1. Introduction
Lymphedema (LE) is a progressive chronic disorder characterized by the accumulation of fluids, proteins, and cell debris in the interstitial compartment, as the result of an impairment of lymphatic functioning. It can affect any part of the body, most commonly the extremities, and in its early phases, it is frequently interpreted as a simple swelling or edema. LE should be clinically distinguished from other causes of extremity edema and enlargement due to fluid retention or osmotic imbalance, skin diseases such as erysipelas, cardiac failure, or venous insufficiency [
1]. It may be congenital (primary) or related to cancer treatments or infectious diseases such as phylariosis (secondary) [
2].
In Western countries, it represents a complication in cancer survivors with a global incidence of 15.5% and a reported rate of up to 60% in women who have undergone breast cancer surgery and radiotherapy [
3]. Major attempts to lower the prevalence of lymphedema have been concentrated on early diagnosis, improvements in surgical procedures for cancer surgeries, and a mix of several imaging modalities that can be performed pre-, intra-, and postoperatively, because there are still few viable therapeutic options. LE remains a chronic condition, characterized by progressive increase in the affected limbs/anatomical areas, with recurrent infections and impaired quality of life.
In recent years, imaging of LE with magnetic resonance and indocyanine green lymphography has led to a significant development. Magnetic resonance lymphography with or without injection of the subcutaneous contrast medium has enabled accurate detection, staging, and characterization of the disease, with identification of aplastic, ipoplastic, and hyperplastic LE patterns allowing doctors to target specific surgical strategies [
4,
5]; indocyanine green lymphography represents a useful tool in surgery as it visualizes superficial lymphatic vessels [
6].
LE has traditionally been treated in conservative ways, including bandages, manual lymphatic drainage, and physiotherapy; however, in recent years, new surgical techniques, namely vascularized lymph-node transfer, and venolymphatic anastomoses have shown promising results for LE treatment and have gained popularity [
7,
8].
Vascularized lymph node transfer consists of the harvesting of superficial lymph nodes and surrounding fat as a tissue flap from a healthy donor site and its implantation into a lymphedematous limb [
9].
A lymph node flap includes lymph nodes and lymphatic-vessel network while avoiding disrupting their normal function. Arterial and venous anastomoses are then created to provide blood supply to the transplanted tissue flap [
9].
A venolymphatic anastomosis, also known as a lymphovenous bypass, is a surgical treatment that involves the creation of an anastomosis between overloaded lymphatic vessels and neighboring venules near the location of the lymphatic obstruction [
10]. This approach is associated with little surgical site morbidity, as the incisions are 1–2 cm in length, but it does require the presence of functioning lymphatic vessels [
11].
Despite the benefits, both of these microsurgical techniques allow a volume reduction of less than 60%, thus requiring further improvement [
12].
Moreover, these surgical approaches are recommended in the early–middle stages of the disease, as in later stages, LE is associated with extensive fibrosis and fat deposition [
13].
A new device called BioBridgeTM (Fibralign Corporation, Union City, CA, USA) (
Figure 1), made up of biocompatible aligned nanofibrillar collagen fibers mimicking a collagen matrix, could improve the benefits of these surgical strategies. BioBridgeTM consists of fibrils of type 1 porcine collagen, produced as ultrathin (1–2 μm) membranes, and further integrated into thin mesh ribbons, with wide surface area and linked cavities, to reinforce soft tissue deficiencies [
14]. Thanks to its peculiar multi-lumen structure, it allows unidirectional capillary flow, driven by hydrostatic and oncotic pressure, which aids the connection between two healthy tissues and the release of growth factors to stimulate lymphatic regeneration [
14]. These grafts are biomimetic, characterized by high mechanical strength, completely absorbable [
15], and designed to facilitate soft tissue strengthening.
The scaffold-aligned collagen fibrils enable endothelial cells to adhere to the scaffold and migrate along it to finally produce functional capillaries within and around the scaffold. These are then integrated into the tissue [
16] (
Figure 2).
According to preliminary research by Huang et al. [
17], BioBridgeTM guides cellular organization, controls endothelium inflammatory response that can actively participate in the phenotypic effects of an abnormal lymphangiogenesis related to fluid accumulation in the pathophysiology of LE [
18] and enhances cell survival following implantation in healthy and ischemic tissues. Collagen scaffolds have been shown to encourage lymphangiogenesis, angiogenesis, and aortigenesis in animal models [
14]. They have also shown a therapeutic and protective function in rat models [
19]; however, the available data on their efficacy in treating lymphedema in human patients in the literature are limited [
19].
We aimed to describe our preliminary experience with adult patients affected by primary LE, who were treated with autologous lymph node transplantation and venolymphatic anastomosis, combined with the positioning of BioBridgeTM, to assess potential lymphatic regeneration.
2. Materials and Methods
This was a retrospective institutional-review-board-approved study, performed following the Helsinki Declaration and its later amendments. We assessed the one-year outcome of three consecutive patients affected by primary LE (
Table 1), who have been previously treated conservatively, with a suboptimal response, and without the desired additional improvement in their quality of life.
All patients were clinically evaluated before the intervention and at one-year follow-up by clinicians experienced in LE management.
Non-contrast MR lymphography was carried out according to our standard protocol using 1.5 Tesla equipment (Magnetom Avanto
fit, Siemens Medical Systems, Erlangen, Germany) with a lower-limb angiographic coil and an eight-channel phased-array body coil on the lower abdomen, with patients positioned supine feet-first [
20] to confirm the diagnosis and assess the LE extent, map the identifiable lymph nodes, and characterize the lymphatic system according to a previously proposed classification [
21,
22]. MR acquisition protocol consisted of T2-weighted high-spatial-resolution turbo spin echo with repetition time (TR) 2870 ms, echo time (TE) 797 ms, field of view (FOV) 380 mm × 380 mm, matrix 358 × 384, slice thickness 1 mm, and a T2-weighted 3D turbo spin echo short tau inversion recovery (STIR) with TR 3000 ms, TE 254 ms, inversion time (TI) 160 m, FOV 460 × 504 mm, matrix 315 × 384, and slice thickness 1 mm [
23].
Indocyanine green fluorescence imaging was used for a pre- and postoperative lymphatic mapping.
Patient 1 showed a complex malformation of the central lymphatics in the abdomen at preoperative non-contrast MR lymphography. He was treated with bilateral inguinal venolymphatic anastomosis with concomitant implantation of BioBridgeTM scaffolds. Five BioBridgeTM tracks were created by tunneling the collagen fibers subcutaneously from the site of intact lymphatic collectors (
Figure 3), to proximal toward the nearest lymph-node basin, using an ENDO CLOSE Trocar Site Closure Device (Covidien). ENDO CLOSE trocars were used to create subcutaneous tunnels, where the BioBridgeTM scaffolds were pulled.
Intra-operative indocyanine green fluorescence lymphography enabled the identification of functioning lymphatic vessels to create anastomoses.
Patient 2 underwent a right inguinal vascularized lymph-node transfer, with the harvesting of autologous lymph nodes from the lateral thoracic site, and the creation of 5 tracks of BioBridgeTM by tunneling the scaffolds subcutaneously in the groin, next to the visualized stenotic lymphatic vessels in the ipsilateral groin. Indocyanine green allowed the identification of functioning lymphatics and BioBridgeTM scaffolds were placed to connect them.
The harvesting flap included lymph nodes clustered in the lateral chest wall, to avoid damaging the lymphatic pathway in the axilla, which is responsible for arm drainage, with flap harvest creation from the soft tissue near the lateral thoracic artery and thoracodorsal arteries [
24].
A precise dissection of the donor location was performed; lymph nodes were then removed along with a portion of the surrounding fat and transplanted into the groin. Utilizing microsurgical methods, an artery and vein were anastomosed with the previously prepared vessels. Suction drainage was placed.
Patient 3 underwent a left popliteal vascularized lymph-node transfer, with the harvesting of autologous lymph nodes from the lateral thoracic site, and the creation of 5 tracks of BioBridgeTM by tunneling the scaffolds subcutaneously, next to the visualized blocked lymphatics in the popliteal cavum. The scaffolds were tunneled from the site of functioning lymphatics toward a proximal site of functioning lymphatics.
Patients suspended all types of conservative treatments and underwent periodic clinical follow-up at 12 months, and a follow-up non-contrast MR lymphography to assess the volume modifications of the affected limbs and the presence of transplanted lymph nodes, and to identify potential lymphangiogenesis.
No patient underwent concurrent liposuction.
MR lymphography images were assessed by a radiologist experienced in LE imaging, who assessed the circumference of the areas affected by LE at the preoperative exam and at the follow-up imaging.
Volume rate excess in comparison to the healthy limb was calculated for patients 2 and 3.
Post BioBridgeTM positioning, lymphangiogenesis was defined as the appearance of new lymphatics in the surgical site, with regular and parallel courses, at follow-up imaging.
Bioimpedance spectroscopy (Imp SFB/bioimpedance device-Impedimex) was performed in the preoperative phase and 1 year after surgery to assess the extracellular fluid component of the total volume of the limbs of interest.
3. Results
No post-surgical complications were reported.
Clinical follow-up identified a significant reduction in the limb sizes, with a marked decrease in the % of excess volume.
Bioimpedance spectroscopy showed a reduction from 10 to 7.5 in patient 1, from 11 to 7.6 in patient 2, and from 8 to 6 in patient 3.
Circumferential measurement of the affected area in the MR lymphography showed a decrease from 37 to 25 cm of the affected region in patient 1, from 42 to 29 cm in the perimalleolar region in patient 2, and from 32 to 28 cm in the perimalleolar region in patient 3. Volume rate excess decreased from 25 to 6% and from 26% to 2% in patients 2 and 3, respectively.
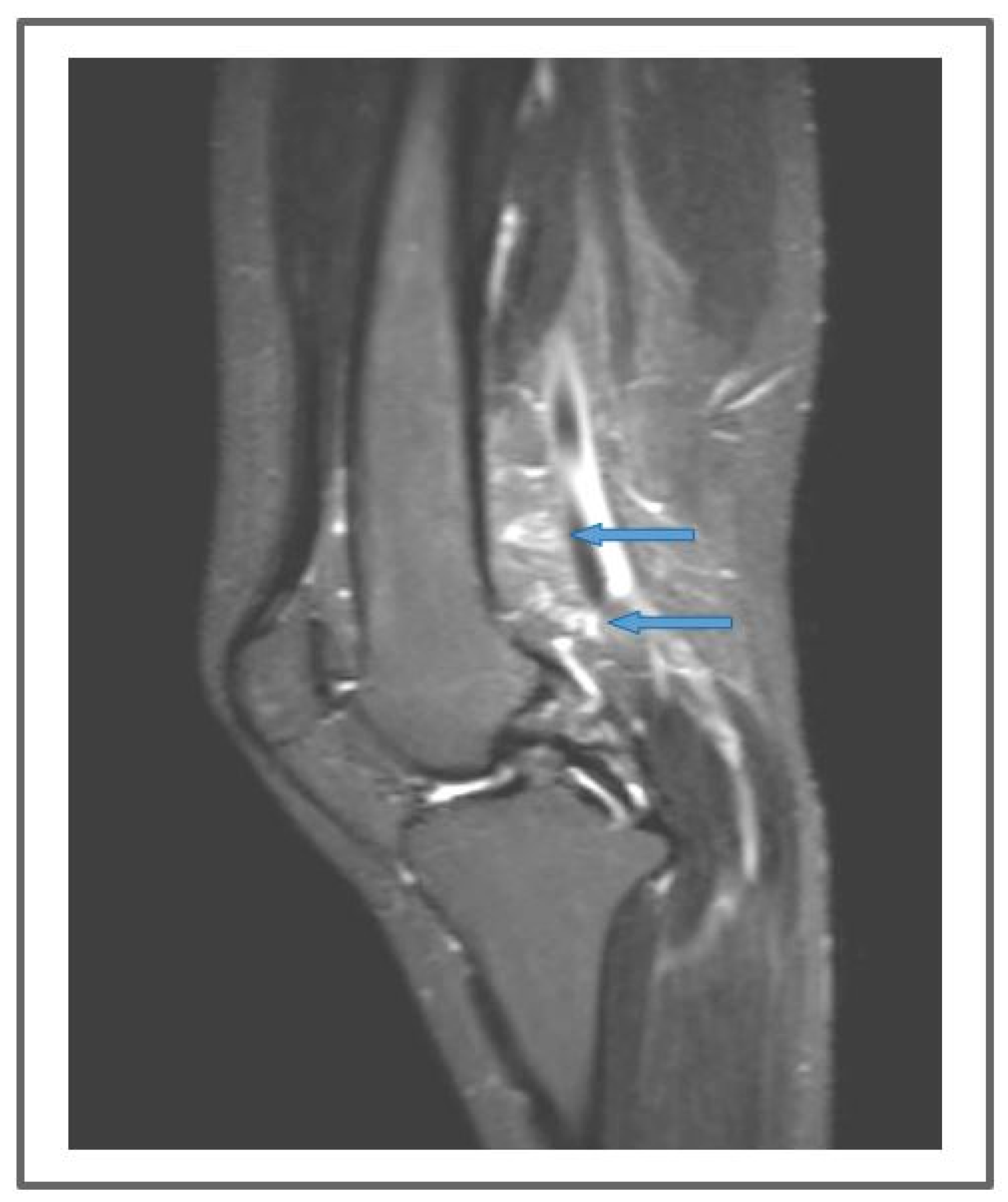
Moreover, imaging showed the appearance of new lymphatic structures in the surgery location with the direction parallel to the implanted BioBridgeTM scaffolds (
Figure 4,
Figure 5 and
Figure 8).
For a comparison (
Figure 10), we also show the preoperative MR lymphography and 1-year follow-up of a 25-year-old woman affected by a right primary LE, treated with venolymphatic anastomosis. The follow-up imaging showed a decrease in the edema, but no new lymphatic vessels were recognizable.
4. Discussion
Microsurgery of LE consisting of venolymphatic anastomoses and vascularized lymph-node transfer has improved the management of LE patients.
In the past, several scaffolds have been applied to help lymphedema patients regain their lymphatic function. In 1908, W. S. Handley suggested utilizing synthetic lymphatic conduits made of sterile silk sutures [
25]. He demonstrated how the subcutaneous implantation of these silk threads into the afflicted limbs assisted lymphatic fluid drainage by capillary action. Although the silk threads had a positive initial effect, they ultimately led to foreign-body reactions that restricted lymphatic fluid drainage shortly after implantation and caused other problems, such as infection. Later, a comparable strategy was started using wicking Teflon multifilament material rather than silk [
26], with a partial benefit. Even while these findings were insufficient to be applied therapeutically as a feasible treatment option, they did support Handley’s hypothesis that the creation of an artificial lymphatic conduit could lead to rapid alleviation of LE.
Interstitial flow has been linked to the growth of new lymphatic capillaries, according to recent research [
27]. The development of lymphatic capillaries that flow in the same direction as the directional interstitial flow occurs first. The BioBridgeTM scaffold might really work by promoting capillary flow, which would make it possible to simultaneously relieve edema and create the ideal environment for lymphangiogenesis. Endothelial cells can thrive in the environment provided by BioBridgeTM.
Our results support the potential application of BioBridgeTM in lymphatic function regeneration. Using non-contrast MR lymphography, we provided both clinical and imaging evidence showing a decrease in the volume of the affected anatomical areas, as well as new lymphatic collectors.
Initial studies by Huang et al. [
17] and Nakayama et al. [
28] provided inferential support for the hypothesis that the addition of collagen scaffolds to autologous lymph-node transfer would provide the necessary support to improve micro-lymphatic vascular engraftment and repair, similarly to its effect on arteriogenesis, through its favorable effect on lymphatic endothelial biology in the repair response.
Chronic scarring is also a known pathologic element of lymphedema in its later stages. In a study by Muthusubramaniam et al., it was discovered that fibril alignment lowers excessive fibroblast proliferation, which in turn minimizes scar formation [
29]. This finding may have clinical implications for lymphedema models.
In a porcine model of acquired lymphedema, Hadamitzky et al. [
30] demonstrated that the implantation of BioBridgeTM aided in the restoration of lymphatic drainage. In the period of observation following BioBridgeTM implantation, the lymphatic tissue was repaired, resulting in restored lymphatic system functionality and reduced fluid stagnation related to the disease. When compared to the surrounding irradiated tissue or to untreated irradiated tissue, immunohistochemical microscopy showed a higher number of lymphatic collectors in the vicinity of the implanted collagen scaffold in all treatment groups. Although there were more blood vessels here as well, the ratio of lymphatic to blood vessels close to the scaffold altered in favor of lymphatics.
In rat models, a recent study looked at the potential of BioBridgeTM to both prevent and alleviate lymphedema. The restoration of lymphatic flow through the collagen scaffolds, in the presence of damaged and interrupted lymphatic channels, allows cellular organization along the graft, resulting in lymphatic regeneration, according to the authors, who speculate that the function of BioBridgeTM in directing capillary flow may be the initial promoter of lymphangiogenesis [
19]. Furthermore, in the rat model, the drainage from the area with damaged lymphatics can be spontaneously repaired by the formation of new functional lymphatic channels, demonstrating that the positioning of BioBridgeTM concurrent with lymphadenectomy inhibits the development of lymphedema. This may present encouraging opportunities for cancer surgery. The use of collagen scaffolds to promote lymphangiogenesis in the surgical routine could improve the outcome of microsurgical procedures in human subjects with lymphedema, with promising results, both in association with vascularized lymph-node transplantation and with venolymphatic anastomoses, leading to a reduction in the L-Dex bioimpedance index and the volume of the affected limbs [
13].
Another study on rat models [
31] utilized mature female Sprague Dawley rats, as the main objective of the models was to generate a situation that resembled the lymphedema condition in women, to assess the effect of BioBridgeTM scaffold implantation in prevention models and treatment models in animals submitted to radiation therapy.
To reduce lymphedema in the rat LE model, Dionyssus et al. used a new propeller vascularized lymphatic tissue flap (pVLNT) in conjunction with BioBridgeTM [
32]: after lymphadenectomy and radiation treatment, 15 female Sprague Dawley rats developed unilateral left hindlimb lymphedema. A skin tunnel was used to transmit an inguinal pVLNT from the unaffected groin to the contralateral groin. The flap was linked to four collagen threads, which were then fan-shaped and implanted beneath the skin of the hind limb. Group A (control), Group B (pVLNT), and Group C (pVLNT + CS) comprised the three study groups. Micro-computed tomography imaging was used to carry out a volumetric examination of both hindlimbs prior to surgery (at the first time point), as well as at 1 and 4 months after surgery. The relative volume difference (excess volume) was assessed for each animal. By using indocyanine green fluoroscopy, lymphatic drainage was evaluated for the quantity and morphology of new collectors as well as the time needed for ICG to travel from the injection point to the midline. The authors observed that a higher volume persisted in group A four months after lymphedema induction, but there was a considerable relative volume decline in groups B (−13.3%) and C (−14%). The viability of pVLNT and the functional repair of lymphatic channels in both the B and C groups was demonstrated by fluoroscopy. Only group C showed statistically significant increases in lymphatic pattern/morphology and lymphatic collector numbers.
In human subjects, BioBridgeTM positioning seems to augment the size reduction of LE limbs compared to vascularized lymph node transplantation or venolymphatic anastomosis alone.
To achieve a better outcome, Deptula et al. [
33] proposed a triple-therapy surgical-treatment algorithm, that incorporated BioBridgeTM, for patients affected by LE stages II and III.
First, liposuction debulking was used to treat patients with stage III lymphedema that had a mostly fibro-adipose component. After a year, patients received lymphatic mapping to look for any obstructed distal superficial lymphatics. If there was a history of cellulitis, patients with obstructed lymphatics were considered candidates for venolymphatic anastomosis with the addition of lymph node transplantation. Stage II patients with fibro-adipose tissue and fluid excess were treated first with a selective liposuction plus physiologic procedure. After surgery, the patients were monitored for 1–2 years to allow for equilibrium. A second liposuction procedure was used to remove any fibro-adipose tissue that was still present. BioBridgeTM implantation was used in cases with persistent volume increase to reduce fluid buildup and further improve lymphatic outflow.
The authors observed that BioBridgeTM positioning helped further reduce the edema and size of the affected limbs, as later-stage LE is characterized by nonfunctional lymphatic channels that run the length of the diseased leg, and these channels were addressed via BioBridgeTM implantation.
A retrospective study by Nguyen et al. included patients who underwent venolymphatic anastomoses and/or vascularized lymph node transfer, followed, or not, by a delayed implant of collagen scaffolds to obtain volume reduction of the affected upper or lower limb. The authors observed a significantly greater edema reduction in the BioBridgeTM cohort, with a major edema reduction in the lymph-node-transfer patient group. They also found the presence of new lymphatic collectors at indocyanine green fluorescence angiography and a decreased dermal backflow at scintigraphy [
34].
The possible mechanism for this is that collagen scaffolds promoted vascular-endothelial-factor release, lymphatic cell migration, and lymphatic network organization along the direction of the lymphatic flow [
27]. This phenomenon is more evident when a lymph node transplantation is performed thanks to the local release of growth factors.
This article describes a preliminary experience, including only three cases, but the use of MR lymphography helped in the confirmation of lymphangiogenesis at follow-up imaging. As already highlighted in a recent literature review, the experiences reported in the literature of the adjunction of nanofibrillar collagen scaffolds included a small number of patients and a high inhomogeneity of patient features, LE stages, surgical techniques, and additional procedures (liposuction) [
14]. Moreover, the studies presented in the literature reported multi-step surgical procedures, with BioBridgeTM positioning after lymph-node transplantation or venolymphatic anastomosis +/− liposuction, whereas we opted for a one-shot surgical technique including nano fibrillar collagen scaffold placement.
Large case series are needed to validate our results and the routine use of BioBridgeTM in the clinical setting and to establish a possible role in LE prevention.