Direct Temperature Measurements of Cardiac Stent during MRI Examinations
Abstract
:1. Introduction
2. Materials and Methods
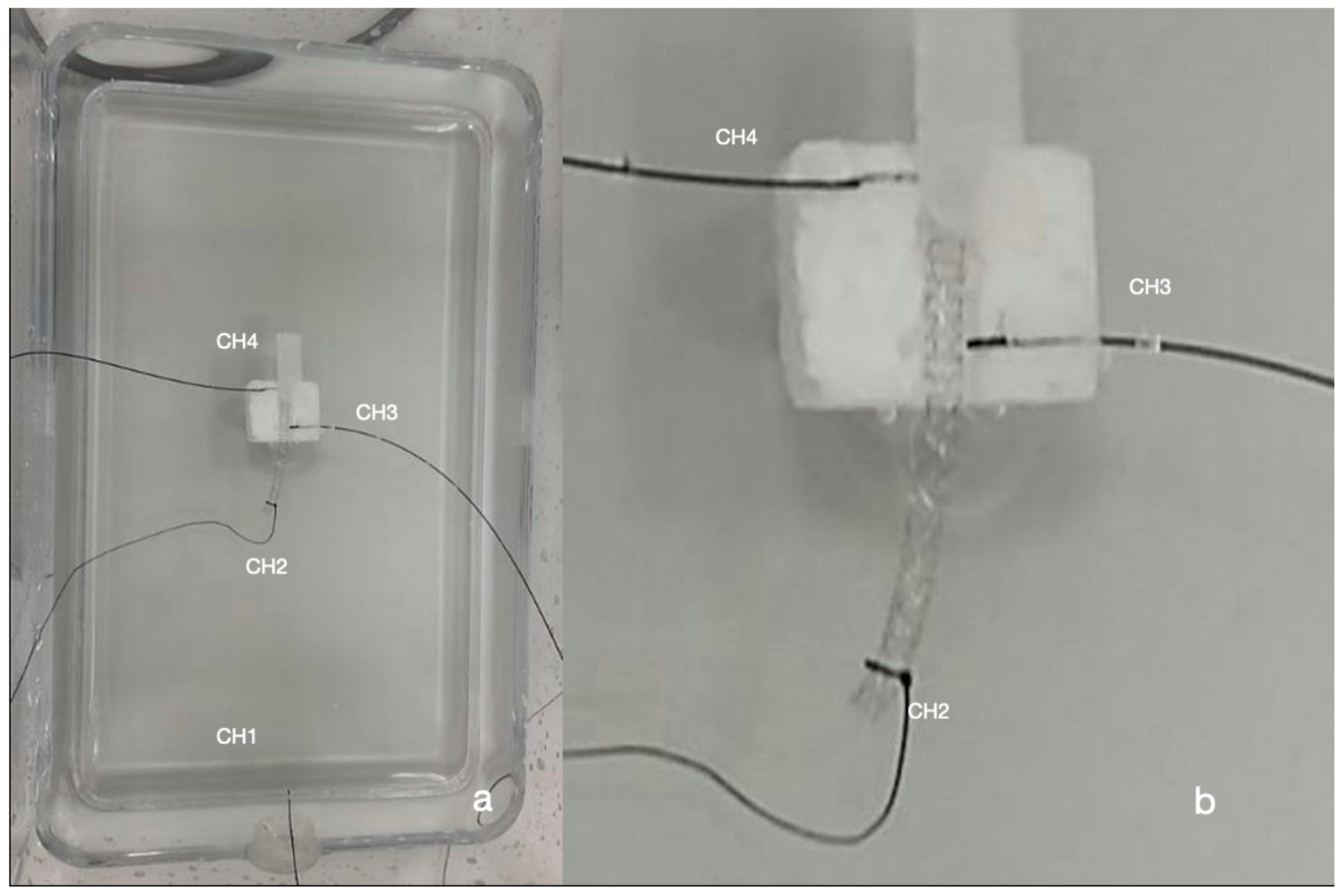
2.1. Temperature Measurements
2.2. Exposure System
2.3. Temperature Data Analysis and SAR Computation
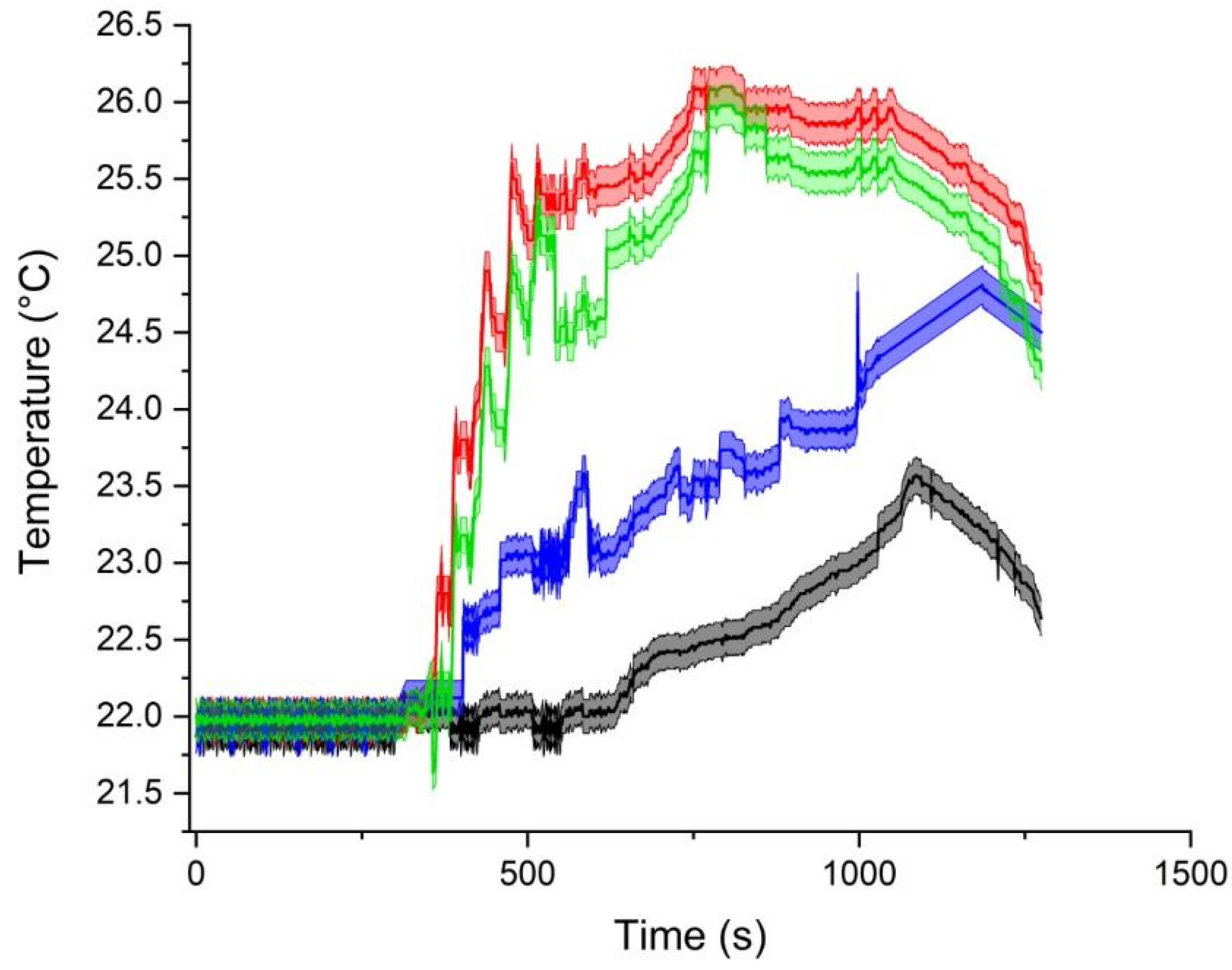
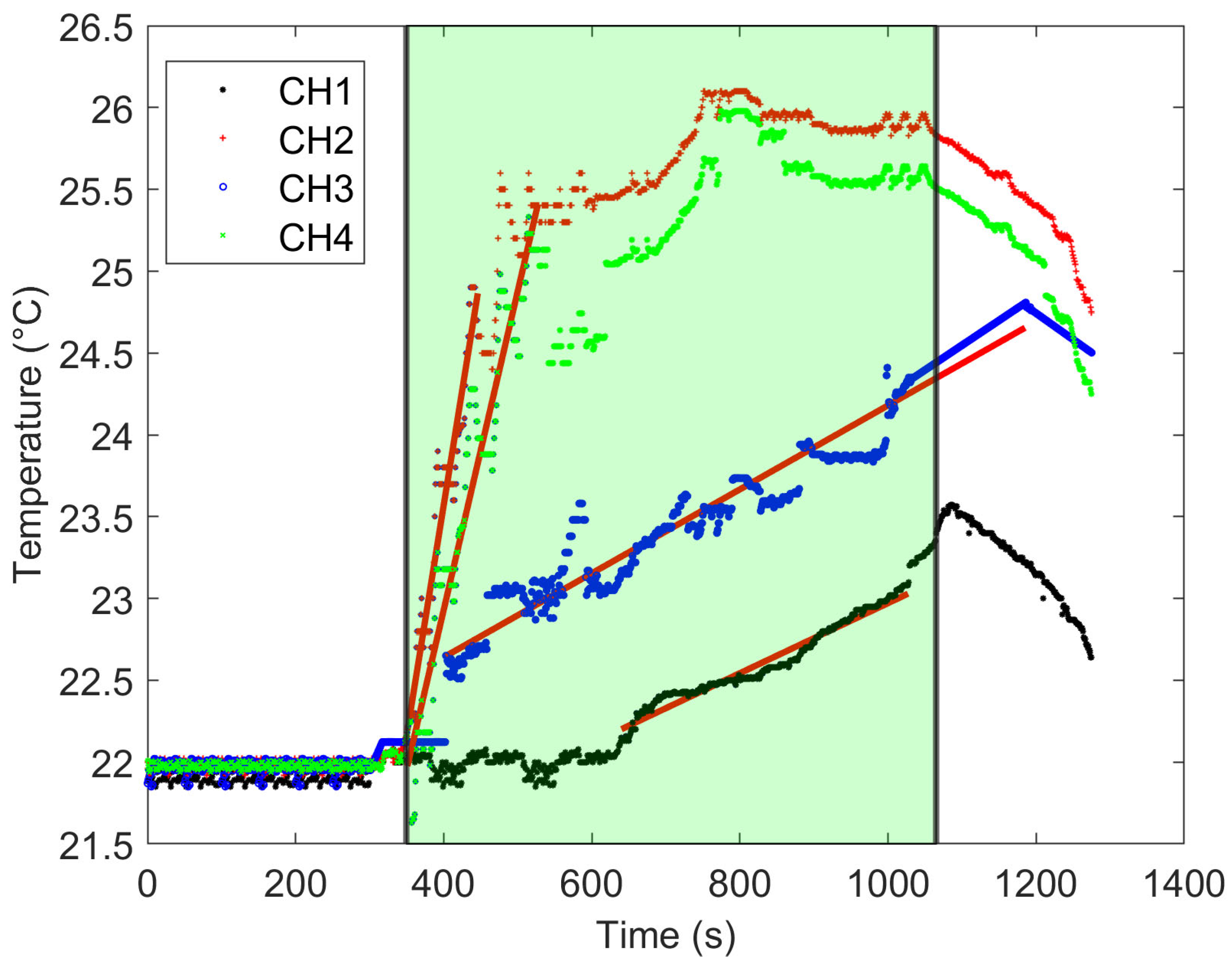
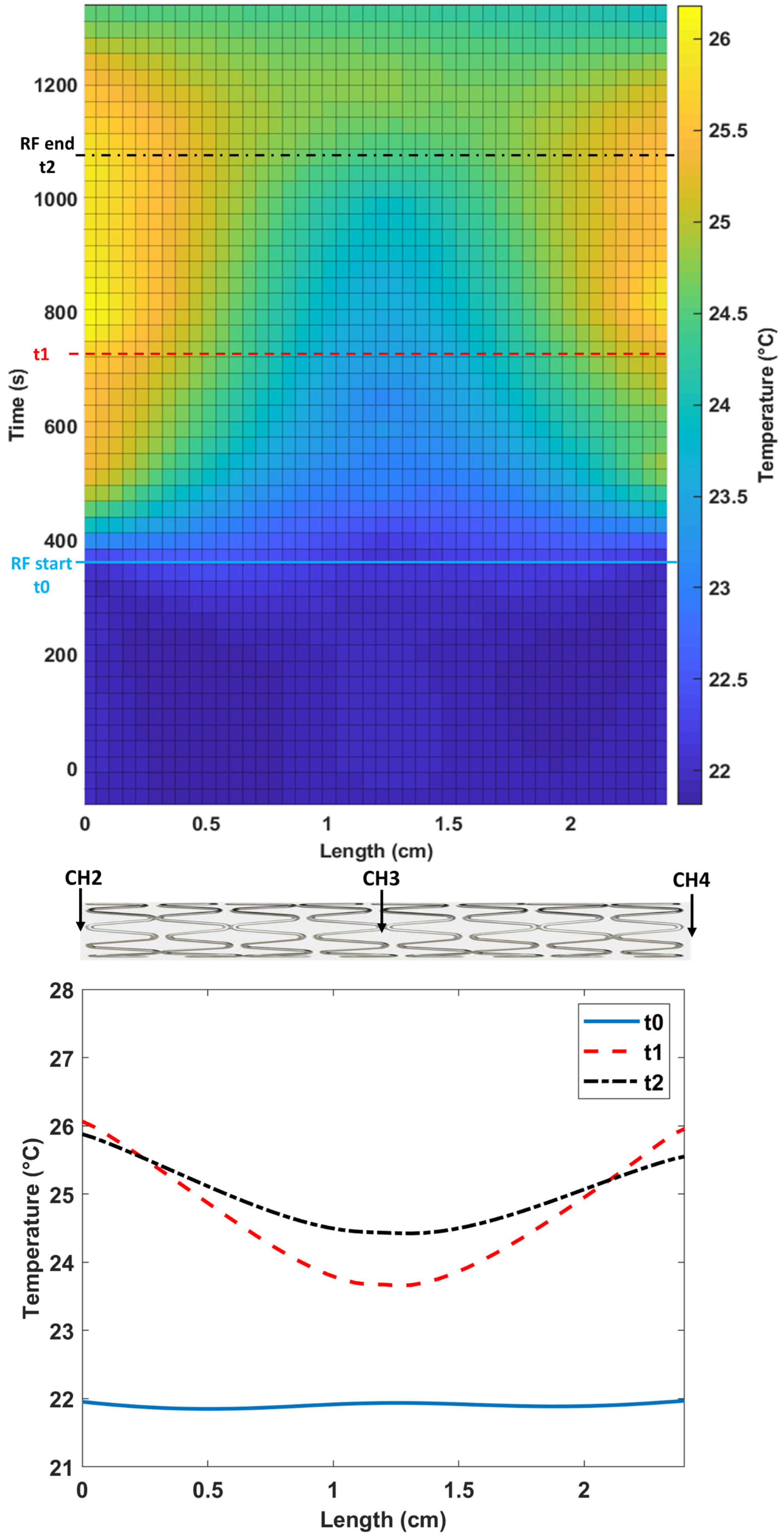
3. Results
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McRobbie, D.W. Essentials of MRI Safety; Wiley-Blackwell: Hoboken, NJ, USA, 2020; ISBN 9781119557173. [Google Scholar]
- Acri, G.; Testagrossa, B.; Vermiglio, G. Personal time-varying magnetic fields evaluation during activities in MRI sites. IFMBE Proc. 2015, 51, 741–744. [Google Scholar] [CrossRef]
- Maralani, P.J.; Schieda, N.; Hecht, E.M.; Litt, H.; Hindman, N.; Heyn, C.; Davenport, M.S.; Zaharchuk, G.; Hess, C.P.; Weinreb, J. MRI safety and devices: An update and expert consensus. J. Magn. Reson. Imaging 2019, 51, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Acri, G.; Inferrera, P.; Denaro, L.; Sansotta, C.; Ruello, E.; Anfuso, C.; Salmeri, F.M.; Garreffa, G.; Vermiglio, G.; Testagrossa, B. dB/dt Evaluation in MRI Sites: Is ICNIRP Threshold Limit (for Workers) Exceeded? Int. J. Environ. Res. Public Health 2018, 15, 1298. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, V.; Sansotta, C.; Morelli, M.S.; Testagrossa, B.; Acri, G. Occupational Exposure Assessment of the Static Magnetic Field Generated by Nuclear Magnetic Resonance Spectroscopy: A Case Study. Int. J. Environ. Res. Public Health 2022, 19, 7674. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef] [PubMed]
- van Rhoon, G.C.; Samaras, T.; Yarmolenko, P.S.; Dewhirst, M.W.; Neufeld, E.; Kuster, N. CEM43°C thermal dose thresholds: A potential guide for magnetic resonance radiofrequency exposure levels? Eur. Radiol. 2013, 23, 2215–2227. [Google Scholar] [CrossRef]
- Baikoussis, N.G.; Apostolakis, E.; Papakonstantinou, N.A.; Sarantitis, I.; Dougenis, D. Safety of Magnetic Resonance Imaging in Patients With Implanted Cardiac Prostheses and Metallic Cardiovascular Electronic Devices. Ann. Thorac. Surg. 2011, 91, 2006–2011. [Google Scholar] [CrossRef]
- Marcu, C.B.; Beek, A.M.; van Rossum, A.C. Clinical applications of cardiovascular magnetic resonance imaging. CMAJ Can. Med. Assoc. J. 2006, 175, 911–917. [Google Scholar] [CrossRef]
- Roguin, A.; Schwitter, J.; Vahlhaus, C.; Lombardi, M.; Brugada, J.; Vardas, P.; Auricchio, A.; Priori, S.; Sommer, T. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace 2008, 10, 336–346. [Google Scholar] [CrossRef]
- Pieri, C.; Bhuva, A.; Moralee, R.; Abiodun, A.; Gopalan, D.; Roditi, G.H.; Moon, J.C.; Manisty, C. Access to MRI for patients with cardiac pacemakers and implantable cardioverter defibrillators. Open Heart 2021, 8, e001598. [Google Scholar] [CrossRef]
- Nitz, W.R.; Oppelt, A.; Renz, W.; Manke, C.; Lenhart, M.; Link, J. On the heating of linear conductive structures as guide wires and catheters in interventional MRI. J. Magn. Reson. Imaging 2001, 13, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M.; Tkach, J.; Phillips, M.; Baker, K.; Shellock, F.G.; Rezai, A.R. Permanent Neurological Deficit Related to Magnetic Resonance Imaging in a Patient with Implanted Deep Brain Stimulation Electrodes for Parkinson’s Disease: Case Report. Neurosurgery 2005, 57, E1063. [Google Scholar] [CrossRef] [PubMed]
- Aissani, S.; Laistler, E.; Felblinger, J. MR safety assessment of active implantable medical devices. Radiologe 2019, 59, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Regulation 2017/745 of the European Parliament of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation EC n. 178/2002 and Regulation EC n. 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745 (accessed on 9 September 2022).
- Mattei, E.; Censi, F.; Calcagnini, G.; Falsaperla, R. Workers with Cardiac AIMD Exposed to EMF: Methods and Case Studies for Risk Analysis in the Framework of the European Regulations. Int. J. Environ. Res. Public Health 2021, 18, 9709. [Google Scholar] [CrossRef] [PubMed]
- ASTM F2182-19e2; Standard Test Method for Measurement of Radio Frequency Induced Heating on or Near Passive Implants during Magnetic Resonance Imaging. ASTM International: West Conshohocken, PA, USA, 2019.
- ISO/TS 10974; Assessment of the Safety of Magnetic Resonance Imaging of Patients with an Active Implantable Medical Device. International Organization for Standardization: Geneva, Switzerland, 2018.
- Bonanno, L.; Marino, S.; Morabito, R.; Barbalace, G.; Sestito, A.; Testagrossa, B.; Acri, G. Evaluation of US and MRI techniques for carotid stenosis: A novel phantom approach. Radiol. Medica 2019, 124, 368–374. [Google Scholar] [CrossRef]
- Och, J.G.; Clarke, G.D.; Sobol, W.T.; Rosen, C.W.; Mun, S.K. Acceptance testing of magnetic resonance imaging systems: Report of AAPM Nuclear Magnetic Resonance Task Group No.6. Med. Phys. 1992, 19, 217–229. [Google Scholar] [CrossRef]
- Acri, G.; Gurgone, S.; Iovane, C.; Romeo, M.B.; Borzelli, D.; Testagrossa, B. A Novel Phantom and a Dedicated Developed Software for Image Quality Controls in X-Ray Intraoral Devices. J. Biomed. Phys. Eng. 2021, 11, 151–162. [Google Scholar] [CrossRef]
- Winter, L.; Oberacker, E.; Özerdem, C.; Ji, Y.; von Knobelsdorff-Brenkenhoff, F.; Weidemann, G.; Ittermann, B.; Seifert, F.; Niendorf, T. On the RF heating of coronary stents at 7.0 Tesla MRI. Magn. Reson. Med. 2015, 74, 999–1010. [Google Scholar] [CrossRef]
- Santoro, D.; Winter, L.; Müller, A.; Vogt, J.; Renz, W.; Özerdem, C.; Grässl, A.; Tkachenko, V.; Schulz-Menger, J.; Niendorf, T. Detailing Radio Frequency Heating Induced by Coronary Stents: A 7.0 Tesla Magnetic Resonance Study. PLoS ONE 2012, 7, e49963. [Google Scholar] [CrossRef]
- Vrtnik, S.; Wencka, M.; Jelen, A.; Kim, H.J.; Dolinšek, J. Coronary stent as a tubular flow heater in magnetic resonance imaging. J. Anal. Sci. Technol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Bassen, H.; Kainz, W.; Mendoza, G.; Kellom, T. MRI-induced heating of selected thin wire metallic implants—Laboratory and computational studies—Findings and new questions raised. Minim. Invasive Ther. Allied Technol. 2006, 15, 76–84. [Google Scholar] [CrossRef]
- Gross, D.C.; Simonetti, O.P. In vivo and post-mortem measurements of radio frequency induced heating during MRI of pigs implanted with vascular stents. J. Cardiovasc. Magn. Reson. 2016, 18, P53. [Google Scholar] [CrossRef]
- EM and Thermal Tissue Database. Available online: https://itis.swiss/virtual-population/tissue-properties/database/database-summary/ (accessed on 17 October 2023).
- Mattei, E.; Triventi, M.; Calcagnini, G.; Censi, F.; Kainz, W.; Mendoza, G.; Bassen, H.I.; Bartolini, P. Complexity of MRI induced heating on metallic leads: Experimental measure-ments of 374 configurations. Biomed. Eng. Online 2008, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Keller, W.; Borkowski, A. Thin plate spline interpolation. J. Geodesy 2019, 93, 1251–1269. [Google Scholar] [CrossRef]
- Panych, L.P.; Madore, B. The physics of MRI safety. J. Magn. Reson. Imaging 2018, 47, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Seifert, F.; Zilberti, L.; Murbach, M.; Ittermann, B. MRI-Related Heating of Implants and Devices: A Review. J. Magn. Reson. Imaging 2021, 53, 1646–1665. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Angelone, L.M.; Lucano, E.; Rajan, S.S.; Iacono, M.I. Radio-Frequency Safety Assessment of Stents in Blood Vessels During Magnetic Resonance Imaging. Front. Physiol. 2018, 9, 1439. [Google Scholar] [CrossRef]
- Scandling, B. Radio Frequency Induced Heating of a Medical Device with Vascular Flow Conditions. Bachelor’s Thesis, The Ohio State University, Columbus, OH, USA, 2016. [Google Scholar]
- Price, R.; Allison, J.; Clarke, G.; Dennis, M.; Keener, C.; Masten, J.; Nessaiver, M.; Och, J.; Reeve, D. 2015 MRI Quality Control Manual; American College of Radiology: Reston, VA, USA, 2015. [Google Scholar]
- Stupic, K.F.; Ainslie, M.; Boss, M.A.; Charles, C.; Dienstfrey, A.M.; Evelhoch, J.L.; Finn, P.; Gimbutas, Z.; Gunter, J.L.; Hill, D.L.G.; et al. A standard system phantom for magnetic resonance imaging. Magn. Reson. Med. 2021, 86, 1194–1211. [Google Scholar] [CrossRef]
- Hellerbach, A.; Schuster, V.; Jansen, A.; Sommer, J. MRI Phantoms—Are There Alternatives to Agar? PLoS ONE 2013, 8, e70343. [Google Scholar] [CrossRef]
- Keenan, K.E.; Ainslie, M.; Barker, A.J.; Boss, M.A.; Cecil, K.M.; Charles, C.; Chenevert, T.L.; Clarke, L.; Evelhoch, J.L.; Finn, P.; et al. Quantitative magnetic resonance imaging phantoms: A review and the need for a system phantom. Magn. Reson. Med. 2017, 79, 48–61. [Google Scholar] [CrossRef]
- Park, S.; Nyenhuis, J.; Smith, C.; Lim, E.; Foster, K.; Baker, K.; Hrdlicka, G.; Rezai, A.; Ruggieri, P.; Sharan, A.; et al. Gelled versus nongelled phantom material for measurement of MRI-induced temperature increases with bioimplants. IEEE Trans. Magn. 2003, 39, 3367–3371. [Google Scholar] [CrossRef]
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Scan duration (s) | 19 | 29 | 40 | 43 | 50 | 23 | 246 | 102 | 130 | 18 |
| Whole body SAR/level (W/kg) | <0.2/normal | <0.2/normal | <0.2/normal | <0.2/normal | <0.2/normal | <0.2/normal | <0.1/normal | <0.1/normal | <0.1/normal | <0.1/normal |
| Max B1 + rms (uT) | 2.38 | 2.34 | 2.39 | 2.38 | 2.34 | 2.33 | 1.60 | 1.28 | 1.81 | 1.54 |
| Sequence | T2W_TSE_Cor_BH | T2W_SPAIR_Cor_BH | T2W_TSE_BH | TW2_SPAIR_BH | BTFE_BH | Dual_FFE_BH | 3D_MRCP_RT | e-THRIVE_dyn_BH | DWI_3b_nav | E_THRIVE_BH |
| TR (ms) | 741 | 14,508 | 20,190 | 21,495 | 3.2 | 146 | 1190 | 3.0 | 6198 | 3.0 |
| TE (ms) | 80 | 80 | 90 | 90 | 1.58 | 1.15 | 600 | 1.43 | 61 | 1.43 |
| Voxel (mm) | 1.30 × 1.50 × 6.00 | 1.70 × 1.91 × 6.00 | 1.20 × 1.40 × 6.00 | 1.20 × 1.40 × 6.00 | 1.59 × 1.70 × 6.00 | 1.99 × 1.90 × 7 | 1.00 × 1.25 × 1.80 | 1.49 × 1.50 × 3.00 | 3.02 × 3.07 × 6.00 | 1.49 × 1.50 × 3.00 |
| FOV (mm) | 375 × 375 × 174 | 375 × 450 × 174 | 375 × 332 × 237 | 375 × 332 × 244 | 375 × 328 × 244 | 375 × 297 × 199 | 260 × 300 × 81 | 375 × 299 × 200 | 375 × 313 × 244 | 375 × 299 × 200 |
| Slice thickness (mm) | 6 | 6 | 6 | 6 | 6 | 7 | 1.80 | 3 | 6 | 3 |
| Number of slices | 25 | 35 | 34 | 35 | 35 | 25 | na | 133 | 35 | 133 |
| Acquisition matrix | 288 × 250 | 220 × 236 | 312 × 237 | 312 × 237 | 236 × 194 | 188 × 156 | 260 × 240 | 252 × 199 | 124 × 102 | 252 × 199 |
| Bandwidth (Hz) | 586.5 | 502.8 | 381.6 | 381.6 | 2037.1 | 2606.9 | 149.1 | 718.9 | 31.9 | 718.9 |
| Probe | dTmax (°C) | SAR (W/kg) | R Pearson | CEM43°C |
|---|---|---|---|---|
| CH1 | 1.642 | 8.965 | 0.982 | 0.011 |
| CH2 | 4.120 | 116.870 | 0.960 | 0.574 |
| CH3 | 2.845 | 10.739 | 0.971 | 0.059 |
| CH4 | 4.004 | 81.297 | 0.961 | 0.360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acri, G.; Campanella, F.; Vermiglio, G.; Anfuso, C.; Testagrossa, B.; Cavallaro, D.; Urzì, D.; Sanzo, A.; D’Avanzo, M.A.; Hartwig, V. Direct Temperature Measurements of Cardiac Stent during MRI Examinations. Appl. Sci. 2023, 13, 11414. https://doi.org/10.3390/app132011414
Acri G, Campanella F, Vermiglio G, Anfuso C, Testagrossa B, Cavallaro D, Urzì D, Sanzo A, D’Avanzo MA, Hartwig V. Direct Temperature Measurements of Cardiac Stent during MRI Examinations. Applied Sciences. 2023; 13(20):11414. https://doi.org/10.3390/app132011414
Chicago/Turabian StyleAcri, Giuseppe, Francesco Campanella, Giuseppe Vermiglio, Carmelo Anfuso, Barbara Testagrossa, Daniela Cavallaro, Domenica Urzì, Antonio Sanzo, Maria Antonietta D’Avanzo, and Valentina Hartwig. 2023. "Direct Temperature Measurements of Cardiac Stent during MRI Examinations" Applied Sciences 13, no. 20: 11414. https://doi.org/10.3390/app132011414






