A Comparative Study of Fourier Transform Near-Infrared Spectroscopy and Physicochemical Methods in Wheat Quality Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Sample Preparation
2.2. Data Analysis
3. Results
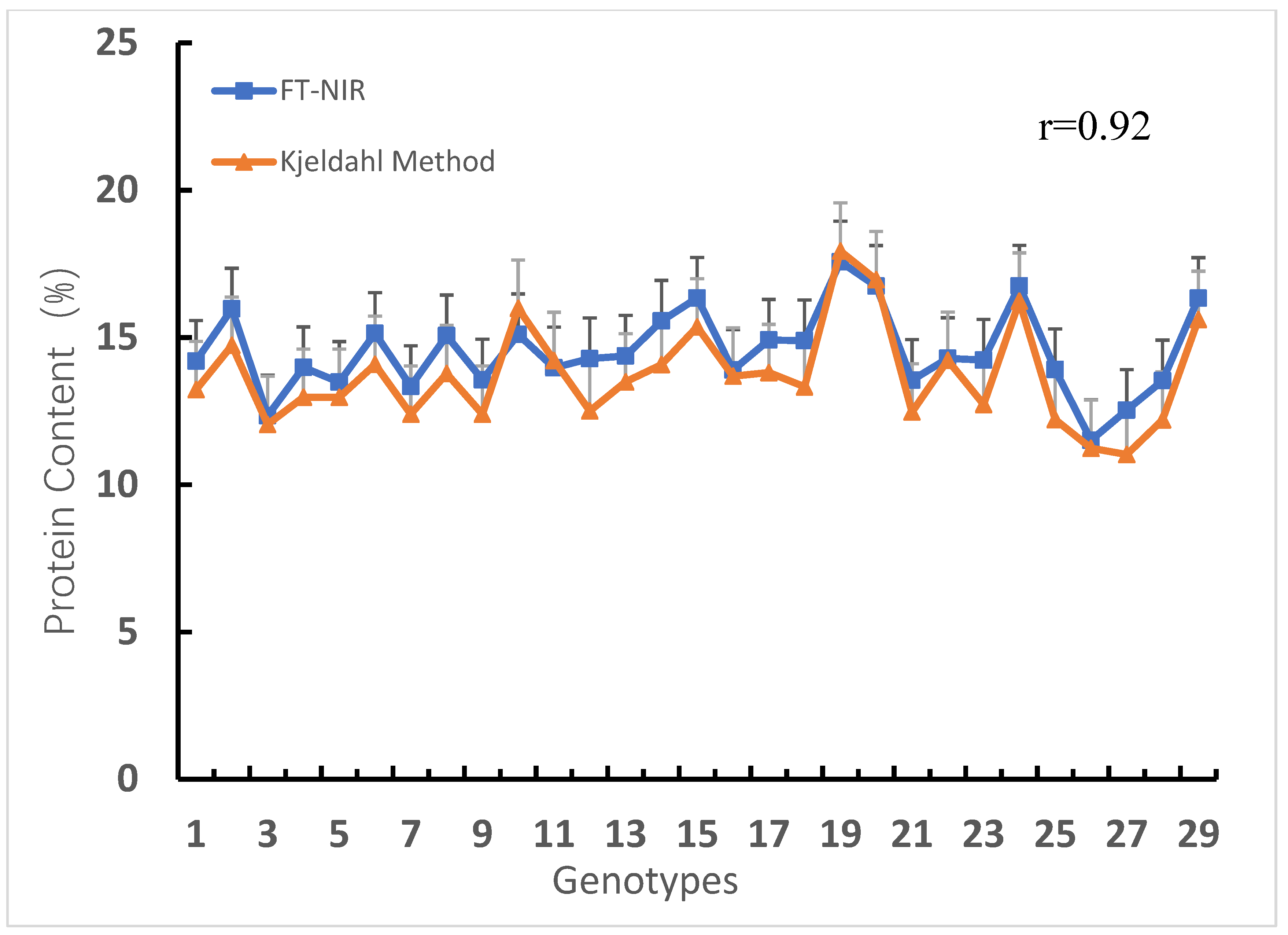
3.1. Protein Content
3.2. Bulk Density
3.3. Extensibility
3.4. Development Time
3.5. Stability Time
3.6. Wet Gluten
3.7. Extension Area
3.8. Correlation Coefficient
3.9. Correlation Analysis between Different Wheat Quality Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, L.; Meng, C.; Yi, T.; Xu, K.; Cao, H.; Zhang, S.; Yang, X.; Zhao, Y. Genome-wide association study reveals the genetic basis of yield- and quality-related traits in wheat. BMC Plant Biol. 2021, 21, 144. [Google Scholar] [CrossRef]
- Zareef, M.; Arslan, M.; Hassan, M.M.; Ahmad, W.; Ali, S.; Li, H.H.; Qin, O.Y.; Wu, X.Y.; Hashim, M.M.; Chen, Q.S. Recent advances in assessing qualitative and quantitative aspects of cereals using nondestructive techniques: A review. Trends Food Sci. Technol. 2021, 116, 815–828. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Shen, L.; Chen, S.; He, L.; Liu, A. Application of near-infrared spectroscopy for the nondestructive analysis of wheat flour: A review. Curr. Res. Food Sci. 2022, 5, 1305–1312. [Google Scholar] [CrossRef]
- Mishra, G.; Srivastava, S.; Panda, B.K.; Mishra, H.N. Rapid Assessment of Quality Change and Insect Infestation in Stored Wheat Grain Using FT-NIR Spectroscopy and Chemometrics. Food Anal. Meth. 2018, 11, 1189–1198. [Google Scholar] [CrossRef]
- De Girolamo, A.; Cervellieri, S.; Mancini, E.; Pascale, M.; Logrieco, A.F.; Lippolis, V. Rapid Authentication of 100% Italian Durum Wheat Pasta by FT-NIR Spectroscopy Combined with Chemometric Tools. Foods 2020, 9, 1551. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, A.; Cortese, M.; Cervellieri, S.; Lippolis, V.; Pascale, M.; Logrieco, A.F.; Suman, M. Tracing the Geographical Origin of Durum Wheat by FT-NIR Spectroscopy. Foods 2019, 8, 450. [Google Scholar] [CrossRef]
- Bekes, F. New Aspects in Quality Related Wheat Research: II. New Methodologies for Better Quality Wheat. Cereal Res. Commun. 2012, 40, 307–333. [Google Scholar] [CrossRef]
- Dowell, F.E.; Maghirang, E.B.; Xie, F.; Lookhart, G.L.; Pierce, R.O.; Seabourn, B.W.; Bean, S.R.; Wilson, J.D.; Chung, O.K. Predicting Wheat Quality Characteristics and Functionality Using Near-Infrared Spectroscopy. Cereal Chem. 2006, 83, 529–536. [Google Scholar] [CrossRef]
- Du, Z.J.; Tian, W.F.; Tilley, M.; Wang, D.H.; Zhang, G.R.; Li, Y.H. Quantitative assessment of wheat quality using near-infrared spectroscopy: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2956–3009. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R. Study on Rapid Measurement Technology of Wheat Main Quality Indexs Based on Near Infrared Spectroscopy. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2022. [Google Scholar]
- Vatter, T.; Gracia-Romero, A.; Kefauver, S.C.; Nieto-Taladriz, M.T.; Aparicio, N.; Araus, J.L. Preharvest phenotypic prediction of grain quality and yield of durum wheat using multispectral imaging. Plant J. 2022, 109, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Molognoni, L.; Ploencio, L.A.D.; Deolindo, C.T.P.; de Oliveira, L.V.A.; Hoff, R.B.; Daguer, H. FT-NIR combined with chemometrics versus classic chemical methods as accredited analytical support for decision-making: Application to chemical compositional compliance of feedingstuffs. Microchem. J. 2020, 158, 105126. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, S.; Zhao, G. Rapid determination of total protein and wet gluten in commercial wheat flour using siSVR-NIR. Food Chem. 2017, 221, 1939–1946. [Google Scholar] [CrossRef]
- GB/T 4882-2001; Statistical Interpretation of Data—Normality Tests. East China Normal University: Shanghai, China, 2001; p. 36.
- Huang, W.-J.; Su, N.-C. A study of generalized normal distributions. Commun. Stat.—Theory Methods 2017, 46, 5612–5632. [Google Scholar] [CrossRef]
- Zhao, J.; Lee, S.K.; Kim, H.-M. Some counterexamples of a skew-normal distribution. Commun. Stat. Appl. Methods 2019, 26, 583–589. [Google Scholar] [CrossRef]
- Zheng, S.; Hardin, J.M.; Gupta, A.K. The inverse problem of multivariate and matrix-variate skew normal distributions. Statistics 2012, 46, 361–371. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, G.; Mei, C.; Chen, Q. Qualitative and quantitative analysis in solid-state fermentation of protein feed by FT-NIR spectroscopy integrated with multivariate data analysis. Anal. Methods 2013, 5, 1872–1880. [Google Scholar] [CrossRef]
- Kasuya, E. On the use of r and r squared in correlation and regression. Ecol. Res. 2019, 34, 235–236. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, B.; Wei, Y.; Zhang, B. Effects of grown origin, genotype, harvest year, and their interactions of wheat kernels on near infrared spectral fingerprints for geographical traceability. Food Chem. 2014, 152, 316–322. [Google Scholar] [CrossRef]
- Wu, Y.F.; Luan, X.L.; Liu, F. FT-NIR online detection performance improvement and evaluation for. Infrared Phys. Technol. 2023, 128, 104514. [Google Scholar] [CrossRef]
- Badaro, A.T.; Tavares, J.; Blasco, J.; Aleixos-Borras, N.; Barbin, D.F. Near infrared techniques applied to analysis of wheat-based products: Recent advances and future trends. Food Control 2022, 140, 109115. [Google Scholar] [CrossRef]
- De Girolamo, A.; Arroyo, M.C.; Cervellieri, S.; Cortese, M.; Pascale, M.; Logrieco, A.F.; Lippolis, V. Detection of durum wheat pasta adulteration with common wheat by infrared spectroscopy and chemometrics: A case study. LWT—Food Sci. Technol. 2020, 127, 109368. [Google Scholar] [CrossRef]
- Pandey, P.; Srivastava, S.; Mishra, H.N. Comparison of FT-NIR and NIR for evaluation of phyisco-chemical properties of stored wheat grains. Food Qual. Saf. 2018, 2, 165–172. [Google Scholar] [CrossRef]
- Amir, R.M.; Anjum, F.M.; Khan, M.I.; Khan, M.R.; Pasha, I.; Nadeem, M. Application of Fourier transform infrared (FTIR) spectroscopy for the identification of wheat varieties. J. Food Sci. Technol. 2013, 50, 1018–1023. [Google Scholar] [CrossRef] [PubMed]







| Number | Sowing Time | Planting Locations | Wheat Genotypes | Sowing Amount/kg·ha−2 |
|---|---|---|---|---|
| 1 | 10.20 | Shanxiayu | VS1403 | 165 |
| 2 | 10.22 | Junbukou | VS1403 | 210 |
| 3 | 10.26 | Xiying | VS5022 | 240 |
| 4 | 10.27 | Miaobu | VS5022 | 255 |
| 5 | 10.27 | Miaobu | VS5022 | 255 |
| 6 | 10.28 | Chenjia | VS1403 | 240 |
| 7 | 10.29 | Louzi | VS1403 | 255 |
| 8 | 11.03 | Zhangfengchaobei | VS44 | 300 |
| 9 | 11.14 | Zhangfengchaobei | VS44 | 300 |
| 10 | 11.15 | Hexiling | VS44 | 300 |
| 11 | 11.15 | Zhangjiaguanzhaung | VS44 | 300 |
| 12 | 11.17 | Dazhai | VS44 | 300 |
| 13 | 10.28 | Changyijiangxiaojie | VS1403 | 180 |
| 14 | 11.4 | Hanting | VS44 | 195 |
| 15 | 10.29 | Anqiuchangxing | Jimai22 | 150 |
| 16 | 10.22 | Junbukou | VS1403 | 210 |
| 17 | 10.24 | Junbukou | Shannong48 | 210 |
| 18 | 11.18 | Xuanjiashiyantian | Yannong15 | 270 |
| 19 | 11.15 | Xuanjiashiyantian | Jiruo116 | 300 |
| 20 | 11.16 | Xuanjiashiyantian | VS44642 | 300 |
| 21 | 11.16 | Xuanjiashiyantian | VS44641 | 300 |
| 22 | 10.23 | Agricultural Bureau | VS7506 | 165 |
| 23 | 10.23 | Agricultural Bureau | VS25053 | 195 |
| 24 | 10.23 | Agricultural Bureau | VS116 | 165 |
| 25 | 10.23 | Agricultural Bureau | VS126 | 195 |
| 26 | 10.23 | Agricultural Bureau | VS40 | 165 |
| 27 | 10.23 | Agricultural Bureau | VS55 | 180 |
| 28 | 10.23 | Agricultural Bureau | VS44641 | 195 |
| 29 | 10.23 | Changyixiadian | VS1403 | 195 |
| Kolmogorov–Smirnov | Shapiro–Wilk | ||||
|---|---|---|---|---|---|
| Methods | Parameters | Statistic | p | Statistic | p |
| FT-NIR | Protein content | 0.131 | 0.2 | 0.976 | 0.724 |
| Bulk density | 0.125 | 0.2 | 0.937 | 0.083 | |
| Dough extensibility | 0.125 | 0.2 | 0.937 | 0.083 | |
| Development time | 0.122 | 0.2 | 0.969 | 0.537 | |
| Stability time | 0.096 | 0.2 | 0.967 | 0.48 | |
| Wet gluten | 0.133 | 0.2 | 0.966 | 0.453 | |
| Extension area | 0.108 | 0.2 | 0.978 | 0.794 | |
| Physicochemical measurement | Protein content | 0.14 | 0.15 | 0.951 | 0.191 |
| Bulk density | 0.193 | 0.007 | 0.909 | 0.016 | |
| Dough extensibility | 0.193 | 0.007 | 0.909 | 0.016 | |
| Development time | 0.301 | 0 | 0.743 | 0 | |
| Stability time | 0.208 | 0.002 | 0.876 | 0.003 | |
| Wet gluten | 0.127 | 0.2 | 0.946 | 0.146 | |
| Extension area | 0.168 | 0.036 | 0.936 | 0.08 | |
| FT-NIR | Protein Content | Bulk Density | Extensibility | Forming Time | Stability Time | Wet Gluten | Extension Area | |
|---|---|---|---|---|---|---|---|---|
| Physicochemical | ||||||||
| Protein Content | 0.916 *** | 0.487 ** | 0.487 ** | 0.364 | −0.175 | 0.872 *** | −0.085 | |
| Bulk Density | 0.415 * | 0.460 * | 0.460 * | 0.264 | −0.230 | 0.436 * | 0.114 | |
| Extensibility | 0.415 * | 0.460 * | 0.460 * | 0.264 | −0.230 | 0.436 * | 0.114 | |
| Forming Time | 0.457 * | 0.289 | 0.289 | 0.370 * | 0.302 | 0.442 * | 0.107 | |
| Stability Time | −0.052 | 0.258 | 0.258 | 0.307 | 0.487 ** | −0.085 | 0.345 | |
| Wet Gluten | 0.506 ** | 0.03 | 0.03 | 0.112 | −0.101 | 0.544 ** | −0.223 | |
| Extension Area | 0.263 | 0.561 ** | 0.56 1** | 0.649 *** | 0.583 *** | 0.311 | 0.630 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Shi, Y. A Comparative Study of Fourier Transform Near-Infrared Spectroscopy and Physicochemical Methods in Wheat Quality Analysis. Appl. Sci. 2023, 13, 11368. https://doi.org/10.3390/app132011368
Hao Z, Shi Y. A Comparative Study of Fourier Transform Near-Infrared Spectroscopy and Physicochemical Methods in Wheat Quality Analysis. Applied Sciences. 2023; 13(20):11368. https://doi.org/10.3390/app132011368
Chicago/Turabian StyleHao, Zenghui, and Yan Shi. 2023. "A Comparative Study of Fourier Transform Near-Infrared Spectroscopy and Physicochemical Methods in Wheat Quality Analysis" Applied Sciences 13, no. 20: 11368. https://doi.org/10.3390/app132011368




