Modelling and Evaluation of the Effect of Pulsed Electric Fields and High Pressure Processing Conditions on the Quality Parameters of Osmotically Dehydrated Tomatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material Selection
2.2. Selection of Appropriate PEF Pre-Treatment Conditions
2.3. Selection of Appropriate HP Pre-Treatment Conditions
2.4. Osmotic Dehydration of Untreated and PEF/HP Pretreated Samples
2.4.1. Determination of Water Loss and Solids Gain and Mathematical Modelling of Osmotic Dehydration
2.4.2. Determination of Physicochemical Parameters
2.4.3. Determination of Tomato Firmness
2.4.4. Determination of Objective Color
2.4.5. Determination of PME and PG Activity
2.4.6. Sensory Evaluation of Tomatoes
2.5. Shelf Life Determination of Untreated and Treated Tomato Samples
2.6. Statistical Analysis
3. Results
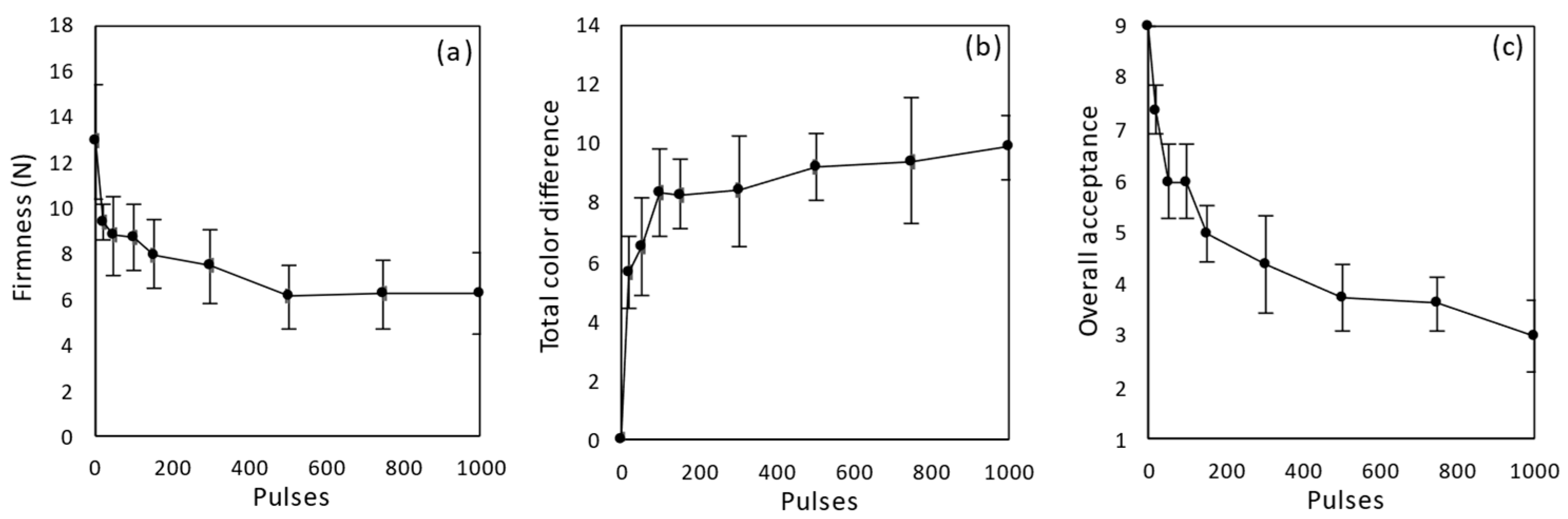
3.1. Selection of PEF Pre-Treatment Conditions for Tomatoes
3.2. Osmotic Dehydration of PEF Pre-Treated Tomatoes
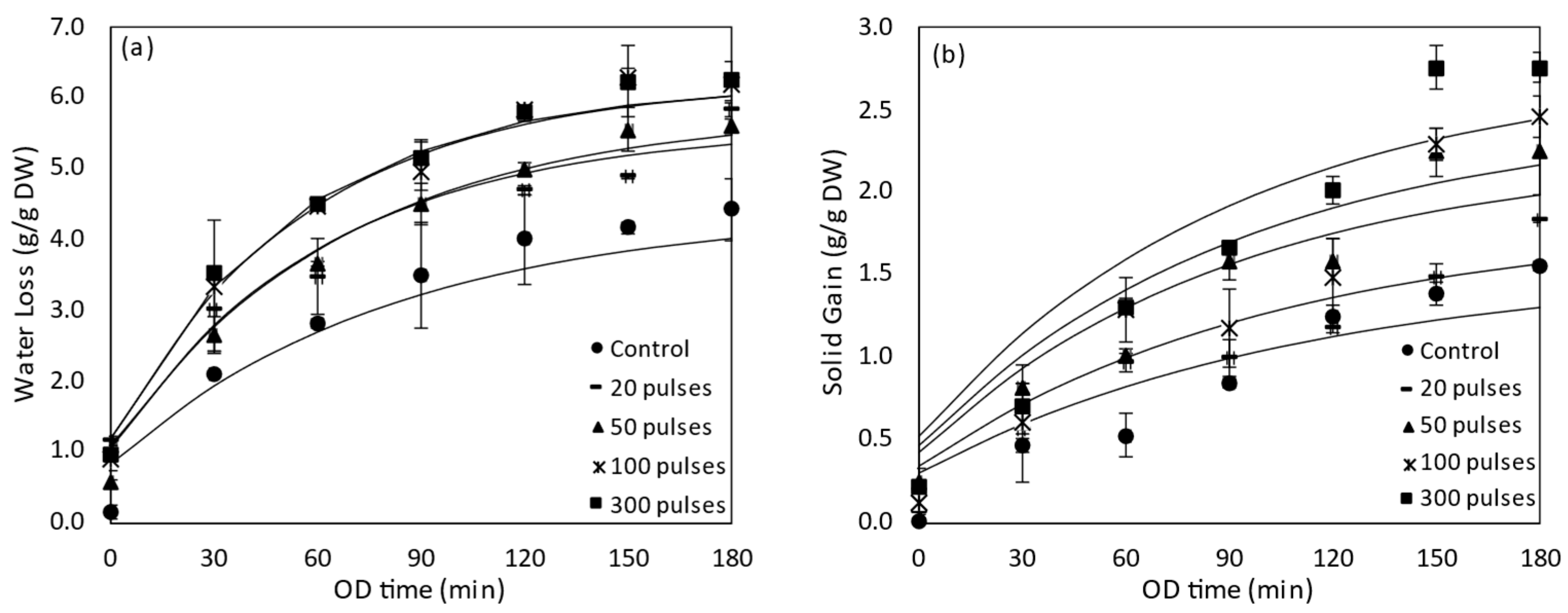
3.2.1. Water Loss and Solids Gain of PEF Pre-Treated Tomatoes during OD
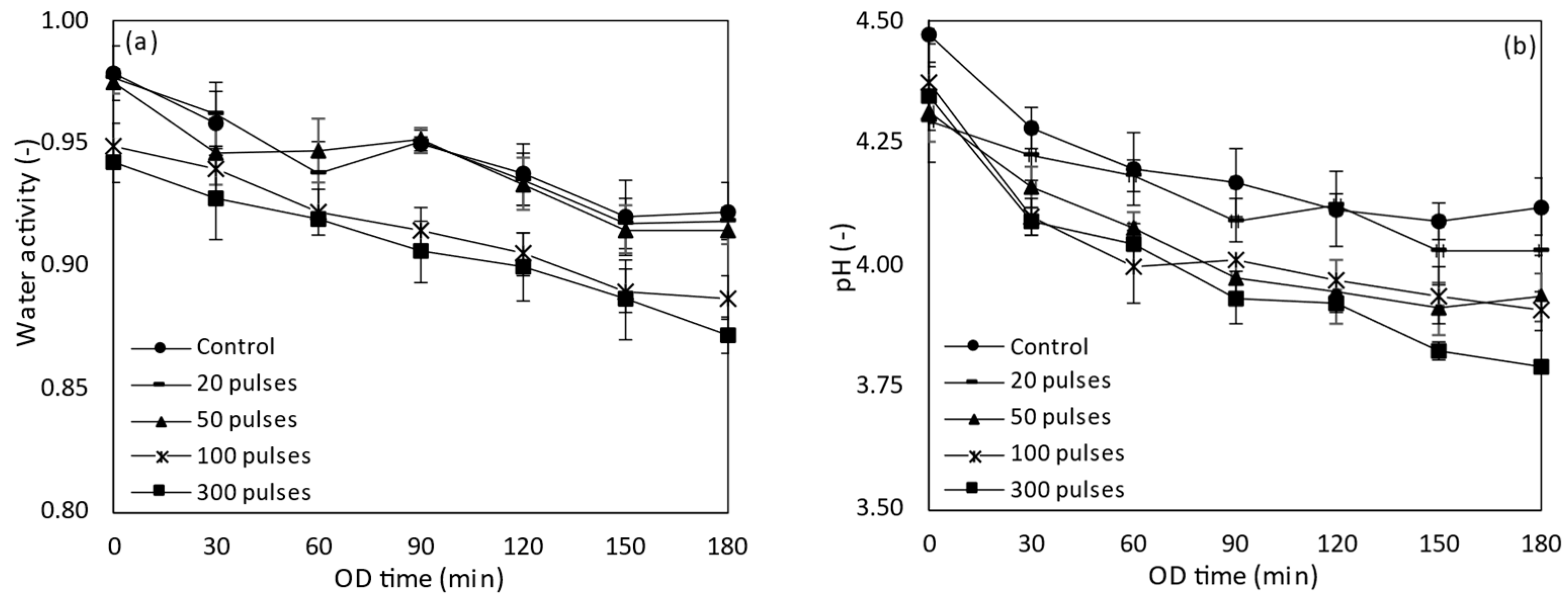
3.2.2. Water Activity, pH and Titratable Acidity of PEF Treated Tomatoes during OD
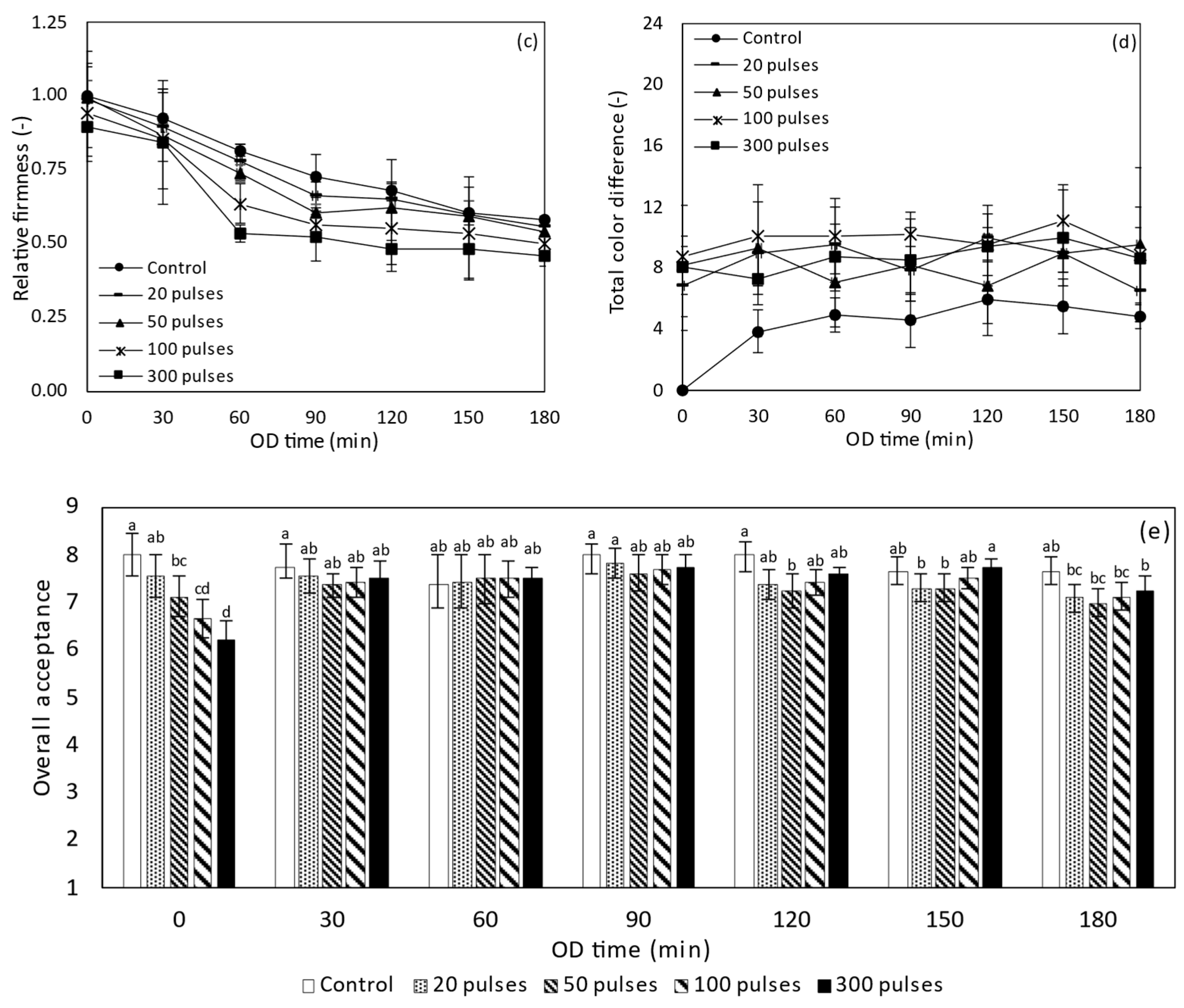
3.2.3. Objective Firmness and Color of PEF Treated Tomatoes during OD
3.2.4. Sensory Evaluation of PEF Treated Tomatoes during OD
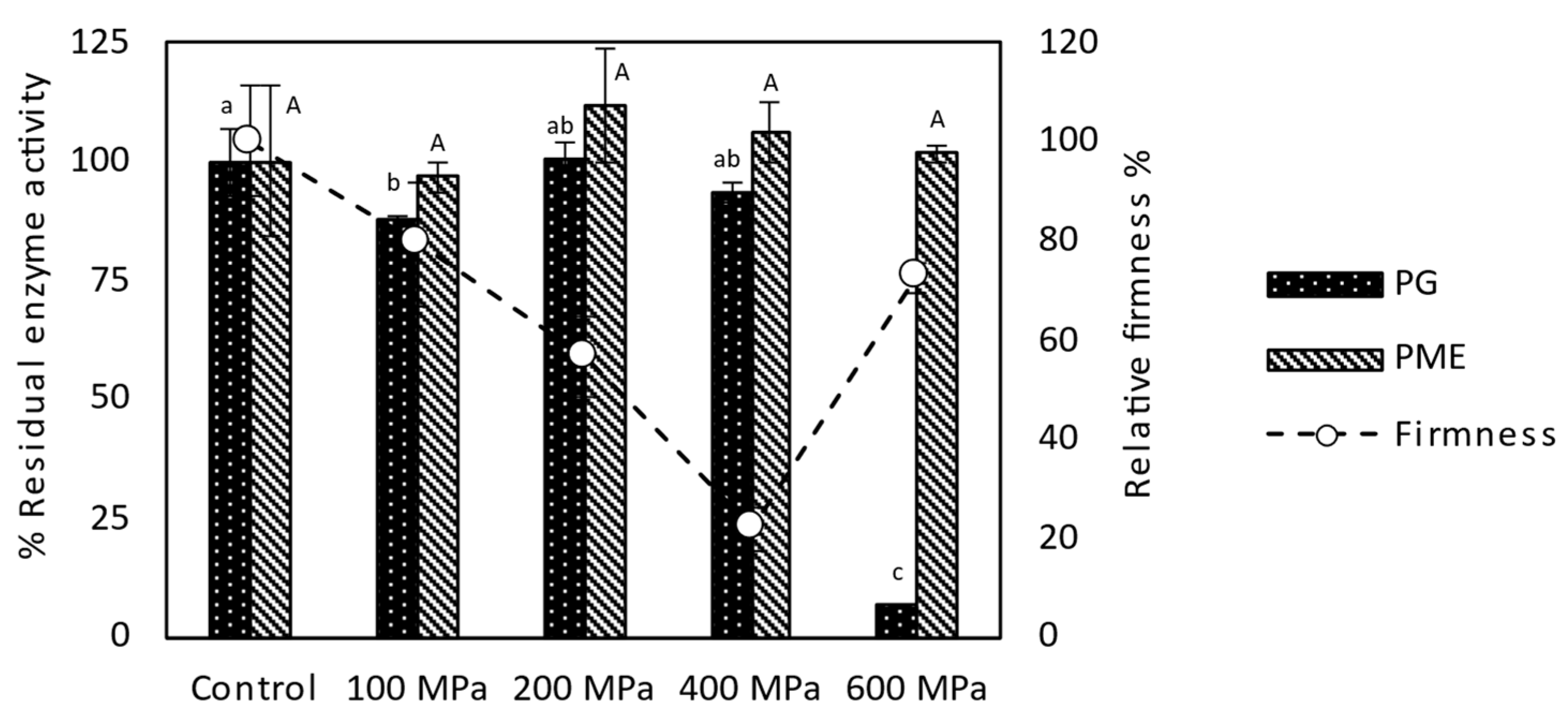
3.3. Selection of HP Pre-Treatment Conditions for OD of Tomatoes
3.4. Osmotic Dehydration of PEF Pre-Treated Tomatoes
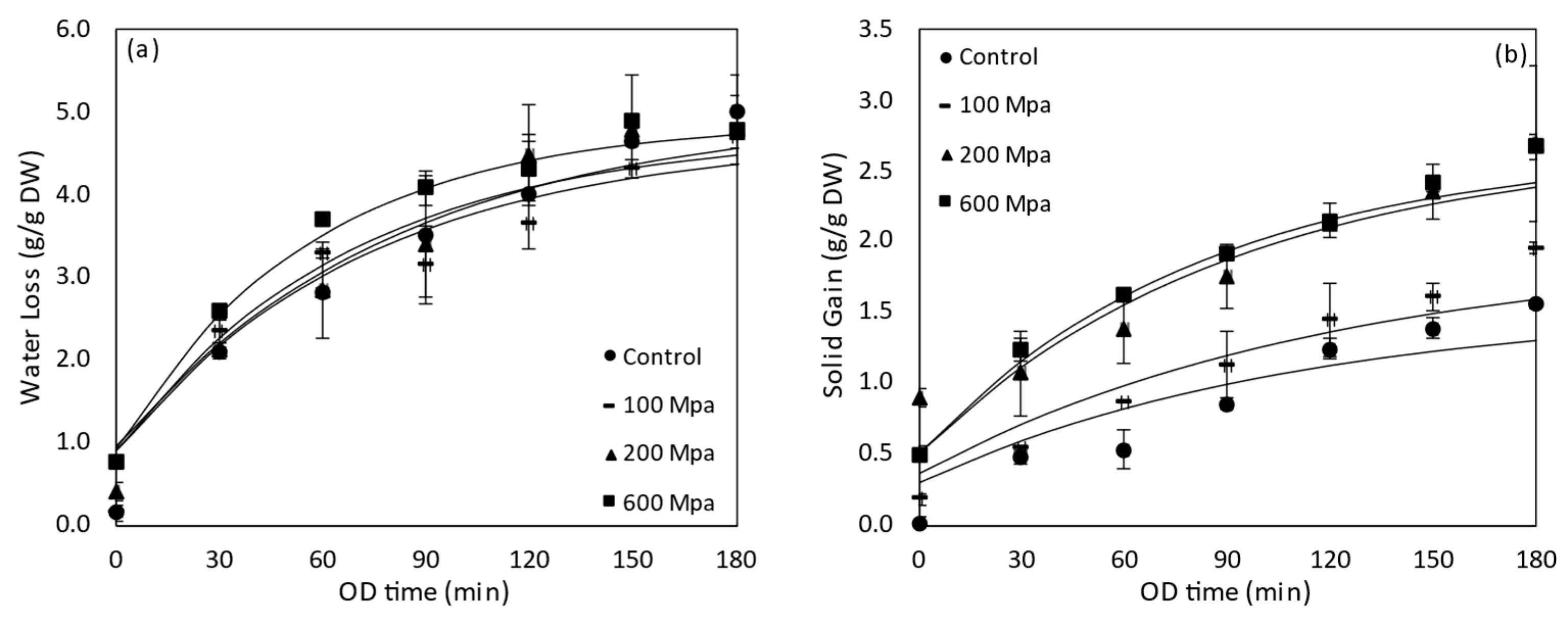
3.4.1. Water Loss and Solids Gain of HP Pre-Treated Tomatoes during OD
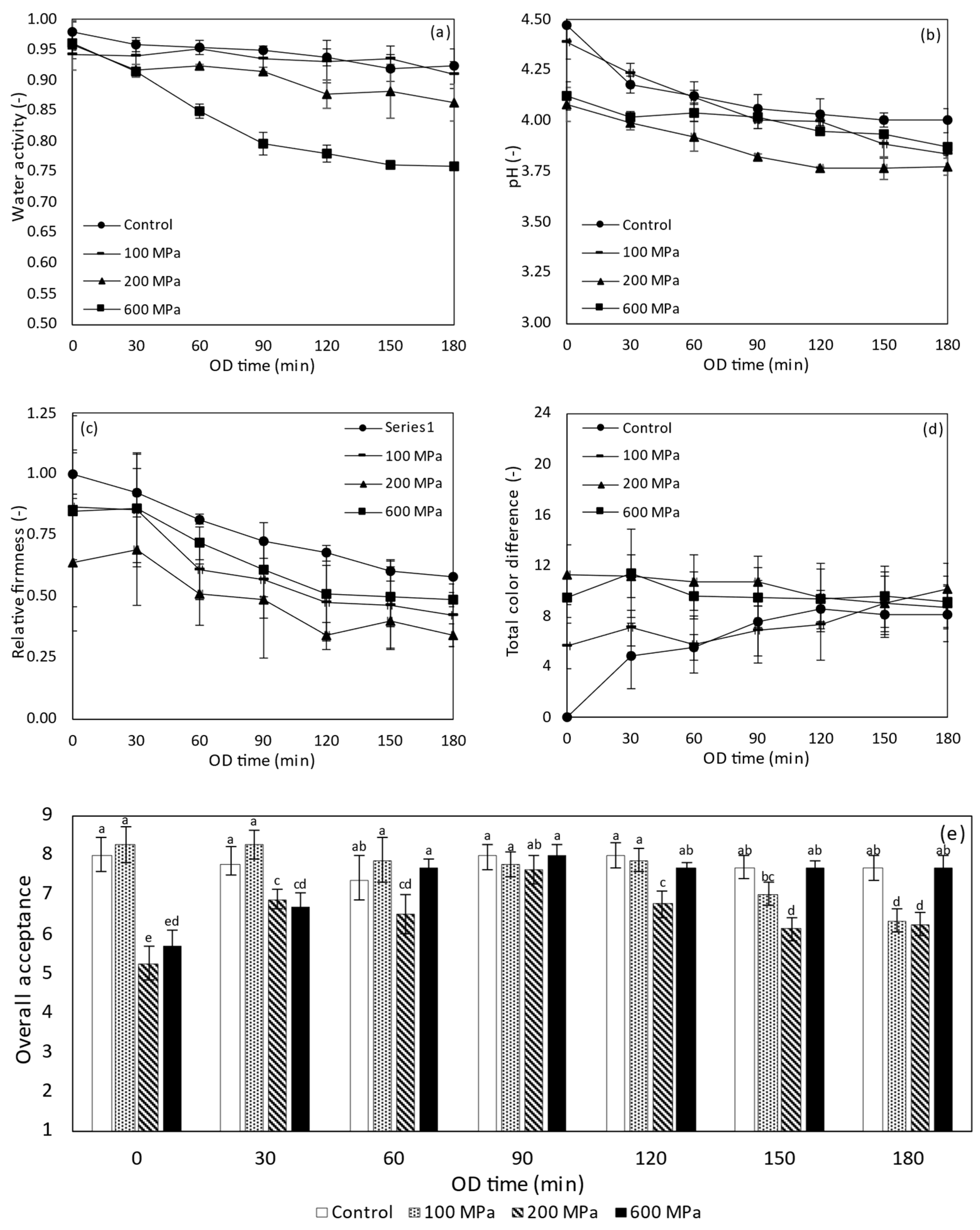
3.4.2. Water Activity, pH and Titratable Acidity of PEF Treated Tomatoes during OD
3.4.3. Objective Firmness and Color of HP Treated Tomatoes during OD
3.4.4. Sensory Evaluation of HP Treated Tomatoes during OD
3.4.5. Selection of the Appropriate Treatment Conditions
3.5. Shelf Life of Untreated and Treated Tomato Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, I.; Qazi, I.M.; Jamal, S. Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov. Food Sci. Emerg. Technol. 2016, 34, 29–43. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Kowalska, H.; Czajkowska, K.; Lenart, A. Osmotic dehydration in production of sustainable and healthy food. Trends Food Sci. Technol. 2016, 50, 186–192. [Google Scholar] [CrossRef]
- Lončar, B.; Nićetin, M.; Filipović, V.; Knežević, V.; Pezo, L.; Šuput, D.; Kuljanin, T. Osmotic dehydration in sugarbeet molasses-Food safety and quality benefits. J. Hyg. Eng. Des. 2021, 34, 16–20. [Google Scholar]
- Chang, C.-K.; Tsai, S.-Y.; Gavahian, M.; Cheng, K.-C.; Hou, C.-Y.; Yudhistira, B.; Lin, S.-H.; Santoso, S.P.; Hsieh, C.-W. Direct and alternating current electric fields affect pectin esterase and cellulase in tomato (Solanum lycopersicum L.) fruit during storage. Postharvest Biol Technol. 2023, 205, 112495. [Google Scholar] [CrossRef]
- Salehi, F. Recent advances in the ultrasound-assisted osmotic dehydration of agricultural products: A review. Food Biosci. 2023, 51, 102307. [Google Scholar] [CrossRef]
- Rastogi, N.K. Developments in osmotic dehydration of foods. In Drying Technology in Food Processing. Unit Operations and Processing Equipment in the Food Industry; Jafari, S.M., Malekjani, N., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 241–304. [Google Scholar] [CrossRef]
- Wiktor, A.; Lammerskitten, A.; Barba, G.; Michalski, M.; Toepfl, S.; Parniakov, O. 1.15-Drying Processes Assisted by PEF for Plant-Based Materials. Innov. Food Process Technol. 2021, 271–280. [Google Scholar] [CrossRef]
- Wiktor, A.; Singh, A.P.; Parniakov, O.; Mykhailyk, V.; Mandal, R.; Witrowa-Rajchert, D. Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow, 1st ed.; Barba, F.J., Parniakov, O., Wiktor, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 155–202. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Dalla Rosa, M. Effect of pulsed electric field (PEF) pre-treatment coupled with osmotic dehydration on physico-chemical characteristics of organic strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of pulsed electric fields to improve product yield and waste valorization in industrial tomato processing. J. Food Eng. 2019, 270, 109778. [Google Scholar] [CrossRef]
- Koch, Y.; Witt, J.; Lammerskitten, A.; Siemer, C.; Toepfl, S. The influence of Pulsed Electric Fields (PEF) on the peeling ability of different fruits and vegetables. J. Food Eng. 2022, 322, 110938. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Wang, C.-Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Mancilla, Y.; Vega-Gálvez, A.; Pérez-Won, M.; Zura, L.; García-Segovia, P.; Di Scala, K. Effect of osmotic dehydration under high hydrostatic pressure on microstructure, functional properties and bioactive compounds of strawberry (Fragaria vesca). Food Bioprocess Technol. 2014, 7, 516–524. [Google Scholar] [CrossRef]
- Crelier, S.; Robert, M.C.; Claude, J.; Juillerat, M.A. Tomato (Lycopersicon esculentum) pectin methylesterase and polygalacturonase behaviors regarding heat- and pressure-induced inactivation. J. Agric. Food Chem. 2001, 49, 5566–5575. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Katsaros, G.; Taoukis, P. Comparison of the application of high pressure and pulsed electric fields technologies on the selective inactivation of endogenous enzymes in tomato products. Innov. Food Sci. Emerg. Technol. 2016, 38, 349–355. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Zachariou, I.; Andreou, V.; Taoukis, P.S. Effect of pulsed electric fields on mass transfer and quality of osmotically dehydrated kiwifruit. Food Bioprod. Proc. 2016, 100, 535–544. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Chalkia, A.; Dimopoulos, G.; Taoukis, P. Combined effect of pulsed electric field and osmotic dehydration pre-treatments on mass transfer and quality of air dried goji berry. Innov. Food Sci. Emerg. Technol. 2018, 49, 106–115. [Google Scholar] [CrossRef]
- Gould, W.A. Tomato Production, Processing and Technology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Dermesonlouoglou, E.K.; Andreou, V.; Alexandrakis, Z.; Katsaros, G.J.; Giannakourou, M.C.; Taoukis, P.S. The hurdle effect of osmotic pretreatment and high-pressure cold pasteurisation on the shelf-life extension of fresh-cut tomatoes. Int. J. Food Sci. Technol. 2017, 52, 916–926. [Google Scholar] [CrossRef]
- Rahaman, A.; Siddeeg, A.; Manzoor, M.F.; Zeng, X.-A.; Ali, S.; Baloch, Z.; Li, J.; Wen, Q.-H. Impact of pulsed electric field treatment on drying kinetics, mass transfer, colour parameters and microstructure of plum. J. Food Sci. Technol. 2019, 56, 2670–2678. [Google Scholar] [CrossRef]
- Artes, F.; Conesa, M.A.; Hernandez, S.; Gil, M.I. Keeping quality of fresh-cut tomato. Postharvest Biol. Technol. 1999, 17, 153–162. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Clavería, R.; Quispe, I.; Vergara, J.; Uribe, E.; Paez, H.; Di Scala, K. Effect of air temperature on drying kinetics and quality characteristics of osmo-treated jumbo squid (Dosidicus gigas). Food Sci. Technol. 2011, 44, 16–23. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. Mathematics of predictive food microbiology. Int. J. Food Microbiol. 1995, 26, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.A.; Gertheiss, J.; Tomasevic, I.; Engelkes, C.; Glaesener, T.; Meyer, J.; Schafer, A.; Wiesen, R.; Morlein, D. Human perception of color differences using computer vision system measurements of raw pork loin. Meat Sci. 2022, 188, 108766. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (aw) on Microbial Stability: As a Hurdle in Food Preservation (Chapter). In Water Activity in Foods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Kempkes, M.A. Industrial pulsed electric field systems. In Handbook of Electroporation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–21. [Google Scholar]
- Cacace, F.; Bottani, E.; Rizzi, A.; Vignali, G. Evaluation of the economic and environmental sustainability of high pressure processing of foods. Innov. Food Sci. Emerg. Technol. 2020, 60, 102281. [Google Scholar] [CrossRef]

| Treatment | Deff Water Loss (m2/s · 10−10) | R2 | Deff Solids Gain (m2/s · 10−10) | R2 | DEI |
|---|---|---|---|---|---|
| Control | 5.17 ± 0.69 a | 0.942 | 3.82 ± 0.42 a | 0.899 | 1.35 ± 0.03 a |
| 20 pulses | 6.16 ± 0.67 ab | 0.945 | 4.10 ± 0.35 ab | 0.855 | 1.46 ± 0.04 b |
| 50 pulses | 6.75 ± 0.52 bc | 0.972 | 4.64 ± 0.39 b | 0.896 | 1.50 ± 0.01 b |
| 100 pulses | 7.91 ± 0.62 c | 0.982 | 4.52 ± 0.40 b | 0.820 | 1.62 ± 0.02 c |
| 300 pulses | 7.40 ± 0.54 c | 0.951 | 4.58 ± 0.43 b | 0.871 | 1.75 ± 0.03 d |
| Treatment | Deff Water Loss (m2/s · 10−10) | R2 | Deff Solids Gain (m2/s · 10−10) | R2 | DEI |
|---|---|---|---|---|---|
| Control | 5.17 ± 0.69 a | 0.955 | 3.82 ± 0.42 ab | 0.920 | 1.35 ± 0.03 a |
| 100 MPa/5 min | 5.74 ± 0.67 a | 0.975 | 3.50 ± 0.70 a | 0.855 | 1.64 ± 0.14 b |
| 200 MPa/5 min | 6.15 ± 0.52 a | 0.966 | 4.61 ± 0.79 ab | 0.896 | 1.33 ± 0.12 a |
| 600 MPa/5 min | 7.40 ± 0.54 b | 0.966 | 5.07 ± 0.85 bc | 0.874 | 1.46 ± 0.14 ab |
| T (°C) | Control | OD | PEF-OD | HP-OD |
|---|---|---|---|---|
| 0 | 44.7 ± 3.2 j | - | - | - |
| 5 | 9.8 ± 0.6 bc | 37.8 ± 3.9 i | 44.8 ± 4.5 j | 50.4 ± 5.3 k |
| 10 | 4.2 ± 0.1 a | 22.6 ± 3.2 fg | 26.3 ± 3.6 gh | 29.6 ± 3.0 h |
| 15 | - | 10.9 ± 3.1 cd | 15.7 ± 2.7 de | 17.7 ± 1.9 ef |
| 25 | - | 5.0 ± 1.1 ab | 5.8 ± 1.7 abc | 6.7 ± 0.7 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsimichas, A.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Modelling and Evaluation of the Effect of Pulsed Electric Fields and High Pressure Processing Conditions on the Quality Parameters of Osmotically Dehydrated Tomatoes. Appl. Sci. 2023, 13, 11397. https://doi.org/10.3390/app132011397
Katsimichas A, Dimopoulos G, Dermesonlouoglou E, Taoukis P. Modelling and Evaluation of the Effect of Pulsed Electric Fields and High Pressure Processing Conditions on the Quality Parameters of Osmotically Dehydrated Tomatoes. Applied Sciences. 2023; 13(20):11397. https://doi.org/10.3390/app132011397
Chicago/Turabian StyleKatsimichas, Alexandros, George Dimopoulos, Efimia Dermesonlouoglou, and Petros Taoukis. 2023. "Modelling and Evaluation of the Effect of Pulsed Electric Fields and High Pressure Processing Conditions on the Quality Parameters of Osmotically Dehydrated Tomatoes" Applied Sciences 13, no. 20: 11397. https://doi.org/10.3390/app132011397






