Insight on Extraction and Preservation of Biological Activity of Cereal β-D-Glucans
Abstract
:1. Introduction
2. The Molecular Structure of Cereal β-D-Glucans
3. Technological and Nutraceutical Value of Cereal β-D-Glucans
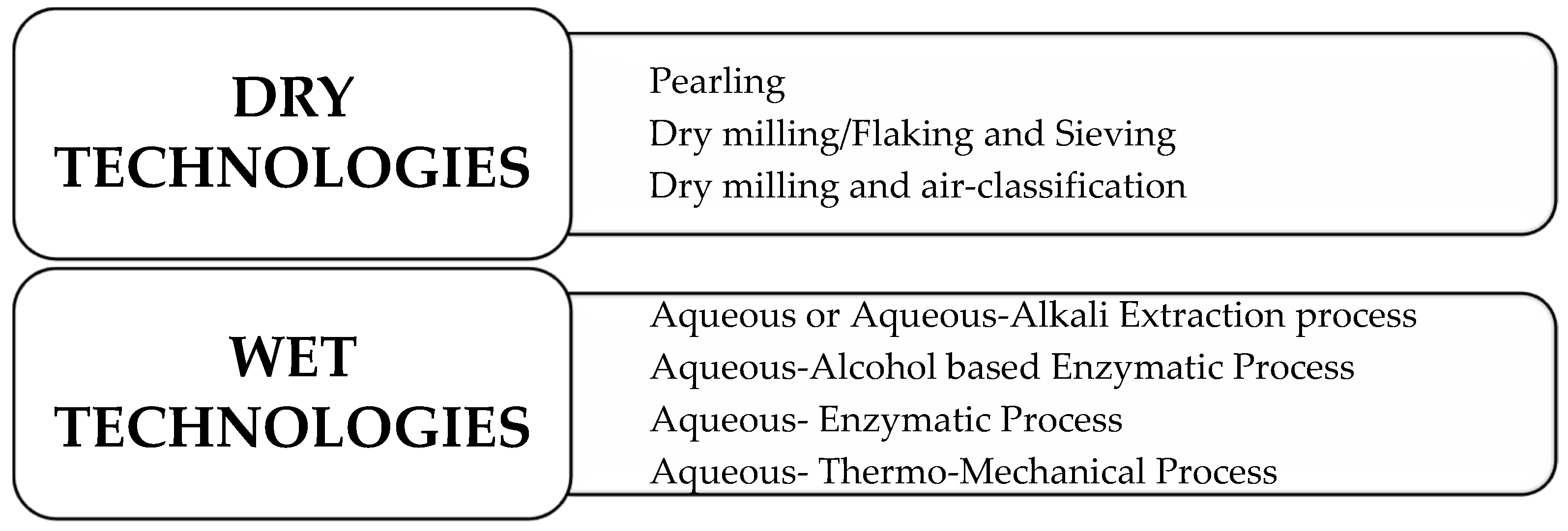
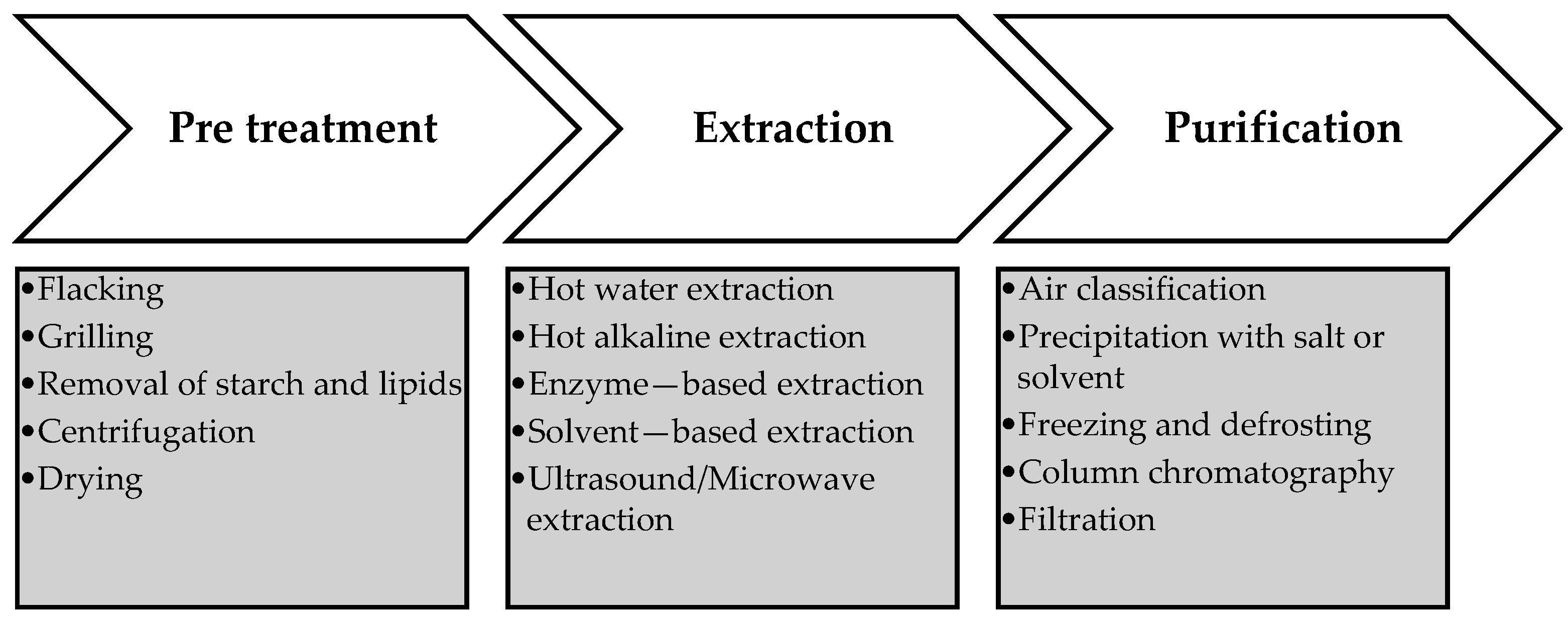
4. Methods of Extraction and Purification of Cereal β-D-Glucans
4.1. Dry Technologies
4.2. Wet Technologies
- Extraction with Water
- Alkali Extraction
- Mixing: The ground wheat/flour/oat bran or barley is mixed in an alkaline aqueous solution (pH~10), generally basified using Na2CO3 or NaOH. The β-D-glucans and proteins are then solubilized.
- Centrifugation of the compound: The insoluble solid particles, starch, and insoluble fiber are separated from the liquid phase containing the solubilized β-D-glucans and proteins.
- Precipitation of proteins once their isoelectric point (pH~4–5) is reached by adding acid. The proteins are then removed from the liquid phase by centrifugation.
- Recovery of β-D-glucan concentrate from the liquid phase by alcohol precipitation and centrifugation, followed by drying [44].
- Hydro-alcoholic Enzymatic Process Extraction
4.3. Emerging Extraction Methods
4.4. Considerations on Different Extraction Methods and Purification of β-D-Glucans in Cereals
5. Causes of Degradation of β-Glucans
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AXs | Arabinoxylans |
| ASE | Accelerated Solvent Extraction |
| DP | Degree of Polymerization |
| EFSA | European Food Safety Authority |
| FDA | Food and Drug Administration |
| MAE | Microwave-Assisted Extraction |
| MW | Molecular Weight |
| ROS | Reactive Oxygen Species |
| SCFAs | Short-Chain Fatty Acids |
| UAE | Ultrasound-Assisted Extraction |
References
- Wood, P.J. Relationships between solution properties of cereal β-glucans and physiological effects—A review. Trends Food Sci. Technol. 2004, 15, 313–320. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hu, M.; Zhao, J.; Lv, L.; Zhang, Y.; Liu, Q.; Zhang, L.; Yu, C.; Wang, P.; Li, Q.; et al. Molecular Characteristics, Synthase, and Food Application of Cereal β-Glucan. J. Food Qual. 2021, 2021, 6682014. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007, 46, 101–118. [Google Scholar] [CrossRef]
- Lante, A.; Canazza, E.; Tessari, P. Beta-Glucans of Cereals: Functional and Technological Properties. Nutrients 2023, 15, 2124. [Google Scholar] [CrossRef]
- Regand, A.; Chowdhury, Z.; Tosh, S.M.; Wolever, T.M.S.; Wood, P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011, 129, 297–304. [Google Scholar] [CrossRef]
- Schmidt, M. Cereal beta-glucans: An underutilized health endorsing food ingredient. Crit. Rev. Food Sci. Nutr. 2022, 62, 3281–3300. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Bobade, H.; Gupta, A.; Sharma, S. Chapter 20—Beta-glucan. In Nutraceuticals and Health Care; Academic Press: Cambridge, MA, USA, 2022; pp. 343–358. [Google Scholar] [CrossRef]
- Mejía, S.M.V.; de Francisco, A.; Bohrer, B.M. A comprehensive review on cereal β-glucan: Extraction, characterization, causes of degradation, and food application. Crit. Rev. Food Sci. Nutr. 2020, 60, 3693–3704. [Google Scholar] [CrossRef]
- Henrion, M.; Francey, C.; Lê, K.A.; Lamothe, L. Cereal B-glucans: The impact of processing and how it affects physiological responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to beta glucans and maintenance of normal blood cholesterol concentrations (ID 754, 755, 757, 801, 1465, 2934) and maintenance or achievement of a normal body weight (ID 820, 823) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1254. [Google Scholar] [CrossRef]
- FDA CFR—Code of Federal Regulations Title 21. 1997. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm (accessed on 3 October 2023).
- Shoukat, M.; Sorrentino, A. Cereal β-glucan: A promising prebiotic polysaccharide and its impact on the gut health. Int. J. Food Sci. Technol. 2021, 56, 2088–2097. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Tunsjø, H.; Charnock, C.; Telle-hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation—Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Dexter, J.E. Barley β-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products—A Review. Food Res. Int. 2008, 41, 850–868. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, S.; You, S.; Shim, J.H.; Yoo, S.H. Effects of barley and oat β-glucan structures on their rheological and thermal characteristics. Carbohydr. Polym. 2012, 89, 1238–1243. [Google Scholar] [CrossRef]
- Sikora, P.; Tosh, S.M.; Brummer, Y.; Olsson, O. Identification of high β-glucan oat lines and localization and chemical characterization of their seed kernel β-glucans. Food Chem. 2013, 137, 83–91. [Google Scholar] [CrossRef]
- Gangopadhyay, N.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Optimisation of yield and molecular weight of β-glucan from barley flour using response surface methodology. J. Cereal Sci. 2015, 62, 38–44. [Google Scholar] [CrossRef]
- Tosh, S.M.; Brummer, Y.; Wolever, T.M.S.; Wood, P.J. Glycemic response to oat bran muffins treated to vary molecular weight of β-glucan. Cereal Chem. 2008, 85, 211–217. [Google Scholar] [CrossRef]
- Mikkelsen, M.S.; Jespersen, B.M.; Larsen, F.H.; Blennow, A.; Engelsen, S.B. Molecular structure of large-scale extracted β-glucan from barley and oat: Identification of a significantly changed block structure in a high β-glucan barley mutant. Food Chem. 2013, 136, 130–138. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Karp, S.; Wyrwisz, J.; Kurek, M.A. Comparative analysis of the physical properties of o/w emulsions stabilised by cereal β-glucan and other stabilisers. Int. J. Biol. Macromol. 2019, 132, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgos, V.; Tosh, S.M.; Wood, P.J. Phase behaviour of high molecular weight oat β-glucan/whey protein isolate binary mixtures. Food Hydrocoll. 2009, 23, 949–956. [Google Scholar] [CrossRef]
- Sharafbafi, N.; Tosh, S.M.; Alexander, M.; Corredig, M. Phase behaviour, rheological properties, and microstructure of oat β-glucan-milk mixtures. Food Hydrocoll. 2014, 41, 274–280. [Google Scholar] [CrossRef]
- Kariluoto, S.; Edelmann, M.; Nyström, L.; Sontag-Strohm, T.; Salovaara, H.; Kivelä, R.; Herranen, M.; Korhola, M.; Piironen, V. In situ enrichment of folate by microorganisms in beta-glucan rich oat and barley matrices. Int. J. Food Microbiol. 2014, 176, 38–48. [Google Scholar] [CrossRef]
- Binou, P.; Yanni, A.E.; Stergiou, A.; Karavasilis, K.; Konstantopoulos, P.; Perrea, D.; Tentolouris, N.; Karathanos, V.T. Enrichment of bread with beta-glucans or resistant starch induces similar glucose, insulin and appetite hormone responses in healthy adults. Eur. J. Nutr. 2021, 60, 455–464. [Google Scholar] [CrossRef]
- Heiniö, R.L.; Noort, M.W.J.; Katina, K.; Alam, S.A.; Sozer, N.; de Kock, H.L.; Hersleth, M.; Poutanen, K. Sensory characteristics of wholegrain and bran-rich cereal foods—A review. Trends Food Sci. Technol. 2016, 47, 25–38. [Google Scholar] [CrossRef]
- Messia, M.C.; De Arcangelis, E.; Candigliota, T.; Trivisonno, M.C.; Marconi, E. Production of β-glucan enriched flour from waxy barley. J. Cereal Sci. 2020, 93, 102989. [Google Scholar] [CrossRef]
- Tessari, P.; Lante, A. A multifunctional bread rich in beta glucans and low in starch improves metabolic control in type 2 diabetes: A controlled trial. Nutrients 2017, 9, 297. [Google Scholar] [CrossRef]
- Vizhi, V.K.; Many, J.N. Study on Estimation, Extraction and Analysis of Barley Beta-glucan. Int. J. Sci. Res. 2014, 3, 1480–1484. [Google Scholar]
- Vasanthan, T.; Temelli, F. Grain fractionation technologies for cereal beta-glucan concentration. Food Res. Int. 2008, 41, 876–881. [Google Scholar] [CrossRef]
- Zheng, G.H.; Rossnagel, B.G.; Tyler, R.T.; Bhatty, R.S. Distribution of β-glucan in the grain of hull-less barley. Cereal Chem. 2000, 77, 140–144. [Google Scholar] [CrossRef]
- Sibakov, J.; Abecassis, J.; Barron, C.; Poutanen, K. Electrostatic separation combined with ultra-fine grinding to produce β-glucan enriched ingredients from oat bran. Innov. Food Sci. Emerg. Technol. 2014, 26, 445–455. [Google Scholar] [CrossRef]
- Gómez-caravaca, A.M.; Verardo, V.; Candigliota, T.; Marconi, E.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Fiorenza, M. Use of air classi fi cation technology as green process to produce functional barley fl ours naturally enriched of alkylresorcinols, β-glucans and phenolic compounds. Food Res. Int. 2015, 73, 88–96. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta glucan: A valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural features, modification, and functionalities of beta-glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef]
- Arzami, A.N.; Ho, T.M.; Mikkonen, K.S. Valorization of cereal by-product hemicelluloses: Fractionation and purity considerations. Food Res. Int. 2022, 151, 110818. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Alonso, E.; Cocero, M.J. Ultrasound-assisted extraction of β-glucans from barley. LWT 2013, 50, 57–63. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Tatke, P.; Jaiswal, Y. An overview of microwave assisted extraction and its applications in herbal drug research. Res. J. Med. Plant 2011, 5, 21–31. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.J.; Weisz, J.; Fedec, P.; Burrows, V.D. Large-scale preparation and properties of oat fractions enriched in β-glucan. Cereal Chem. 1989, 66, 97–103. [Google Scholar]
- Du, B.; Zhu, F.; Xu, B. β-Glucan extraction from bran of hull-less barley by accelerated solvent extraction combined with response surface methodology. J. Cereal Sci. 2014, 59, 95–100. [Google Scholar] [CrossRef]
- Yoo, H.U.; Ko, M.J.; Chung, M.S. Hydrolysis of beta-glucan in oat flour during subcritical-water extraction. Food Chem. 2020, 308, 125670. [Google Scholar] [CrossRef] [PubMed]
- Messia, M.C.; Candigliota, T.; De Arcangelis, E.; Marconi, E. Arabinoxylans and β-glucans assessment in cereals. Ital. J. FoodSci. 2017, 29, 112–122. [Google Scholar] [CrossRef]
- Harasym, J.; Dziendzikowska, K.; Gromadzka-Ostrowska, J. Proteinaceous Residue Removal from Oat β-Glucan Extracts Obtained by Alkaline Water Extraction. Molecules 2019, 24, 1729. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S.; Jenkins, A.L.; Prudence, K.; Johnson, J.; Duss, R.; Chu, Y.; Steinert, R.E. Effect of adding oat bran to instant oatmeal on glycaemic response in humans—A study to establish the minimum effective dose of oat β-glucan. Food Funct. 2018, 9, 1692–1700. [Google Scholar] [CrossRef]
- Liu, K. Fractionation of oats into products enriched with protein, beta-glucan, starch, or other carbohydrates. J. Cereal Sci. 2014, 60, 317–322. [Google Scholar] [CrossRef]
- Limberger, V.M.; de Francisco, A.; Borges, M.R.; Oro, T.; Ogliari, P.J.; Scheuer, P.M.; Noronha, C.M. Extração de β-glucanas de cevada e caracterização parcial do amido residual. Tecnol. Aliment. Ciên. Rural 2011, 41, 2217–2223. [Google Scholar] [CrossRef]
- Rieder, A.; Ballance, S.; Knutsen, S.H. Viscosity based quantification of endogenous β-glucanase activity in flour. Carbohydr. Polym. 2015, 115, 104–111. [Google Scholar] [CrossRef]
- Gamel, T.H.; Badali, K.; Tosh, S.M. Changes of β-glucan physicochemical characteristics in frozen and freeze dried oat bran bread and porridge. J. Cereal Sci. 2013, 58, 104–109. [Google Scholar] [CrossRef]
- Faure, A.M.; Werder, J.; Nyström, L. Reactive oxygen species responsible for beta-glucan degradation. Food Chem. 2013, 141, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.M.; Sánchez-Ferrer, A.; Zabara, A.; Andersen, M.L.; Nyström, L. Modulating the structural properties of β-d-glucan degradation products by alternative reaction pathways. Carbohydr. Polym. 2014, 99, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhan, R.; Sontag-Strohm, T.; Maina, N.H. The protective role of phytate in the oxidative degradation of cereal beta-glucans. Carbohydr. Polym. 2017, 169, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, R.; Henniges, U.; Sontag-Strohm, T.; Potthast, A. Oxidation of oat β-glucan in aqueous solutions during processing. Carbohydr. Polym. 2012, 87, 589–597. [Google Scholar] [CrossRef]
- Huth, M.; Dongowski, G.; Gebhardt, E.; Flamme, W. Functional properties of dietary fibre enriched extrudates from Barley. J. Cereal Sci. 2000, 32, 115–128. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S. Extrusion of Hulled Barley Affecting β-Glucan and Properties of Extrudates. Food Bioprocess Technol. 2013, 6, 1374–1389. [Google Scholar] [CrossRef]
- Sayanjali, S.; Ying, D.; Sanguansri, L.; Buckow, R.; Augustin, M.A.; Gras, S.L. The effect of extrusion on the functional properties of oat fibre. LWT 2017, 84, 106–113. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Zhang, Z. Extrusion process improves the functionality of soluble dietary fiber in oat bran. J. Cereal Sci. 2011, 54, 98–103. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S.; Rosell, C.M. Effects of roasting on barley β-glucan, thermal, textural and pasting properties. J. Cereal Sci. 2011, 53, 25–30. [Google Scholar] [CrossRef]
- Brummer, Y.; Duss, R.; Wolever, T.M.S.; Tosh, S.M. Glycemic response to extruded oat bran cereals processed to vary in molecular weight. Cereal Chem. 2012, 89, 255–261. [Google Scholar] [CrossRef]
- Beer, M.U.; Wood, P.J.; Weisz, J.; Fillion, N. Effect of cooking and storage on the amount and molecular weight of (1→3)(1→4)-β-d-glucan extracted from oat products by an in vitro digestion system. Cereal Chem. 1997, 74, 705–709. [Google Scholar] [CrossRef]
- Tosh, S.M.; Brummer, Y.; Wood, P.J.; Wang, Q.; Weisz, J. Evaluation of structure in the formation of gels by structurally diverse (1→3)(1→4)-β-d-glucans from four cereal and one lichen species. Carbohydr. Polym. 2004, 57, 249–259. [Google Scholar] [CrossRef]
- Moriartey, S.; Temelli, F.; Vasanthan, T. Effect of Storage Conditions on the Solubility and Viscosity of β-Glucan Extracted from Bread under In Vitro Conditions. J. Food Sci. 2011, 76, C1–C7. [Google Scholar] [CrossRef] [PubMed]
- Lan-Pidhainy, X.; Brummer, Y.; Tosh, S.M.; Wolever, T.M.; Wood, P.J. Reducing beta-glucan solubility in oat bran muffins by freeze-thaw treatment attenuates its hypoglycemic effect. Cereal Chem. 2007, 84, 512–517. [Google Scholar] [CrossRef]

| Company | Source | Processes | Registered Name |
|---|---|---|---|
| Natraceutical Edmonton, AB, Canada | Barley and oats | Enzymatic semialcoholic | Viscofiber ® (Up to 65%) |
| GTC Nutrition, Golden, CO, USA | Oats | Watery | OatVantage ™ (Up to 54%) |
| Cargill Inc., Minneapolis, MN, USA | Barley | Watery | Βfiber ™ (Up to 70%) |
| GraceLinc Ltd., Canterbury, New Zealand | Barley | Watery | Glucagel ™ (Up to 75%) |
| Van Drunen Farms, Momence, IL, USA | Oats | Aqueous-thermomechanical | Nutrim ™ (Up to 6%) |
| Danisco, Thomson, IL, USA | Oats | Aqueous-enzymatic | Oatrim ™ (Up to 25%) |
| Nutrition Inc., Boulder, CO, USA | Oats | Air grinding and classification | Oatwell ™ (Up to 22%) |
| Sources | Arabinoxylans | Glucans (β-D-Glucans) | |
|---|---|---|---|
| Bran | Barley | 19.0–21.4 | 6.15–7.58 |
| Oat | 3.0 | 5.4–8.5 | |
| Rice | 4.8–5.1 | 0.04–0.21 | |
| Wheat | 18.0–24.0 | 2.1 | |
| Rye | 12.0–18.0 | 2.9 | |
| Corn | 27.2–29.9 | 0.1 | |
| Husk | Barley | 19.0–20.0 | 37–40 |
| Oat | 3.5 | 37.10 | |
| Rice | 8.4–9.2 | 34.2 | |
| Wheat | NR | 38.2 | |
| Soybean | 13.1 | NR | |
| Straw | Barley | 11.0–14.0 | 33.6 |
| Oat | NR | 32.1 | |
| Rice | 11.0–18.3 | 34.1 | |
| Wheat | NR | 30.4 | |
| Rye | NR | 33.12 | |
| Corn | 27.0–30.0 | 40.9 | |
| Corn cob | 12.8 | 41.6 | |
| Cause of Degradation and Functionality of Cereal β-D-Glucans | How to Avoid Depolymerization/Degradation |
|---|---|
| Activity of endogenous β-D-glucanases |
|
| Oxidative depolymerization in the presence of ROS |
|
| Thermal degradation |
|
| Germination |
|
| Extrusion firing |
|
| Toasting |
|
| Freezing and thawing cycle |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lante, A.; Canazza, E. Insight on Extraction and Preservation of Biological Activity of Cereal β-D-Glucans. Appl. Sci. 2023, 13, 11080. https://doi.org/10.3390/app131911080
Lante A, Canazza E. Insight on Extraction and Preservation of Biological Activity of Cereal β-D-Glucans. Applied Sciences. 2023; 13(19):11080. https://doi.org/10.3390/app131911080
Chicago/Turabian StyleLante, Anna, and Elisa Canazza. 2023. "Insight on Extraction and Preservation of Biological Activity of Cereal β-D-Glucans" Applied Sciences 13, no. 19: 11080. https://doi.org/10.3390/app131911080






