectrometry. Talanta 2018, 181, 265–270. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Villar-Blanco, L.; Lavilla, I.; Bendicho, C. Test for Arsenic Speciation in Waters Based on a Paper-Based Analytical Device with Scanometric Detection. Anal. Chim. Acta 2018, 1011, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Munonde, T.S.; Maxakato, N.W.; Nomngongo, P.N. Preparation of Magnetic Fe3O4 Nanocomposites Modified with MnO2, Al2O3, Au and Their Application for Preconcentration of Arsenic in River Water Samples. J. Environ. Chem. Eng. 2018, 6, 1673–1681. [Google Scholar] [CrossRef]
- Lee, H.G.; Kwon, J.Y.; Chung, D.S. Sensitive Arsenic Speciation by Capillary Electrophoresis Using UV Absorbance Detection with On-Line Sample Preconcentration Techniques. Talanta 2018, 181, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Baranik, A.; Gagor, A.; Queralt, I.; Marguí, E.; Sitko, R.; Zawisza, B. Determination and Speciation of Ultratrace Arsenic and Chromium Species Using Aluminium Oxide Supported on Graphene Oxide. Talanta 2018, 185, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ren, T.; Lin, Y.; Zheng, C. Application of Solid-Phase Microextraction in Atomic Spectrometry. Adv. Sample Prep. 2022, 3, 100033. [Google Scholar] [CrossRef]
- Meira, L.A.; Almeida, J.S.; Oliveira, M.S.; Dias, F.D.S.; Queiroz, A.F.S.; Soares, S.A.R.; Teixeira, L.S.G. Biodiesel Trace Element Analysis by Energy Dispersive X-Ray Fluorescence Spectrometry Using Magnetic Solid-Phase Microextraction. Energy Fuels 2021, 35, 510–518. [Google Scholar] [CrossRef]
- López-García, I.; Marín-Hernández, J.J.; Hernández-Córdoba, M. Solid-Phase Dispersive Microextraction Using Reduced Graphene Oxide for the Sensitive Determination of Cadmium and Lead in Waters. Anal. Methods 2019, 11, 635–641. [Google Scholar] [CrossRef]
- Saqaf Jagirani, M.; Soylak, M. A Review: Recent Advances in Solid Phase Microextraction of Toxic Pollutants Using Nanotechnology Scenario. Microchem. J. 2020, 159, 105436. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; Hakami, H.M.; Alanazi, A.G. Influence of Synthesis-Heating Conditions on the Porosity and Performance of a Carbon Nanotube/SDS-Alumina Nanocomposite for Effective Wastewater Treatment: Fabrication, Characterization, and Adsorption Modeling. Ind. Eng. Chem. Res. 2023, 62, 12571–12588. [Google Scholar] [CrossRef]
- Rani, L.; Kaushal, J.; Srivastav, A.L.; Mahajan, P. A Critical Review on Recent Developments in MOF Adsorbents for the Elimination of Toxic Heavy Metals from Aqueous Solutions. Environ. Sci. Pollut. Res. 2020, 27, 44771–44796. [Google Scholar] [CrossRef]
- Luebbers, M.T.; Wu, T.; Shen, L.; Masel, R.I. Trends in the Adsorption of Volatile Organic Compounds in a Large-Pore Metal-Organic Framework, IRMOF-1. Langmuir 2010, 26, 11319–11329. [Google Scholar] [CrossRef]
- Zhang, S.; Du, Z.; Li, G. Metal-Organic Framework-199/Graphite Oxide Hybrid Composites Coated Solid-Phase Microextraction Fibers Coupled with Gas Chromatography for Determination of Organochlorine Pesticides from Complicated Samples. Talanta 2013, 115, 32–39. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Wang, W.; Wang, C.; Wang, Z. Covalent Bonding of Metal-Organic Framework-5/Graphene Oxide Hybrid Composite to Stainless Steel Fiber for Solid-Phase Microextraction of Triazole Fungicides from Fruit and Vegetable Samples. J. Agric. Food Chem. 2016, 64, 2792–2801. [Google Scholar] [CrossRef]
- Wang, X.; Ye, N. Recent Advances in Metal-Organic Frameworks and Covalent Organic Frameworks for Sample Preparation and Chromatographic Analysis. Electrophoresis 2017, 38, 3059–3078. [Google Scholar] [CrossRef] [PubMed]
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, Separation, and Catalytic Properties of Densified Metal-Organic Frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef]
- Ma, M.; Lu, X.; Guo, Y.; Wang, L.; Liang, X. Combination of Metal-Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs): Recent Advances in Synthesis and Analytical Applications of MOF/COF Composites. TrAC Trends Anal. Chem. 2022, 157, 116741. [Google Scholar] [CrossRef]
- Miao, Q.; Jiang, L.; Yang, J.; Hu, T.; Shan, S.; Su, H.; Wu, F. MOF/Hydrogel Composite-Based Adsorbents for Water Treatment: A Review. J. Water Process Eng. 2022, 50, 103348. [Google Scholar] [CrossRef]
- Cui, S.; Qin, M.; Marandi, A.; Steggles, V.; Wang, S.; Feng, X.; Nouar, F.; Serre, C. Metal-Organic Frameworks as Advanced Moisture Sorbents for Energy-Efficient High Temperature Cooling. Sci. Rep. 2018, 8, 15284. [Google Scholar] [CrossRef]
- Fakhraei Ghazvini, M.; Vahedi, M.; Najafi Nobar, S.; Sabouri, F. Investigation of the MOF Adsorbents and the Gas Adsorptive Separation Mechanisms. J. Environ. Chem. Eng. 2021, 9, 104790. [Google Scholar] [CrossRef]
- Wu, X.M.; Liu, L.X.; Liu, L.; You, Z.H.; Guo, H.X.; Chen, Z.X. Ultrasound Assisted Synthesis of Nanoscale NH2-MIL-53(Fe) Forthe Adsorption of Dye. Jiegou Huaxue 2021, 40, 42–46. [Google Scholar] [CrossRef]
- Samadi Kazemi, M.; Sobhani, A. CuMn2O4/Chitosan Micro/Nanocomposite: Green Synthesis, Methylene Blue Removal, and Study of Kinetic Adsorption, Adsorption Isotherm Experiments, Mechanism and Adsorbent Capacity. Arab. J. Chem. 2023, 16, 104754. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A.; Abdi, J.; Hayati, B.; Shekarchi, A.A. Bio-Based Magnetic Metal-Organic Framework Nanocomposite: Ultrasound-Assisted Synthesis and Pollutant (Heavy Metal and Dye) Removal from Aqueous Media. Appl. Surf. Sci. 2019, 480, 288–299. [Google Scholar] [CrossRef]
- Levasseur, B.; Petit, C.; Bandosz, T.J. Reactive Adsorption of NO2 on Copper-Based Metal-Organic Framework and Graphite Oxide/Metal-Organic Framework Composites. ACS Appl. Mater. Interfaces 2010, 2, 3606–3613. [Google Scholar] [CrossRef]
- Kondo, A.; Yashiro, T.; Okada, N.; Hiraide, S.; Ohkubo, T.; Tanaka, H.; Maeda, K. Selective Molecular-Gating Adsorption in a Novel Copper-Based Metal–Organic Framework. J. Mater. Chem. A Mater. 2018, 6, 5910–5918. [Google Scholar] [CrossRef]
- Srivastava, S.; Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. Molecular Simulation of Copper Based Metal-Organic Framework (Cu-MOF) for Hydrogen Adsorption. Int. J. Hydrog. Energy 2022, 47, 15820–15831. [Google Scholar] [CrossRef]
- Chiadmi, F.; Schlatter, J. Determination and Validation of a Solid-Phase Extraction Gas Chromatography-Mass Spectrometry for the Quantification of Methadone and Its Principal Metabolite in Human Plasma. Anal. Chem. Insights 2015, 10, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Islas, G.; Ibarra, I.S.; Hernandez, P.; Miranda, J.M.; Cepeda, A. Dispersive Solid Phase Extraction for the Analysis of Veterinary Drugs Applied to Food Samples: A Review. Int. J. Anal. Chem. 2017, 2017, 8215271. [Google Scholar] [CrossRef] [PubMed]
- Tuzen, M.; Soylak, M.; Citak, D.; Ferreira, H.S.; Korn, M.G.A.; Bezerra, M.A. A Preconcentration System for Determination of Copper and Nickel in Water and Food Samples Employing Flame Atomic Absorption Spectrometry. J. Hazard. Mater. 2009, 162, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.F.; Huang, Z.Q.; Sun, X.Y.; Zhang, X.Y.; Zhao, S.M.; Zhao, Y.; Wang, Z.L.; Sun, W.Y. Cd(II) Frameworks with Tetracarboxylate and Imidazole-Containing Ligands: Syntheses, Structures, Adsorption and Sensing Properties. J. Solid. State Chem. 2022, 312, 123243. [Google Scholar] [CrossRef]
- Xin, X.; Jing, Y.; Li, P.; Zhang, X.; Li, J.; Li, L.; Zhang, L. Imidazole-Based Metal-Organic Framework for the “On1-Off-On2” Fluorescence-Based Detection of Picric Acid and the Adsorption of Congo Red. Cryst. Growth Des. 2023, 23, 3988–3995. [Google Scholar] [CrossRef]
- Baziar, M.; Zakeri, H.R.; Ghalehaskar, S.; Nejad, Z.D.; Shams, M.; Anastopoulos, I.; Giannakoudakis, D.A.; Lima, E.C. Metal-Organic and Zeolitic Imidazole Frameworks as Cationic Dye Adsorbents: Physicochemical Optimizations by Parametric Modeling and Kinetic Studies. J. Mol. Liq. 2021, 332, 115832. [Google Scholar] [CrossRef]
- Mohamed El-Toni, A.; Habila, M.A.; Abbas Ibrahim, M.; Puzon Labis, J.; ALOthman, Z.A. Simple and Facile Synthesis of Amino Functionalized Hollow Core—Mesoporous Shell Silica Spheres Using Anionic Surfactant for Pb(II), Cd(II), and Zn(II) Adsorption and Recovery. Chem. Eng. J. 2014, 251, 441–451. [Google Scholar] [CrossRef]
- Alomar, T.S.; Habila, M.A.; Almasoud, N.; Alothman, Z.A.; Sheikh, M.; Soylak, M. Biomass-Derived Adsorbent for Dispersive Solid-Phase Extraction of Cr(III), Fe(III), Co(II) and Ni(II) from Food Samples Prior to ICP-MS Detection. Appl. Sci. 2021, 11, 7792. [Google Scholar] [CrossRef]
- Hou, J.; Hao, J.; Wang, Y.; Liu, J. Synthesis of CuII/ZIF-8 Metal-Organic Framework Catalyst and Its Application in the Aerobic Oxidation of Alcohols. Chem. Res. Chin. Univ. 2019, 35, 860–865. [Google Scholar] [CrossRef]
- Charoensuk, S.; Tan, J.; Sain, M.; Manuspiya, H. A Single Crystal Hybrid Ligand Framework of Copper(Ii) with Stable Intrinsic Blue-Light Luminescence in Aqueous Solution. Nanomaterials 2021, 11, 2281. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Esfanidiary, N.; Yliniemi, J. Porous SB-Cu1 Two-Dimensional Metal-Organic Framework: The Green Catalyst towards CN Bond-Forming Reactions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128202. [Google Scholar] [CrossRef]
- Li, Z.H.; Xue, L.P.; Wang, L.; Zhang, S.T.; Zhao, B.T. Two-Dimensional Copper-Based Metal–Organic Framework as a Robust Heterogeneous Catalyst for the N-Arylation of Imidazole with Arylboronic Acids. Inorg. Chem. Commun. 2013, 27, 119–121. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A.; Samanidou, V.F. Applications of Metal-Organic Frameworks in Food Sample Preparation. Molecules 2018, 23, 2896. [Google Scholar] [CrossRef]
- Li, D.; He, M.; Chen, B.; Hu, B. Metal Organic Frameworks-Derived Magnetic Nanoporous Carbon for Preconcentration of Organophosphorus Pesticides from Fruit Samples Followed by Gas Chromatography-Flame Photometric Detection. J. Chromatogr. A 2019, 1583, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, H.; Liu, D.; Xiao, J.; Qian, Y.; Xi, H. Effective Ligand Functionalization of Zirconium-Based Metal-Organic Frameworks for the Adsorption and Separation of Benzene and Toluene: A Multiscale Computational Study. ACS Appl. Mater. Interfaces 2015, 7, 5775–5787. [Google Scholar] [CrossRef]
- Habila, M.; Alhenaki, B.; El-Marghany, A.; Sheikh, M.; Ghfar, A.; ALOthman, Z.; Soylak, M. Metal Organic Frameworks Enhanced Dispersive Solid Phase Microextraction of Malathion before Detection by UHPLC-MS/MS. J. Sep. Sci. 2020, 43, 3103–3109. [Google Scholar] [CrossRef]
- Moradi, S.E.; Shabani, A.M.H.; Dadfarnia, S.; Emami, S. Sulfonated Metal Organic Framework Loaded on Iron Oxide Nanoparticles as a New Sorbent for the Magnetic Solid Phase Extraction of Cadmium from Environmental Water Samples. Anal. Methods 2016, 8, 6337–6346. [Google Scholar] [CrossRef]
- Habila, M.A.; AlMasoud, N.; Alomar, T.S.; AlOthman, Z.A.; Yilmaz, E.; Soylak, M. Deep Eutectic Solvent-Based Microextraction of Lead(II) Traces from Water and Aqueous Extracts before FAAS Measurements. Molecules 2020, 25, 4794. [Google Scholar] [CrossRef]
- Habila, M.A.; Alabdullkarem, E.A.; ALOthman, Z.A.; Yilmaz, E.; Soylak, M. Thiomalic Acid/Ferric Chloride-Based Deep Eutectic Solvent for Microextraction of Chromium in Natural Water Samples Prior to FAAS Analysis. Int. J. Environ. Anal. Chem. 2020, 102, 1825–1833. [Google Scholar] [CrossRef]
- Habila, M.A.; Alothman, Z.A.; Soylak, M. Fe3O4 nanoparticles and Ultrasound Assisted Dispersive Liquid-Liquid Microextraction of Lead(Ii) for Its Microsampling Flame Atomic Absorption Spectrometric Determination in Food and Environmental Samples. RSC Adv. 2014, 4, 55610–55614. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Tuzen, M. Assessment of Toxic Arsenic in Water, Rice and Honey Samples Using New and Green Vortex-Assisted Liquid Phase Microextraction Procedure Based on Deep Eutectic Solvent Followed by Hydride Generation-Atomic Absorption Spectrometry: Multivariate Study. SSRN Electron. J. 2022, 179, 107541. [Google Scholar] [CrossRef]
- da Silva, F.L.F.; Lima, F.E.H.; Andrade Neto, D.M.; de Menezes, F.L.; Fechine, L.M.U.D.; Akhdhar, A.; Ribeiro, L.P.D.; Nogueira, A.R.A.; Fechine, P.B.A.; Lopes, G.S.; et al. Magnetic Solid Phase Extraction as a Nonchromatographic Method for the Quantification of Ultratrace Inorganic Arsenic in Rice by Inductively Coupled Plasma-Optical Emission Spectrometry (ICP OES). Food Chem. 2023, 412, 135461. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lin, Y.; Su, Y.; Li, Y.; Deng, Y.; Zheng, C. Sensitive and Environmentally Friendly Field Analysis of Waterborne Arsenic by Electrochemical Hydride Generation Microplasma Optical Emission Spectrometry. Anal. Chem. 2022, 94, 17514–17521. [Google Scholar] [CrossRef] [PubMed]
- Dam, G.K.; Fajal, S.; Dutta, S.; Let, S.; Desai, A.V.; Ghosh, S.K. Hydrolytically Stable Luminescent Cationic MOF for Selective Detection of Toxic Organic Arsenic in Water. ACS Appl. Opt. Mater. 2023, 1, 1217–1226. [Google Scholar] [CrossRef]
- Reich, N.D.; Nghiem, A.A.; Nicholas, S.; Bostick, B.C.; Campbell, M.G. Determination of Arsenic Content in Water Using a Silver Coordination Polymer. ACS Environ. Au 2022, 2, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.; Alam, I. Arsenic Chemistry in a Ground Water Aquifer from the Eastern Province of Saudi Arabia. Water Air Soil. Pollut. 1996, 89, 67–76. [Google Scholar] [CrossRef]
- Ali, I.; Hasan, M.A.; Alharbi, O.M.L. Toxic Metal Ions Contamination in the Groundwater, Kingdom of Saudi Arabia. J. Taibah Univ. Sci. 2020, 14, 1571–1579. [Google Scholar] [CrossRef]
- Alharbi, T.; Al-Kahtany, K.; Nour, H.E.; Giacobbe, S.; El-Sorogy, A.S. Contamination and Health Risk Assessment of Arsenic and Chromium in Coastal Sediments of Al-Khobar Area, Arabian Gulf, Saudi Arabia. Mar. Pollut. Bull. 2022, 185, 114255. [Google Scholar] [CrossRef]
- Almutairi, M.; Alsaleem, T.; Jeperel, H.; Alsamti, M.; Alowaifeer, A.M. Determination of Inorganic Arsenic, Heavy Metals, Pesticides and Mycotoxins in Indian Rice (Oryza Sativa) and a Probabilistic Dietary Risk Assessment for the Population of Saudi Arabia. Regul. Toxicol. Pharmacol. 2021, 125, 104986. [Google Scholar] [CrossRef] [PubMed]
- Al-Farraj, A.S.; Al-Wabel, M.I.; El-Saeid, M.H.; El-Naggar, A.H.; Ahmed, Z. Evaluation of Groundwater for Arsenic Contamination Using Hydrogeochemical Properties and Multivariate Statistical Methods in Saudi Arabia. J. Chem. 2013, 2013, 812365. [Google Scholar] [CrossRef]
| Interfering Ions | Added as | Concentration (mg·L−1) | Recovery% |
|---|---|---|---|
| NO3− | Al(NO3)3 | 500 | 98.2 ± 0.1 |
| Cl− | KCl | 1500 | 99.5 ± 0.5 |
| F− | NaF | 400 | 94.2 ± 1.1 |
| CO32− | Na2CO3 | 500 | 96.0 ± 0.6 |
| SO42− | Na2SO4 | 800 | 92.9 ± 0.9 |
| K+ | KCl | 1000 | 93.3 ± 1.0 |
| Mg2+ | MgCl2 | 800 | 100.0 ± 0.2 |
| Ca2+ | CaCl2 | 500 | 99.5 ± 0.1 |
| Na+ | Na3PO4 | 5000 | 94.2 ± 0.8 |
| Fe3+ | Fe(NO3)3 | 15 | 96.0 ± 0.2.3 |
| Zn2+ | Ni(NO3)2 | 20 | 93.3 ± 0.5 |
| Cd2+ | Cd(NO3)2 | 10 | 91.1 ± 0.9 |
| Pb2+ | Pb(NO3)2 | 10 | 95.6 ± 0.5 |
| Ni2+ | Ni(NO3)2 | 10 | 97.0 ± 1.5 |
| Cu2+ | Cu(NO3)2 | 20 | 97.8 ± 1.1 |
| Concentration of Standard Solution (mg·L−1) | Intra-Day Validation | Inter-Day Validation | ||||||
|---|---|---|---|---|---|---|---|---|
| First Day | Second Day | Third Day | ||||||
| Detected Concentration (mg·L−1) | Precision (RSD%) | Detected Concentration (mg·L−1) | Precision (RSD%) | Detected Concentration (mg·L−1) | Precision (RSD%) | Detected Concentration (mg·L−1) | Precision (RSD%) | |
| 0.01 | 0.0100 | 1.59 | 0.0099 | 1.91 | 0.0101 | 2.22 | 0.0099 | 2.46 |
| 0.03 | 0.0300 | 1.38 | 0.0299 | 1.92 | 0.0300 | 2.47 | 0.0299 | 2.56 |
| 0.05 | 0.0499 | 2.32 | 0.0495 | 1.97 | 0.0497 | 2.14 | 0.0499 | 2.92 |
| Real Sample Kind | Detected Arsenic(III) (mg·L−1) | Additions/Recovery Investigations | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spiking of 0.05 mg·L−1 | Spiking of 0.5 mg·L−1 | Spiking of 1.0 mg·L−1 | Spiking of 1.5 mg·L−1 | |||||||
| Detected | Recovery% | Detected | Recovery% | Detected | Recovery% | Detected | Recovery% | |||
| Water | Tap Water | BDL * | 0.049 ± 0.002 | 98 | 0.495 ± 0.001 | 99 | 0.999 ± 0.020 | 100 | 1.486 ± 0.015 | 99 |
| Ground Water | 0.005 ± 0.001 | 0.050 ± 0.005 | 100 | 0.491 ± 0.009 | 98 | 0.984 ± 0.015 | 98 | 1.487 ± 0.020 | 99 | |
| Food | Lettuce | BDL * | 0.048 ± 0.001 | 96 | 0.499 ± 0.010 | 100 | 0.987 ± 0.005 | 99 | 1.49 ± 0.018 | 99 |
| Dill | BDL * | 0.048 ± 0.005 | 96 | 0.489 ± 0.005 | 98 | 0.957 ± 0.014 | 96 | 1.489 ± 0.005 | 99 | |
| Coriander | BDL * | 0.049 ± 0.004 | 98 | 0.475 ± 0.008 | 95 | 0.953 ± 0.002 | 95 | 1.499 ± 0.009 | 100 | |
| Parsley | BDL * | 0.046 ± 0.004 | 92 | 0.495 ± 0.001 | 99 | 0.94 ± 0.009 | 94 | 1.5 ± 0.012 | 100 | |
| Tomatoes | BDL * | 0.050 ± 0.006 | 100 | 0.496 ± 0.010 | 99 | 0.972 ± 0.010 | 97 | 1.482 ± 0.025 | 99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habila, M.A.; ALOthman, Z.A.; Sheikh, M.; Alaswad, S.O. Fabrication of Spiny-like Spherical Copper Metal–Organic Frameworks for the Microextraction of Arsenic(III) from Water and Food Samples before ICP-MS Detection. Appl. Sci. 2023, 13, 10036. https://doi.org/10.3390/app131810036
Habila MA, ALOthman ZA, Sheikh M, Alaswad SO. Fabrication of Spiny-like Spherical Copper Metal–Organic Frameworks for the Microextraction of Arsenic(III) from Water and Food Samples before ICP-MS Detection. Applied Sciences. 2023; 13(18):10036. https://doi.org/10.3390/app131810036
Chicago/Turabian StyleHabila, Mohamed A., Zeid A. ALOthman, Mohamed Sheikh, and Saleh O. Alaswad. 2023. "Fabrication of Spiny-like Spherical Copper Metal–Organic Frameworks for the Microextraction of Arsenic(III) from Water and Food Samples before ICP-MS Detection" Applied Sciences 13, no. 18: 10036. https://doi.org/10.3390/app131810036
APA StyleHabila, M. A., ALOthman, Z. A., Sheikh, M., & Alaswad, S. O. (2023). Fabrication of Spiny-like Spherical Copper Metal–Organic Frameworks for the Microextraction of Arsenic(III) from Water and Food Samples before ICP-MS Detection. Applied Sciences, 13(18), 10036. https://doi.org/10.3390/app131810036







