Hormonal Interactions Underlying Rootstock-Induced Vigor Control in Horticultural Crops
Abstract
:1. Introduction
2. Rootstocks Control Scion Morphology
3. Role of Plant Growth Retardants (PGRs) in Plant Development
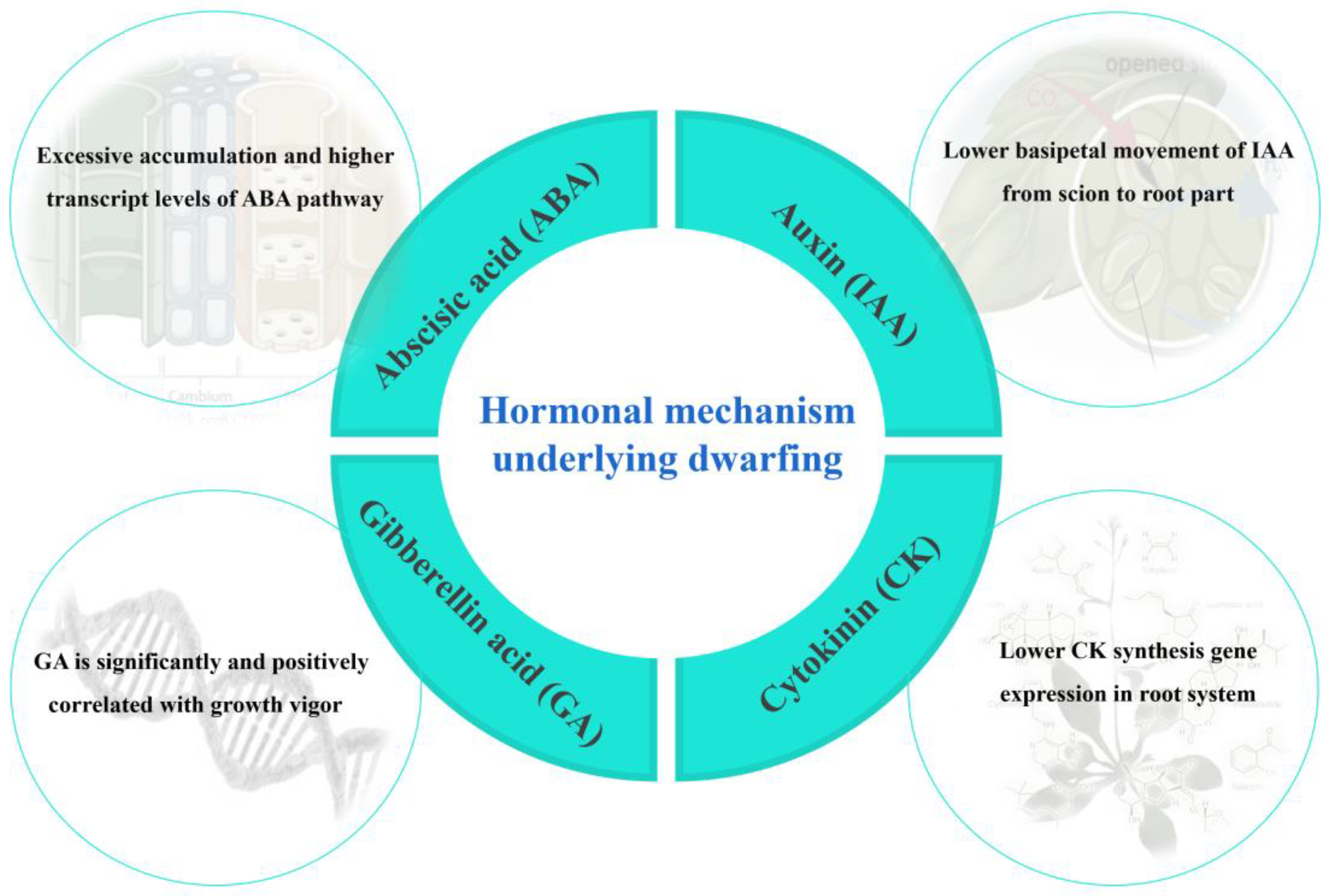
4. Effect of Multiple Plant Hormones in Rootstock-Induced Dwarfing
4.1. Auxin (IAA)
4.2. Gibberellins (GAs)
4.3. Abscisic Acid (ABA)
4.4. Cytokinin (CK)
4.5. Brassinosteroid (BR)
4.6. Phenols
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayat, F.; Iqbal, S.; Coulibaly, D.; Razzaq, M.K.; Nawaz, M.A.; Jiang, W.; Shi, T.; Gao, Z. An insight into dwarfing mechanism: Contribution of scion-rootstock interactions toward fruit crop improvement. Fruit Res. 2021, 1, 3. [Google Scholar] [CrossRef]
- Hayat, F.; Ma, C.; Iqbal, S.; Huang, X.; Omondi, O.K.; Ni, Z.; Shi, T.; Tariq, R.; Khan, U.; Gao, Z. Rootstock-Mediated Transcriptional Changes Associated with Cold Tolerance in Prunus mume Leaves. Horticulturae 2021, 7, 572. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A technique to modify ion accumulation in horticultural crops. Front. Plant Sci. 2016, 7, 1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Habran, A.; Commisso, M.; Helwi, P.; Hilbert, G.; Negri, S.; Ollat, N.; Gomès, E.; Van Leeuwen, C.; Guzzo, F.; Delrot, S. Roostocks/scion/nitrogen interactions affect secondary metabolism in the grape berry. Front. Plant Sci. 2016, 7, 1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.N.; Hayat, F.; Asim, M.; Iqbal, S.; Ashraf, T.; Asghar, S. Influence of citrus rootstocks on growth performance and leaf mineral nutrition of ‘Salustiana’ sweet orange [Citrus sinensis (L). obsek]. J. Pure Appl. Agric. 2020, 5, 46–53. [Google Scholar]
- Hayat, F.; Qiu, C.; Xu, X.; Wang, Y.; Wu, T.; Zhang, X.; Nawaz, M.A.; Han, Z. Rootstocks influence morphological and biochemical changes in young ‘Red Fuji’apple plants. Int. J. Agric. Biol. 2019, 21, 1097–1105. [Google Scholar]
- Hayat, F.; Li, J.; Iqbal, S.; Peng, Y.; Hong, L.; Balal, R.M.; Khan, M.N.; Nawaz, M.A.; Khan, U.; Farhan, M.A.; et al. A mini review of citrus rootstocks and their role in high-density orchards. Plants 2022, 11, 2876. [Google Scholar] [CrossRef]
- Pereira Costa, D.; Sanches Stuchi, E.; Girardi, E.A.; Moreira, A.S.; da Silva Gesteira, A.; Coelho Filho, M.A.; da Silva Ledo, C.A.; da Silva, A.L.V.; de Leão, H.C.; Sampaio Passos, O. Less is more: A hard way to get potential dwarfing hybrid rootstocks for Valencia sweet orange. Agriculture 2021, 11, 354. [Google Scholar] [CrossRef]
- Hayat, F.; Asghar, S.; Yanmin, Z.; Xue, T.; Nawaz, M.A.; Xu, X.; Wang, Y.; Wu, T.; Zhang, X.; Qiu, C.; et al. Rootstock Induced Vigour is Associated with Physiological, Biochemical and Molecular Changes in ‘Red Fuji’Apple. Int. J. Agric. Biol. 2020, 24, 1823–1834. [Google Scholar]
- Brown, P.; Zhang, Q.; Ferguson, L. Influence of rootstock on nutrient acquisition by pistachio. J. Plant Nutr. 1994, 17, 1137–1148. [Google Scholar] [CrossRef]
- Zhang, H.; An, H.S.; Wang, Y.; Zhang, X.Z.; Han, Z.H. Low expression of PIN gene family members is involved in triggering the dwarfing effect in M9 interstem but not in M9 rootstock apple trees. Acta Physiol. Plant. 2015, 37, 104. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, X.; Wu, T.; Xu, X.; Han, Z.; Wang, Y. Methylation effect on IPT5b gene expression determines cytokinin biosynthesis in apple rootstock. Biochem. Biophys. Res. Commun. 2017, 482, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Z.; Shao, J.; Sun, J.; Zha, Q.; Zhang, X. Interaction of MdWRKY24 and MdRGL in Response to Tree Dwarfing in Malus domestica. Agronomy 2022, 12, 2345. [Google Scholar] [CrossRef]
- Chen, Y.; An, X.; Zhao, D.; Li, E.; Ma, R.; Li, Z.; Cheng, C. Transcription profiles reveal sugar and hormone signaling pathways mediating tree branch architecture in apple (Malus domestica Borkh.) grafted on different rootstocks. PloS ONE 2020, 15, e0236530. [Google Scholar] [CrossRef]
- Hao, S.; Lu, Y.; Liu, J.; Bu, Y.; Chen, Q.; Ma, N.; Zhou, Z.; Yao, Y. GIBBERELLIN INSENSITIVE DWARF1 plays an important role in the growth regulation of dwarf apple rootstocks. HortScience 2019, 54, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Underhill, S.J. Expression of gibberellin metabolism genes and signalling components in dwarf phenotype of breadfruit (Artocarpus altilis) plants growing on marang (Artocarpus odoratissimus) rootstocks. Plants 2020, 9, 634. [Google Scholar] [CrossRef]
- Zhou, Y.; Underhill, S.J. Differential transcription pathways associated with rootstock-induced dwarfing in breadfruit (Artocarpus altilis) scions. BMC Plant Biol. 2021, 21, 261. [Google Scholar] [CrossRef]
- Hu, F.; Chen, Z.; Zhao, J.; Wang, X.; Su, W.; Qin, Y.; Hu, G. Differential gene expression between the vigorous and dwarf litchi cultivars based on RNA-Seq transcriptome analysis. PloS ONE 2018, 13, e0208771. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.; Yan, Q.; Zhao, S.; He, F.; Xu, J.; Qi, B.; Zhang, Y. Knockout of the S-acyltransferase gene, PbPAT14, confers the dwarf yellowing phenotype in first generation pear by ABA accumulation. Int. J. Mol. Sci. 2019, 20, 6347. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.-K.; Sun, M.-Y.; Li, J.-M.; Song, B.-B.; Liu, Y.-Y.; Tian, Y.-K.; Wang, C.-H.; Jun, W. Comparative transcriptome analysis provides insights into the mechanism of pear dwarfing. J. Integr. Agric. 2022, 21, 1952–1967. [Google Scholar] [CrossRef]
- Liu, J.-L.; Zhang, C.-X.; Li, T.-T.; Liang, C.-L.; Yang, Y.-J.; Ding-Li, L.; Cui, Z.-H.; Ran, W.; Song, J.-K. Phenotype and mechanism analysis of plant dwarfing in pear regulated by abscisic acid. J. Integr. Agric. 2022, 21, 1346–1356. [Google Scholar] [CrossRef]
- Qi, L.; Chen, L.; Wang, C.; Zhang, S.; Yang, Y.; Liu, J.; Li, D.; Song, J.; Wang, R. Characterization of the auxin efflux transporter PIN proteins in pear. Plants 2020, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ye, X.; Cao, L.; Yu, X.; Qu, S. The regulation of DKGA2ox1 and miR171f_3 in scion dwarfing with Diospyros kaki Thunb. cv.‘Nan-tong-xiao-fang-shi’as interstocks. Planta 2021, 254, 113. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhuang, W.; Tu, X.; Gao, Z.; Xiong, A.; Yu, X.; Li, X.; Li, F.; Qu, S. Transcriptomic analysis of interstock-induced dwarfism in Sweet Persimmon (Diospyros kaki Thunb.). Hortic. Res. 2019, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Joo, J.H.; Bae, Y.S.; Lee, J.S. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001, 126, 1055–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchant, A.; Kargul, J.; May, S.T.; Muller, P.; Delbarre, A.; Perrot-Rechenmann, C.; Bennett, M.J. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999, 18, 2066–2073. [Google Scholar] [CrossRef]
- Moore, I. Gravitropism: Lateral thinking in auxin transport. Curr. Biol. 2002, 12, R452–R454. [Google Scholar] [CrossRef] [Green Version]
- Band, L.R.; Wells, D.M.; Larrieu, A.; Sun, J.; Middleton, A.M.; French, A.P.; Brunoud, G.; Sato, E.M.; Wilson, M.H.; Péret, B. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc. Natl. Acad. Sci. USA 2012, 109, 4668–4673. [Google Scholar] [CrossRef] [Green Version]
- Gray, W.M. Hormonal regulation of plant growth and development. PLoS Biol. 2004, 2, e311. [Google Scholar] [CrossRef] [Green Version]
- Karlidağ, H.; Aslantaş, R.; Eşitken, A. Effects of interstock (M9) length grafted onto MM106 rootstock on sylleptic shoot formation, growth and yield in some apple cultivars. J. Agric. Sci. 2014, 20, 331–336. [Google Scholar]
- Marini, R.P.; Black, B.; Crassweller, R.M.; Domoto, P.A.; Hampson, C.; Johnson, S.; Kosola, K.; McArtney, S.; Masabni, J.; Moran, R.; et al. Performance of ‘golden delicious’ apple on 23 rootstocks at 12 locations: A five-year summary of the 2003 nc-140 dwarf rootstock trial. J. Am. Pomol. Soc. 2009, 63, 115. [Google Scholar]
- Santos, A.; Santos-Ribeiro, R.; Cavalheiro, J.; Cordeiro, V.; Lousada, J.L. Initial growth and fruiting of ‘Summit’sweet cherry (Prunus avium) on five rootstocks. N. Z. J. Crop Hortic. Sci. 2006, 34, 269–277. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Li, J.; Liu, M.-M.; Yao, Q.; Chen, J.-Z. Transcriptome profiling to understand the effect of citrus rootstocks on the growth of ‘Shatangju’mandarin. PLoS ONE 2017, 12, e0169897. [Google Scholar]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernández-Fernández, F.; Harrison, R.J.; Schmidt, S. Else, Felicidad Fernández-Fernández, Richard J. Harrison, and Sonja Schmidt. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weibel, A.; Johnson, R.S.; DeJong, T.M. Comparative vegetative growth responses of two peach cultivars grown on size-controlling versus standard rootstocks. J. Am. Soc. Hortic. Sci. 2003, 128, 463–471. [Google Scholar] [CrossRef]
- Webster, T. Dwarfing rootstocks: Past, present and future. Compact. Fruit Tree 2002, 35, 67–72. [Google Scholar]
- Sitarek, M.; Bartosiewicz, B. Influence of a few seedling rootstocks on the growth, yield and fruit quality of apricot trees. J. Fruit Ornam. Plant Res. 2011, 19, 81–86. [Google Scholar]
- Hayat, F.; Li, J.; Liu, W.; Li, C.; Song, W.; Iqbal, S.; Khan, U.; Umer Javed, H.; Ahsan Altaf, M.; Tu, P.; et al. Influence of Citrus Rootstocks on Scion Growth, Hormone Levels, and Metabolites Profile of ‘Shatangju’ Mandarin (Citrus reticulata Blanco). Horticulturae 2022, 8, 608. [Google Scholar] [CrossRef]
- Bruckner, C.H.; DeJong, T.M. Proposed pre-selection method for identification of dwarfing peach rootstocks based on rapid shoot xylem vessel analysis. Sci. Hortic. 2014, 165, 404–409. [Google Scholar] [CrossRef]
- Umar, I.; Akash, S. Control of height through growth retardants in fruit trees. Asian J. Hortic. 2008, 3, 473–478. [Google Scholar]
- Koukourikou-Petridou, M.A. Paclobutrazol affects the extension growth and the levels of endogenous IAA of almond seedlings. Plant Growth Regul. 1996, 18, 187–190. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, C.; Mao, J.; Song, C.; Ma, J.; Zhang, D.; Han, M.; An, N. Genome-wide identification and expression analysis of brassinosteroid biosynthesis and metabolism genes regulating apple tree shoot and lateral root growth. J. Plant Physiol. 2018, 231, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tahir, M.M.; Li, S.; Tang, T.; Mao, J.; Li, K.; Shao, Y.; Yang, W.; Niu, J.; Zhang, D. Effect of exogenous abscisic acid (ABA) on the morphology, phytohormones, and related gene expression of developing lateral roots in ‘Qingzhen 1’apple plants. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 148, 23–34. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, P.; Jiang, M.; Qu, S. Antioxidant and the dwarfing candidate gene of “Nantongxiaofangshi”(Diospyros kaki Thunb.). Oxidative Med. Cell. Longev. 2019, 2019, 1629845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, T.; Gao, Z.; Wang, L.; Zhang, Z.; Zhuang, W.; Sun, H.; Zhong, W. Identification of differentially-expressed genes associated with pistil abortion in Japanese apricot by genome-wide transcriptional analysis. PLoSe ONE 2012, 7, e47810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, S.R.; Chandel, K.P.S. In vitro conservation of Musa germplasm: Effects of mannitol and temperature on growth and storage. J. Hortic. Sci. 1993, 68, 841–846. [Google Scholar] [CrossRef]
- Davies, P.J. Plant Hormones: Physiology, Biochemistry and Molecular Biology; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Sauer, M.; Robert, S.; Kleine-Vehn, J. Auxin: Simply complicated. J. Exp. Bot. 2013, 64, 2565–2577. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.M.; Zhang, X.; Shah, K.; Hayat, F.; Li, S.; Mao, J.; Liu, Y.; Shao, Y.; Zhang, D. Nitrate application affects root morphology by altering hormonal status and gene expression patterns in B9 apple rootstock nursery plants. Fruit Res. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Allen, J.; Baker, D. Free tryptophan and indole-3-acetic acid levels in the leaves and vascular pathways of Ricinus communis L. Planta 1980, 148, 69–74. [Google Scholar] [CrossRef]
- Bose, T.; Jana, B.; Mukhopadhyay, T. Effects of growth regulators on growth and flowering in Hippeastrum hybridum hort. Sci. Hortic. 1980, 12, 195–200. [Google Scholar] [CrossRef]
- Yu, C.; Dong, W.; Zhan, Y.; Huang, Z.-A.; Li, Z.; Kim, I.S.; Zhang, C. Genome-wide identification and expression analysis of ClLAX, ClPIN and ClABCB genes families in Citrullus lanatus under various abiotic stresses and grafting. BMC Genet. 2017, 18, 33. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Kamiya, T.; Fujiwara, T. Differential roles of PIN1 and PIN2 in root meristem maintenance under low-B conditions in Arabidopsis thaliana. Plant Cell Physiol. 2015, 56, 1205–1214. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Zhang, D.; Zhang, J.; Zheng, L.; Zhao, C.; Ma, J.; An, N.; Han, M. Expression analysis of key auxin synthesis, transport, and metabolism genes in different young dwarfing apple trees. Acta Physiol. Plant. 2016, 38, 43. [Google Scholar] [CrossRef]
- Rudikovskii, A.V.; Stolbicova, A.V.; Rudikovskaya, E.G.; Dudareva, L.V. Role of phytohormones in the formation of dwarf and tall Siberian crabapple (Malus baccata L. Borkh.). Zemdirb.-Agric. 2019, 106, 167–172. [Google Scholar] [CrossRef]
- Lockard, R. Stock and scion growth relationship and the dwarfing mechanism in apple. Hort. Rev. 1981, 3, 315–375. [Google Scholar]
- Kamboj, J.; Quinlan, J.; Baker, D. Identification and quantitation by GC-MS of zeatin and zeatin riboside in xylem sap from rootstock and scion of grafted apple trees. Plant Growth Regul. 1999, 28, 199–205. [Google Scholar] [CrossRef]
- Michalczuk, L. Indole-3-acetic acid level in wood, bark and cambial sap of apple rootstocks differing in growth vigour. Acta Physiol. Plant. 2002, 24, 131–136. [Google Scholar] [CrossRef]
- Soumelidou, K.; Battey, N.; John, P.; Barnett, J. The anatomy of the developing bud union and its relationship to dwarfing in apple. Ann. Bot. 1994, 74, 605–611. [Google Scholar] [CrossRef]
- Benjamins, R.; Quint, A.; Weijers, D.; Hooykaas, P.; Offringa, R. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 2001, 128, 4057–4067. [Google Scholar] [CrossRef] [PubMed]
- Scarpella, E.; Marcos, D.; Friml, J.; Berleth, T. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006, 20, 1015–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Berkel, K.; de Boer, R.J.; Scheres, B.; ten Tusscher, K. Polar auxin transport: Models and mechanisms. Development 2013, 140, 2253–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.L.; Zhang, H.; Yu, C.; Ma, L.; Wang, Y.; Zhang, X.Z.; Han, Z.H. Possible roles of auxin and zeatin for initiating the dwarfing effect of M9 used as apple rootstock or interstock. Acta Physiol. Plant. 2012, 34, 235–244. [Google Scholar] [CrossRef]
- Friml, J.; Vieten, A.; Sauer, M.; Weijers, D.; Schwarz, H.; Hamann, T.; Offringa, R.; Jürgens, G. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature 2003, 426, 147–153. [Google Scholar] [CrossRef]
- Tanaka, H.; Dhonukshe, P.; Brewer, P.; Friml, J. Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell. Mol. Life Sci. CMLS 2006, 63, 2738–2754. [Google Scholar] [CrossRef]
- Viaene, T.; Delwiche, C.F.; Rensing, S.A.; Friml, J. Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci. 2013, 18, 5–10. [Google Scholar] [CrossRef]
- Gan, Z.; Wang, Y.; Wu, T.; Xu, X.; Zhang, X.; Han, Z. MdPIN1b encodes a putative auxin efflux carrier and has different expression patterns in BC and M9 apple rootstocks. Plant Mol. Biol. 2018, 96, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Ueda, J.; Komaki, M.K.; Bell, C.J.; Shimura, Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 1991, 3, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Cazzonelli, C.I.; Vanstraelen, M.; Simon, S.; Yin, K.; Carron-Arthur, A.; Nisar, N.; Tarle, G.; Cuttriss, A.J.; Searle, I.R.; Benkova, E. Role of the Arabidopsis PIN6 auxin transporter in auxin homeostasis and auxin-mediated development. PLoS ONE 2013, 8, e70069. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Liu, Y.; Maere, S.; Lee, E.; Van Isterdael, G.; Xie, Z.; Xuan, W.; Lucas, J.; Vassileva, V.; Kitakura, S. A coherent transcriptional feed-forward motif model for mediating auxin-sensitive PIN3 expression during lateral root development. Nat. Commun. 2015, 6, 8821. [Google Scholar] [CrossRef]
- Webster, A.; Altkinson, C.; Lucas, A.; Vaughan, S.; Taylor, L. Interactions between root restriction, irrigation and rootstock treatments on the growth and cropping of ‘Queen Cox’apple trees: Effects on orchard growth and cropping. J. Hortic. Sci. Biotechnol. 2000, 75, 181–189. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Fang, P.; Ma, C.; White, J.C.; Xiang, Z.; Wang, H.; Zhang, Z.; Rui, Y.; Xing, B. Engineered nanomaterials inhibit Podosphaera pannosa infection on rose leaves by regulating phytohormones. Environ. Res. 2019, 170, 1–6. [Google Scholar] [CrossRef]
- Liu, J.; Sherif, S.M. Hormonal orchestration of bud dormancy cycle in deciduous woody perennials. Front. Plant Sci. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Regnault, T.; Davière, J.-M.; Wild, M.; Sakvarelidze-Achard, L.; Heintz, D.; Carrera Bergua, E.; Lopez Diaz, I.; Gong, F.; Hedden, P.; Achard, P. The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nat. Plants 2015, 1, 15073. [Google Scholar] [CrossRef] [PubMed]
- Bulley, S.M.; Wilson, F.M.; Hedden, P.; Phillips, A.L.; Croker, S.J.; James, D.J. Modification of gibberellin biosynthesis in the grafted apple scion allows control of tree height independent of the rootstock. Plant Biotechnol. J. 2005, 3, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Motosugi, H.; Nishijima, T.; Hiehata, N.; Koshioka, M.; Sugiura, A. Endogenous gibberellins in the xylem exudate from apple trees. Biosci. Biotechnol. Biochem. 1996, 60, 1500–1502. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Löhr, B.; Streitner, C.; Steffen, A.; Lange, T.; Staiger, D. A glycine-rich RNA-binding protein affects gibberellin biosynthesis in Arabidopsis. Mol. Biol. Rep. 2014, 41, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Kamiya, Y. Gibberellin biosynthesis: Enzymes, genes and their regulation. Annu. Rev. Plant Biol. 1997, 48, 431–460. [Google Scholar] [CrossRef]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Fagoaga, C.; Tadeo, F.R.; Iglesias, D.J.; Huerta, L.; Lliso, I.; Vidal, A.M.; Talon, M.; Navarro, L.; García-Martínez, J.L.; Pena, L. Engineering of gibberellin levels in citrus by sense and antisense overexpression of a GA 20-oxidase gene modifies plant architecture. J. Exp. Bot. 2007, 58, 1407–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tworkoski, T.; Fazio, G. Hormone and growth interactions of scions and size-controlling rootstocks of young apple trees. Plant Growth Regul. 2016, 78, 105–119. [Google Scholar] [CrossRef]
- Van Hooijdonk, B.; Woolley, D.; Warrington, I.; Tustin, S. Rootstocks modify scion architecture, endogenous hormones, and root growth of newly grafted ‘Royal Gala’apple trees. J. Am. Soc. Hortic. Sci. 2011, 136, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Hooijdonk, V.; Woolley, D.; Warrington, I.; Tustin, D. Initial alteration of scion architecture by dwarfing apple rootstocks may involve shoot-root-shoot signalling by auxin, gibberellin, and cytokinin. J. Hortic. Sci. Biotechnol. 2010, 85, 59–65. [Google Scholar] [CrossRef]
- Griffiths, J.; Murase, K.; Rieu, I.; Zentella, R.; Zhang, Z.-L.; Powers, S.J.; Gong, F.; Phillips, A.L.; Hedden, P.; Sun, T.-P. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 2006, 18, 3399–3414. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, K.; Ramadan, A.; Arimura, G.-i.; Imai, K.; Tomii, K.; Shinozaki, K.; Sawasaki, T. Tyrosine phosphorylation of the GARU E3 ubiquitin ligase promotes gibberellin signalling by preventing GID1 degradation. Nat. Commun. 2017, 8, 1004. [Google Scholar] [CrossRef] [Green Version]
- Schwechheimer, C. Gibberellin signaling in plants–the extended version. Front. Plant Sci. 2012, 2, 107. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Mei, C.; Song, H.; Shan, D.; Sun, Y.; Hu, Z.; Wang, L.; Zhang, T.; Wang, J.; Kong, J. Potential roles of melatonin and ABA on apple dwarfing in semi-arid area of Xinjiang China. Peer J. 2022, 10, e13008. [Google Scholar] [CrossRef]
- Boss, P.K.; Thomas, M.R. Association of dwarfism and floral induction with a grape ‘green revolution’mutation. Nature 2002, 416, 847–850. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A. Evolution of the stomatal regulation of plant water content. Plant Physiol. 2017, 174, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodger, J.Q.; Schachtman, D.P. Re-examining the role of ABA as the primary long-distance signal produced by water-stressed roots. Plant Signal. Behav. 2010, 5, 1298–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 2014, 202, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Tworkoski, T.; Fazio, G. Effects of size-controlling apple rootstocks on growth, abscisic acid, and hydraulic conductivity of scion of different vigor. Int. J. Fruit Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- Sharp, R.E.; LeNoble, M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002, 53, 33–37. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Gampala, S.S.; Rock, C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell 2002, 14, S15–S45. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, R. Abscisic acid synthesis and response. Arab. Book/Am. Soc. Plant Biol. 2013, 11, e0166. [Google Scholar] [CrossRef] [Green Version]
- Jia, D.; Gong, X.; Li, M.; Li, C.; Sun, T.; Ma, F. Overexpression of a novel apple NAC transcription factor gene, MdNAC1, confers the dwarf phenotype in transgenic apple (Malus domestica). Genes 2018, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Finlayson, S.A. Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiol. 2015, 169, 611–626. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Ahmad, R. Influence of endogenous plant hormones on physiological and growth attributes of Kinnow mandarin grafted on nine rootstocks. J. Plant Growth Regul. 2022, 41, 1254–1264. [Google Scholar] [CrossRef]
- Kamboj, J.; Browning, G.; Blake, P.; Quinlan, J.; Baker, D. GC-MS-SIM analysis of abscisic acid and indole-3-acetic acid in shoot bark of apple rootstocks. Plant Growth Regul. 1999, 28, 21–27. [Google Scholar] [CrossRef]
- Noda, K.; Okuda, H.; Iwagaki, I. Indole acetic acid and abscisic acid levels in new shoots and fibrous roots of citrus scion-rootstock combinations. Sci. Hortic. 2000, 84, 245–254. [Google Scholar] [CrossRef]
- Noiton, D.; Vine, J.; Mullins, M. Effects of serial subculture in vitro on the endogenous levels of indole-3-acetic acid and abscisic acid and rootability in microcuttings of ‘Jonathan’apple. Plant Growth Regul. 1992, 11, 377–383. [Google Scholar] [CrossRef]
- Moghadam, E.G.; Shabani, Z. The relation of endogenous abscisic acid and indole acetic acid on vigor of some selected dwarf mahaleb (Prunus mahaleb L.) genotypes. J. Hortic. For. 2014, 6, 107–111. [Google Scholar]
- Aloni, R. The induction of vascular tissues by auxin. In Plant Hormones; Springer: Berlin, Germany, 2010; pp. 485–518. [Google Scholar]
- Avery, D. Comparisons of fruiting and deblossomed maiden apple trees, and of non-fruiting trees on a dwarfing and an invigorating rootstock. New Phytol. 1969, 68, 323–336. [Google Scholar] [CrossRef]
- Jones, O. Mode-of-Action of Rootstock/Scion Interactions in Apple and Cherry Trees. Int. Workshop Control. Vigor Fruit Trees 1983, 146, 175–182. [Google Scholar] [CrossRef]
- Skene, K.; Antcliff, A. A comparative study of cytokinin levels in bleeding sap of Vitis vinifera (L.) and the two grapevine rootstocks, Salt Creek and 1613. J. Exp. Bot. 1972, 23, 283–293. [Google Scholar] [CrossRef]
- Saidha, T.; Goldschmidt, E.; Monselise, S. Endogenous cytokinins from developing ‘Shamouti’orange fruits derived from leafy and leafless inflorescences. Sci. Hortic. 1985, 26, 35–41. [Google Scholar] [CrossRef]
- Sorce, C.; Massai, R.; Picciarelli, P.; Lorenzi, R. Hormonal relationships in xylem sap of grafted and ungrafted Prunus rootstocks. Sci. Hortic. 2002, 93, 333–342. [Google Scholar] [CrossRef]
- Barry, G.; Rogers, S.; Fraley, R.; Brand, L. Identification of a cloned cytokinin biosynthetic gene. Proc. Natl. Acad. Sci. USA 1984, 81, 4776–4780. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Letham, D.S.; Jameson, P.E.; Zhang, R.; Parker, C.W.; Bandenoch-Jones, J.; Noodén, L.D. Cytokinin biochemistry in relation to leaf senescence: IV. Cytokinin metabolism in soybean explants. Plant Physiol. 1988, 88, 788–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuelson, M.E.; Larsson, C.-M. Nitrate regulation of zeation riboside levels in barley roots: Effects of inhibitors of N assimilation and comparison with ammonium. Plant Sci. 1993, 93, 77–84. [Google Scholar] [CrossRef]
- Takei, K.; Sakakibara, H.; Taniguchi, M.; Sugiyama, T. Nitrogen-dependent accumulation of cytokinins in root and thetranslocation to leaf: Implication of cytokinin species that induces geneexpression of maize responseregulator. Plant Cell Physiol. 2001, 42, 85–93. [Google Scholar] [CrossRef]
- Martineau, B.; Houck, C.M.; Sheehy, R.E.; Hiatt, W.R. Fruit-specific expression of the A. tumefaciens isopentenyl transferase gene in tomato: Effects on fruit ripening and defense-related gene expression in leaves. Plant J. 1994, 5, 11–19. [Google Scholar] [CrossRef]
- Tokunaga, H.; Kojima, M.; Kuroha, T.; Ishida, T.; Sugimoto, K.; Kiba, T.; Sakakibara, H. Arabidopsis lonely guy (LOG) multiple mutants reveal a central role of the LOG-dependent pathway in cytokinin activation. Plant J. 2012, 69, 355–365. [Google Scholar] [CrossRef]
- Tubbs, F. Growth relations of rootstock and scion in apples. J. Hortic. Sci. 1980, 55, 181–189. [Google Scholar] [CrossRef]
- Abod, S.; Webster, A. Root and shoot growth of newly-transplanted apple trees as affected by rootstock cultivar, defoliation and time after transplanting. J. Hortic. Sci. 1989, 64, 655–666. [Google Scholar] [CrossRef]
- Fujioka, S.; Yokota, T. Biosynthesis and metabolism of brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef]
- Tanabe, S.; Ashikari, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Yano, M.; Yoshimura, A.; Kitano, H.; Matsuoka, M.; Fujisawa, Y. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 2005, 17, 776–790. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Zhang, D.; Li, K.; Liu, Z.; Liu, X.; Song, C.; Li, G.; Zhao, C.; Ma, J.; Han, M. Effect of exogenous Brassinolide (BR) application on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis. Plant Growth Regul. 2017, 82, 391–401. [Google Scholar] [CrossRef]
- Zheng, L.; Ma, J.; Mao, J.; Fan, S.; Zhang, D.; Zhao, C.; An, N.; Han, M. Genome-wide identification of SERK genes in apple and analyses of their role in stress responses and growth. BMC Genom. 2018, 19, 962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Xue, H.; Zhang, L.; Zhang, F.; Ou, C.; Wang, F.; Zhang, Z. Involvement of auxin and brassinosteroid in dwarfism of autotetraploid apple (Malus× domestica). Sci. Rep. 2016, 6, 26719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Ma, J.; Zhang, L.; Gao, C.; Zhang, D.; Zhao, C.; Han, M. Revealing critical mechanisms of BR-mediated apple nursery tree growth using iTRAQ-based proteomic analysis. J. Proteom. 2018, 173, 139–154. [Google Scholar] [CrossRef]
- Yamamoto, R.; Fujioka, S.; Iwamoto, K.; Demura, T.; Takatsuto, S.; Yoshida, S.; Fukuda, H. Co-regulation of brassinosteroid biosynthesis-related genes during xylem cell differentiation. Plant Cell Physiol. 2007, 48, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Vert, G.; Walcher, C.L.; Chory, J.; Nemhauser, J.L. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc. Natl. Acad. Sci. USA 2008, 105, 9829–9834. [Google Scholar] [CrossRef] [Green Version]
- McKay, M.J.; Ross, J.J.; Lawrence, N.L.; Cramp, R.E.; Beveridge, C.A.; Reid, J.B. Control of internode length in Pisum sativum (further evidence for the involvement of indole-3-acetic acid). Plant Physiol. 1994, 106, 1521–1526. [Google Scholar] [CrossRef]
- Kim, T.-W.; Wang, Z.-Y. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu. Rev. Plant Biol. 2010, 61, 681–704. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Zhang, M.; Tan, B.; Jiang, Y.; Zheng, X.; Ye, X.; Guo, Z.; Xiong, T.; Wang, W.; Li, J. A single nucleotide mutation in GID 1c disrupts its interaction with DELLA 1 and causes a GA-insensitive dwarf phenotype in peach. Plant Biotechnol. J. 2019, 17, 1723–1735. [Google Scholar] [CrossRef] [Green Version]
- Prassinos, C.; Ko, J.-H.; Lang, G.; Iezzoni, A.F.; Han, K.-H. Rootstock-induced dwarfing in cherries is caused by differential cessation of terminal meristem growth and is triggered by rootstock-specific gene regulation. Tree Physiol. 2009, 29, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Lockard, R.; Schneider, G.; Kemp, T. Phenolic Compounds in Two Size-controlling Apple Rootstocks1. J. Am. Soc. Hortic. Sci. 1982, 107, 183–186. [Google Scholar] [CrossRef]
- Moghadam, E.G.; Khalighi, A. Relationship between vigor of Iranian Prunus mahaleb L. selected dwarf rootstocks and some morphological characters. Sci. Hortic. 2007, 111, 209–212. [Google Scholar] [CrossRef]
- Mendel, K.; Cohen, A. Methods for the rapid evaluation of rootstocks for citrus; Ministry of Agriculture, Agricultural Research Station: 1962. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C10&q=Mendel%2C+K.%3B+Cohen%2C+A.+Methods+for+the+rapid+evaluation+of+rootstocks+for+citrus%3B+Ministry+of+Agriculture%2C+Agricultural+Research+Station%3A+1962.&btnG= (accessed on 16 December 2022).
- Butkeviciute, A.; Abukauskas, V.; Janulis, V.; Kviklys, D. Phenolic content and antioxidant activity in apples of the ‘galaval’cultivar grown on 17 different rootstocks. Antioxidants 2022, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Mainla, L.; Moor, U.; Karp, K.; Puessa, T. The effect of genotype and rootstock on polyphenol composition of selected apple cultivars in Estonia. Zemdirb. Agric. 2011, 98, 63–70. [Google Scholar]
- Nachit, M.; Feucht, W. Suitability of phenolic and amino acids as selection criteria for the vigour of Malus rootstocks. Mitt. Rebe Wein Obstbau Fruchteverwert. 1976, 26, 199–204. [Google Scholar]
| Crop | Treatments Combinations | Reported Effect | References |
|---|---|---|---|
| Apple | Different graft combinations with or without interstock. | Dwarfing was caused by decreased MdPIN8 expression in the M9 interstem or inadequate root zeatin synthesis. | [12] |
| Different apple rootstocks | Dwarfing may be triggered by low IPT5b expression and high levels of methylation in the promoter region in the M9 rootstock. | [13] | |
| M.9 and SH40 interstocks | The DELLA protein RGA-like (RGL) gene represses the gibberellin signaling pathway. Further research revealed that MdWRKY24 might influence plant dwarfing via the synergistic effect of MdRGL1/2/3. | [14] | |
| Dwarfing self-rootstock, dwarfing interstock, and vigorous rootstock | The complex process of growth reduction is mediated by several transcription factors, genes involved in sugar metabolism, and IAA, CK, ABA, and GA pathways. | [15] | |
| Malus domestica, Malus honanensis, M. honanensis cv. S19, and ‘SH6’ | GID1c gene expression in the ‘SH6’ rootstock decreased with GA content compared with other tested rootstocks. Moreover, the upregulation of GID1c gene triggered the significant genes involved in hormone biosynthesis and metabolism, improving the coregulation of several hormones involved in plant growth and inhibiting dwarfing characteristics. | [16] | |
| Breadfruit | Breadfruit plants grafted onto Marang rootstocks | Breadfruit plants grafted on marang rootstocks may exhibit a dwarf phenotype due to the downregulation of AaGA20ox3 gene. GA deficiency may contribute to rootstock-induced breadfruit dwarfing. | [17] |
| 1. Breadfruit scions grafted onto marang (Artocarpus odoratissimus) rootstock. 2. Breadfruit scions grafted onto breadfruit rootstocks, self-graft (control) 3. Breadfruit seedlings (non-graft) | In breadfruit scions, disruption of pathways regulating nutrient transport, cell wall biosynthesis, sucrose utilization, and networks of hormone transduction may inhibit cell proliferation and stem elongation, resulting in a dwarf phenotype. | [18] | |
| Litchi | Vigorous cultivar (FZX) and dwarf cultivar (ZNX). | GA2ox gene was only found to be upregulated in ZNX samples, implying that GA might play an essential role in regulating huge variation between vigorous and dwarf litchi cultivars. | [19] |
| Pear | Mutant and wild type | Mutant lines with high levels of endogenous ABA and ABA pathway gene transcripts showed that the PbPAT14 function is related to the ABA pathway. | [20] |
| Dwarf phenotype and arborescent phenotype | When dwarf pears were compared to arborescent, their heights were reduced by 62.8%, and their internode lengths were noticeably shorter. Changes in genes expression in GA and BR degradation and signal transmission may explain the reduced number of cells in dwarf plants. | [21] | |
| Dwarfing ‘601D’ and vigorous ‘601T’ | Morphological traits showed that ‘601D’ had shorter internodal length, lower stomata density, greater stomata size, and increased photosynthetic capacity. In addition, an excessive accumulation of ABA is responsible for the dwarfing mechanism of ‘601D’. | [22] | |
| ‘Xueqing’/‘QN101’/‘Douli’, ‘Xueqing’/‘OHF51’/‘Douli’ | Differences in expression in shoot tips and stems between ‘QN101’ and ‘OHF51’ reveal a connection between IAA polar transport and dwarfing potential. | [23] | |
| Persimmon | Three different grafting combinations. | When interstock lengths were between 20 and 25 cm, dwarfism was more prominent. DKGA2ox1 and MIR171f_3 influence persimmon dwarfism via regulating scion GA content. | [24] |
| Sweet Persimmon | Two different graft combinations | IAA and GA levels were lower in ‘Kanshu’ grafted onto the ‘Nantong-xiaofangshi’ interstock compared with no interstock. | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, F.; Li, J.; Iqbal, S.; Khan, U.; Ali, N.A.; Peng, Y.; Hong, L.; Asghar, S.; Javed, H.U.; Li, C.; et al. Hormonal Interactions Underlying Rootstock-Induced Vigor Control in Horticultural Crops. Appl. Sci. 2023, 13, 1237. https://doi.org/10.3390/app13031237
Hayat F, Li J, Iqbal S, Khan U, Ali NA, Peng Y, Hong L, Asghar S, Javed HU, Li C, et al. Hormonal Interactions Underlying Rootstock-Induced Vigor Control in Horticultural Crops. Applied Sciences. 2023; 13(3):1237. https://doi.org/10.3390/app13031237
Chicago/Turabian StyleHayat, Faisal, Juan Li, Shahid Iqbal, Ummara Khan, Nadia Ahmed Ali, Yang Peng, Leming Hong, Sumeera Asghar, Hafiz Umer Javed, Caiqin Li, and et al. 2023. "Hormonal Interactions Underlying Rootstock-Induced Vigor Control in Horticultural Crops" Applied Sciences 13, no. 3: 1237. https://doi.org/10.3390/app13031237
APA StyleHayat, F., Li, J., Iqbal, S., Khan, U., Ali, N. A., Peng, Y., Hong, L., Asghar, S., Javed, H. U., Li, C., Song, W., Tu, P., Chen, J., & Shahid, M. A. (2023). Hormonal Interactions Underlying Rootstock-Induced Vigor Control in Horticultural Crops. Applied Sciences, 13(3), 1237. https://doi.org/10.3390/app13031237








