Conductive Polymer and Nanoparticle-Promoted Polymer Hybrid Coatings for Metallic Bipolar Plates in Proton Membrane Exchange Water Electrolysis
Abstract
1. Introduction
2. Silane Treatment Technology
3. Conductive Polymer Based Coating
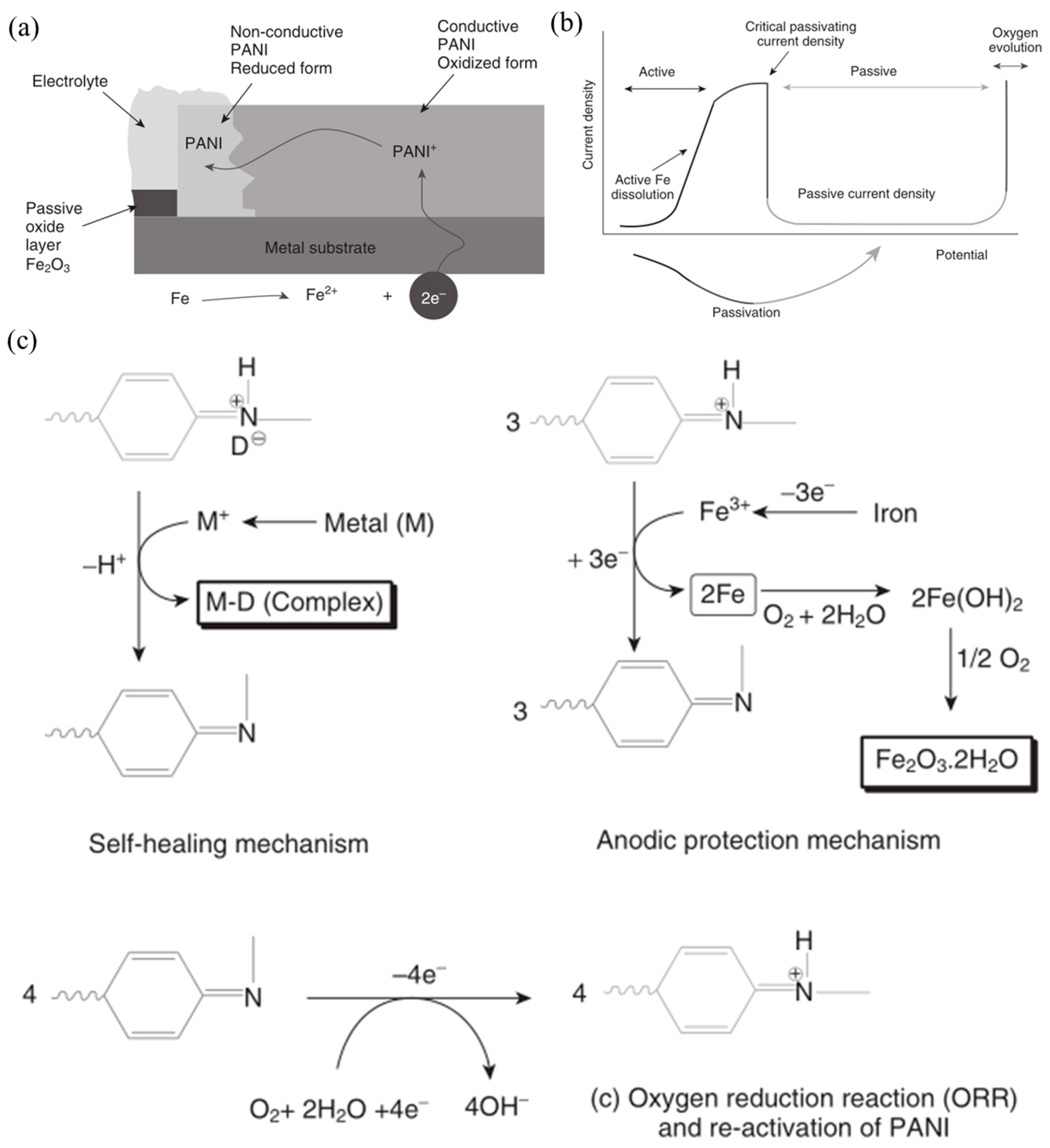
3.1. Polyaniline (PANI)
3.2. Polypyrrole (PPy)
4. Nanoparticles-Promoted Polymer Hybrid Coatings
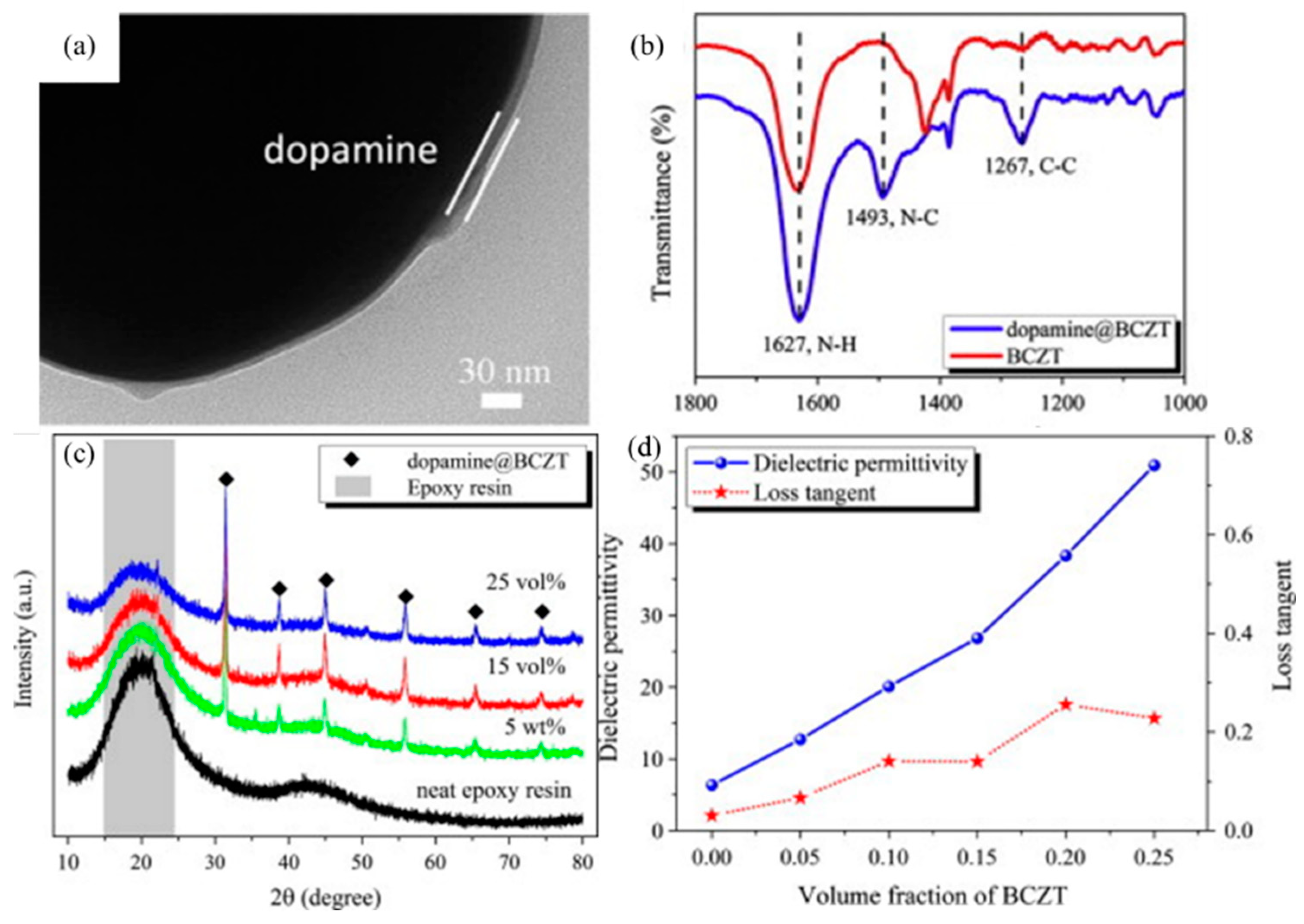
4.1. Epoxy Resin (EP)-Based Hybrid Coatings
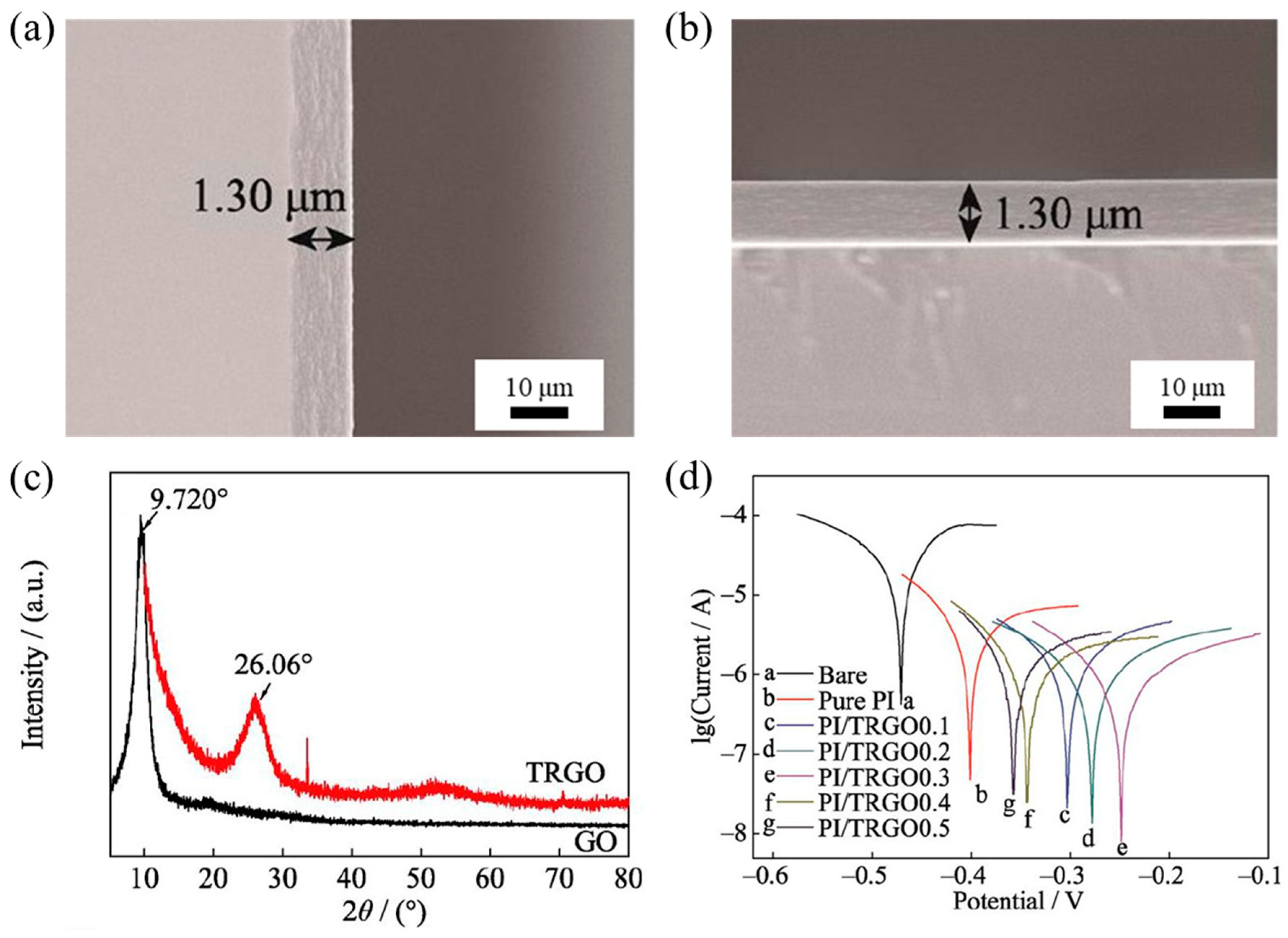
4.2. Polyimide (PI) Based Hybrid Coatings
4.3. Other Polymer-Based Hybrid Coatings
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| PEMWE | Proton exchange membrane water electrolysis |
| BPs | Bipolar plates |
| PANI | Polyaniline |
| PPy | Polypyrrole |
| ICR | Interface contact resistance |
| PEMFC | Proton exchange membrane fuel cell |
| OER | Oxygen evolution reaction |
| HER | Hydrogen evolution reaction |
| CVD | Chemical vapor deposition |
| PVD | Physical vapor deposition |
| EIS | Electrochemical impedance spectroscopy |
| CV | Cyclic voltammetry |
| PAMT | 2-amino-5-mercapto-1,3,4-thiadiazole |
| EP | Epoxy resin |
| GO | Grapheme oxide |
| TRGO | thermal reduced grapheme oxide |
| BCZT | Ba0.85Ca0.15Zr0.1Ti0.9O3 |
| FTIR | Fourier transform infrared spectroscopy |
| PI | Polyimide |
| EPD | Electrophoretic deposition |
| PPD | P-phenylenediamine |
| TiN | Titanium nitride |
| RMGO | Reduced magnesium oxide |
| PDADMAC | Polydimethyldiallylammonium chloride |
| Polydopamine | PDA |
References
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Bezdek, R.H. The hydrogen economy and jobs of the future. Renew. Energy Environ. Sustain. 2020, 96, 107. [Google Scholar] [CrossRef]
- Li, C.; Liao, G.; Fang, B. Magnetic-field-promoted photocatalytic overall water-splitting systems. Chem. Catal. 2022, 2, 238–241. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Fang, B. Donor-acceptor organic semiconductor heterojunction nanoparticles for efficient photocatalytic H2 evolution. Matter 2022, 5, 1635–1637. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.Y.; Fang, B.; Yang, H. Z-scheme systems: From fundamental principles to characterization, synthesis, and photocatalytic fuel-conversion applications. Phys. Rep. 2022, 983, 1–41. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.Y.; Fang, B.; Yang, H. Emerging frontiers of Z-scheme photocatalytic systems. Trends Chem. 2022, 4, 111–127. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, G.; Ma, D.; Li, Z.; Yan, Z.; Xu, A.; Zhong, W.; Fang, B. Synergistic effects of g-C3N4 three-dimensional inverse opals and Ag modification toward high-efficiency photocatalytic H2 evolution. J. Clean. Prod. 2021, 328, 129745. [Google Scholar] [CrossRef]
- Reilly, K.; Adeli, B.; Fang, B.; Wilkinson, D.P.; Taghipour, F. Advanced titanium dioxide fluidizable nanowire photocatalysts. RSC Adv. 2022, 12, 4240–4252. [Google Scholar] [CrossRef]
- Xu, G.; Cai, X.; Wang, L.; Zhang, Q.; Fang, B.; Zhong, X.; Yao, J. Thermogravimetric-infrared analysis and performance optimization of co-pyrolysis of oily sludge and rice husks. Int. J. Hydrogen Energy 2022, 47, 27437–27451. [Google Scholar] [CrossRef]
- Smith, D.W.; Oladoyinbo, F.O.; Mortimore, W.A.; Colquhoun, H.M.; Thomassen, M.S.; Ødegård, A.; Guillet, N.; Mayousse, E.; Klicpera, T.; Hayes, W. A Microblock Ionomer in Proton Exchange Membrane Electrolysis for the Production of High Purity Hydrogen. Macromolecules 2013, 46, 1504–1511. [Google Scholar] [CrossRef]
- Moschovi, A.M.; Zagoraiou, E.; Polyzou, E.; Yakoumis, I. Recycling of Critical Raw Materials from Hydrogen Chemical Storage Stacks (PEMWE), Membrane Electrode Assemblies (MEA) and Electrocatalysts. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1024, 012008. [Google Scholar] [CrossRef]
- Gohil, J.M.; Dutta, K. Structures and properties of polymers in ion exchange membranes for hydrogen generation by water electrolysis. Polym. Adv. Technol. 2021, 32, 4598–4615. [Google Scholar] [CrossRef]
- Liu, G.; Hou, F.; Wang, X.; Fang, B. Stainless Steel-Supported Amorphous Nickel Phosphide/Nickel as an Electrocatalyst for Hydrogen Evolution Reaction. Nanomaterials 2022, 12, 3328. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hou, F.; Peng, S.; Wang, X.; Fang, B. Synthesis, Physical Properties and Electrocatalytic Performance of Nickel Phosphides for Hydrogen Evolution Reaction of Water Electrolysis. Nanomaterials 2022, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Peng, S.; Hou, F.; Wang, X.; Fang, B. Preparation and Performance Study of the Anodic Catalyst Layer via Doctor Blade Coating for PEM Water Electrolysis. Membranes 2023, 13, 24. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Li, X.; Fang, B. Emerging polymeric carbon nitride Z-scheme systems for photocatalysis. Cell Rep. Phys. Sci. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Teuku, H.; Alshami, I.; Goh, J.; Masdar, M.S.; Loh, K.S. Review on bipolar plates for low-temperature polymer electrolyte membrane water electrolyzer. Int. J. Energy Res. 2021, 45, 20583–20600. [Google Scholar] [CrossRef]
- Khatib, F.N.; Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; El-Hassan, Z.; Durrant, A.; Thompson, J.; Olabi, A.G. Material degradation of components in polymer electrolyte membrane (PEM) electrolytic cell and mitigation mechanisms: A review. Renew. Sustain. Energy Rev. 2019, 111, 1–14. [Google Scholar] [CrossRef]
- Liu, G.; Hou, F.; Wang, X.; Fang, B. Ir-IrO2 with heterogeneous interfaces and oxygen vacancies-rich surfaces for highly efficient oxygen evolution reaction. Appl. Surf. Sci. 2023, 615, 156333. [Google Scholar] [CrossRef]
- Liu, G.; Hou, F.; Wang, X.; Fang, B. Robust Porous TiN Layer for Improved Oxygen Evolution Reaction Performance. Materials 2022, 15, 7602. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Wei, Z.; Han, X. Optimization scheduling control strategy of wind-hydrogen system considering hydrogen production efficiency. J. Energy Storage 2022, 47, 103609. [Google Scholar] [CrossRef]
- Hu, S.; Feng, C.; Wang, S.; Liu, J.; Wu, H.; Zhang, L.; Zhang, J. Ni3N/NF as Bifunctional Catalysts for Both Hydrogen Generation and Urea Decomposition. ACS Appl. Mater. Interfaces 2019, 11, 13168–13175. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef]
- Štrbac, S.; Smiljanić, M.; Wakelin, T.; Potočnik, J.; Rakočević, Z. Hydrogen evolution reaction on bimetallic Ir/Pt(poly) electrodes in alkaline solution. Electrochim. Acta 2019, 306, 18–27. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, H.; Li, Y.; Zhou, Y.; Fang, B. Ligand-free synthesis of noble metal nanocatalysts for electrocatalysis. Chem. Eng. J. 2023, 451, 138668. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Cavaliere, S.; Dupont, M.; Jones, D.; Rozière, J. On the stability of antimony doped tin oxide supports in proton exchange membrane fuel cell and water electrolysers. Sustain. Energy Fuels 2019, 3, 1526–1535. [Google Scholar] [CrossRef]
- Liu, G.; Hou, F.; Peng, S.; Wang, X.; Fang, B. Process and challenges of stainless steel based bipolar plates for proton exchange membrane fuel cells. Int. J. Miner. Metall. Mater. 2022, 29, 1099–1119. [Google Scholar] [CrossRef]
- Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.G. Fuel Cell Technologies, Applications, and State of the Art. A Reference Guide. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Das, P.K.; Li, X.; Liu, Z.S. Analytical approach to polymer electrolyte membrane fuel cell performance and optimization. J. Electroanal. Chem. 2007, 604, 72–90. [Google Scholar] [CrossRef]
- Khan, M.A.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent Progresses in Electrocatalysts for Water Electrolysis. Electrochem. Energy Rev. 2018, 1, 483–530. [Google Scholar] [CrossRef]
- Shirvanian, P.; van Berkel, F. Novel components in Proton Exchange Membrane (PEM) Water Electrolyzers (PEMWE): Status, challenges and future needs. A mini review. Electrochem. Commun. 2020, 114, 106704. [Google Scholar] [CrossRef]
- Corona-Guinto, J.L.; Cardeño-García, L.; Martínez-Casillas, D.C.; Sandoval-Pineda, J.M.; Tamayo-Meza, P.; Silva-Casarin, R.; González-Huerta, R.G. Performance of a PEM electrolyzer using RuIrCoOx electrocatalysts for the oxygen evolution electrode. Int. J. Hydrogen Energy 2013, 38, 12667–12673. [Google Scholar] [CrossRef]
- Song, H.J.; Yoon, H.; Ju, B.; Kim, D.W. Highly Efficient Perovskite-Based Electrocatalysts for Water Oxidation in Acidic Environments: A Mini Review. Adv. Energy Mater. 2021, 11, 2002428. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Parra-Puerto, A.; Ng, K.L.; Fahy, K.; Goode, A.E.; Ryan, M.P.; Kucernak, A. Supported Transition Metal Phosphides: Activity Survey for HER, ORR, OER, and Corrosion Resistance in Acid and Alkaline Electrolytes. ACS Catal. 2019, 9, 11515–11529. [Google Scholar] [CrossRef]
- Rojas, N.; Sanchez-Molina, M.; Sevilla, G.; Amores, E.; Almandoz, E.; Esparza, J.; Cruz Vivas, M.R.; Colominas, C. Coated stainless steels evaluation for bipolar plates in PEM water electrolysis conditions. Int. J. Hydrogen Energy 2021, 46, 25929–25943. [Google Scholar] [CrossRef]
- Asri, N.F.; Husaini, T.; Sulong, A.B.; Majlan, E.H.; Daud, W.R.W. Coating of stainless steel and titanium bipolar plates for anticorrosion in PEMFC: A review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Wang, S.H.; Peng, J.; Lui, W.B. Surface modification and development of titanium bipolar plates for PEM fuel cells. J. Power Sources 2006, 160, 485–489. [Google Scholar] [CrossRef]
- Ursúa, A.; Gandía, L.M.; Sanchis, P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE 2011, 100, 410–426. [Google Scholar] [CrossRef]
- Ayers, K.E.; Anderson, E.B.; Capuano, C.; Carter, B.; Dalton, L.; Hanlon, G.; Manco, J.; Niedzwiecki, M. Research Advances towards Low Cost, High Efficiency PEM Electrolysis. ECS Trans. 2010, 33, 3–15. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Siva, T.; Rajkumar, S.; Muralidharan, S.; Sathiynarayanan, S. Bipolar properties of coatings to enhance the corrosion protection performance. Prog. Org. Coat 2019, 137, 105379. [Google Scholar] [CrossRef]
- Dongfang, S.; Guixin, S.; Shanlong, P.; Dongdong, W.; Yue, L.; Heng, Z.; Xindong, W. Electrophoretic deposition of TiN coating on titanium bipolar plate used for PEM water electrolysis. Electroplat. Finish. 2022, 41, 497–505. [Google Scholar] [CrossRef]
- Müller, A.; Kauranen, P.; von Ganski, A.; Hell, B. Injection moulding of graphite composite bipolar plates. J Power Sources 2006, 154, 467–471. [Google Scholar] [CrossRef]
- Ijaodola, O.; Ogungbemi, E.; Khatib, F.N.; Wilberforce, T.; Ramadan, M.; El Hassan, Z.; Thompson, J.; Olabi, A.G. Evaluating the Effect of Metal Bipolar Plate Coating on the Performance of Proton Exchange Membrane Fuel Cells. Energies 2018, 11, 3203. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Schuller, G.; Boaventura, M.; Mendes, A. Synergetic integration of a methanol steam reforming cell with a high temperature polymer electrolyte fuel cell. Int. J. Hydrogen Energy 2017, 42, 13902–13912. [Google Scholar] [CrossRef]
- Stein, T.; Ein-Eli, Y. Challenges and Perspectives of Metal-Based Proton Exchange Membrane’s Bipolar Plates: Exploring Durability and Longevity. Energy Technol. 2020, 8, 2000007. [Google Scholar] [CrossRef]
- Stroebel, R. Approach to Provide a Metallic Bipolar Plate Module to the Industry; NADA: Woolloomooloo, NSW, Australia, 2017. [Google Scholar]
- LaConti, A.B.; Griffith, A.E.; Cropley, C.C.; Kosek, J.A. Titanium Carbide Bipolar Plate for Electrochemical Devices. U.S. Patent 6083641, 1998. [Google Scholar]
- Gago, A.; Ansar, A.; Wagner, N.; Arnold, J.; Friedrich, K.A. Titanium coatings deposited by thermal spraying for bipolar plates of PEM electrolysers. In Proceedings of the 4th European PEFC and H2 Forum, Lucerne, Switzerland, 2–5 July 2013. [Google Scholar]
- Bi, J.; Yang, J.; Liu, X.; Wang, D.; Yang, Z.; Liu, G.; Wang, X. Development and evaluation of nitride coated titanium bipolar plates for PEM fuel cells. Int. J. Hydrogen Energy 2021, 46, 1144–1154. [Google Scholar] [CrossRef]
- Barrera, O.; Bombac, D.; Chen, Y.; Daff, T.D.; Galindo-Nava, E.; Gong, P.; Haley, D.; Horton, R.; Katzarov, I.; Kermode, J.R.; et al. Understanding and mitigating hydrogen embrittlement of steels: A review of experimental, modelling and design progress from atomistic to continuum. J. Mater. Sci. 2018, 53, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Liang, C.H.; Wang, H. Hydrogen embrittlement of titanium and its alloys. Corros. Sci. Prot. Technol. 1970, 18, 561–576. [Google Scholar]
- Li, Y.S.; Zhang, C.Z.; Ma, H.T.; Yang, L.Z.; Zhang, L.L.; Tang, Y.; Li, X.J.; He, L.L.; Feng, R.; Yang, Q.; et al. CVD nanocrystalline diamond coatings on Ti alloy: A synchrotron-assisted interfacial investigation. Mater Chem. Phys. 2012, 134, 145–152. [Google Scholar] [CrossRef]
- Hu, X.; Xu, D.; Byun, T.S.; Wirth, B.D. Modeling of irradiation hardening of iron after low-dose and low-temperature neutron irradiation. Model Simul. Mat. Sci. Eng. 2014, 22, 065002. [Google Scholar] [CrossRef]
- Liu, G.; Peng, S.; Hou, F.; Fang, B.; Wang, X. Au-TiO2/Ti Hybrid Coating as a Liquid and Gas Diffusion Layer with Improved Performance and Stability in Proton Exchange Membrane Water Electrolyzer. Molecules 2022, 27, 6644. [Google Scholar] [CrossRef] [PubMed]
- Lædre, S.; Kongstein, O.E.; Oedegaard, A.; Karoliussen, H.; Seland, F. Materials for Proton Exchange Membrane water electrolyzer bipolar plates. Int. J. Hydrogen Energy 2017, 42, 2713–2723. [Google Scholar] [CrossRef]
- Joseph, S.; McClure, J.C.; Chianelli, R.; Pich, P.; Sebastian, P.J. Conducting polymer-coated stainless steel bipolar plates for proton exchange membrane fuel cells (PEMFC). Int. J. Hydrogen Energy 2005, 30, 1339–1344. [Google Scholar] [CrossRef]
- Wang, S.H.; Peng, J.; Lui, W.B.; Zhang, J.S. Performance of the gold-plated titanium bipolar plates for the light weight PEM fuel cells. J. Power Sources 2006, 162, 486–491. [Google Scholar] [CrossRef]
- Song, S.H.; Min, B.K.; Hong, M.-H.; Kwon, T.-Y. Application of a novel CVD TiN coating on a biomedical Co–Cr Alloy: An evaluation of coating layer and substrate characteristics. Materials 2020, 13, 1145. [Google Scholar] [CrossRef]
- Jin, N.; Yang, Y.; Luo, X.; Xia, Z. Development of CVD Ti-containing films. Prog. Mater Sci. 2013, 58, 1490–1533. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Ali, N. PVD Technology in Fabrication of Micro- and Nanostructured Coatings. Compr. Mater. Process. 2014, 7, 49–84. [Google Scholar]
- Mansoor, N.S.; Fattah-alhosseini, A.; Elmkhah, H.; Shishehian, A. Comparison of the mechanical properties and electrochemical behavior of TiN and CrN single-layer and CrN/TiN multi-layer coatings deposited by PVD method on a dental alloy. Mater Res. Express 2020, 6, 126433. [Google Scholar] [CrossRef]
- Li, P.; Ding, X.; Yang, Z.; Chen, M.; Wang, M.; Wang, X. Electrochemical synthesis and characterization of polyaniline-coated PEMFC metal bipolar plates with improved corrosion resistance. Ionics 2018, 24, 1129–1137. [Google Scholar] [CrossRef]
- Wang, Y.; Northwood, D.O. An investigation into polypyrrole-coated 316L stainless steel as a bipolar plate material for PEM fuel cells. J. Power Sources 2006, 163, 500–508. [Google Scholar] [CrossRef]
- Zhang, T.; Zeng, C.L. Corrosion protection of 1Cr18Ni9Ti stainless steel by polypyrrole coatings in HCl aqueous solution. Electrochim. Acta 2005, 50, 4721–4727. [Google Scholar] [CrossRef]
- García, M.A.L.; Smit, M.A. Study of electrodeposited polypyrrole coatings for the corrosion protection of stainless steel bipolar plates for the PEM fuel cell. J. Power Sources 2006, 158, 397–402. [Google Scholar] [CrossRef]
- Deyab, M.A. Corrosion protection of aluminum bipolar plates with polyaniline coating containing carbon nanotubes in acidic medium inside the polymer electrolyte membrane fuel cell. J. Power Sources 2014, 268, 50–55. [Google Scholar] [CrossRef]
- Joseph, S.; McClure, J.C.; Sebastian, P.J.; Moreira, J.; Valenzuela, E. Polyaniline and polypyrrole coatings on aluminum for PEM fuel cell bipolar plates. J. Power Sources 2008, 177, 161–166. [Google Scholar] [CrossRef]
- Chen, W.C.; Lo, Y.; Chen, H.S. Effects of Ti surface treatments with silane and arginylglycylaspartic acid peptide on bone cell progenitors. Odontology 2014, 103, 322–332. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S.; Fedrizzi, L. Silane pre-treatments on copper and aluminium. Electrochim. Acta 2006, 51, 6097–6103. [Google Scholar] [CrossRef]
- Cieślik, M.; Engvall, K.; Pan, J.; Kotarba, A. Silane–parylene coating for improving corrosion resistance of stainless steel 316L implant material. Corros. Sci. 2011, 53, 296–301. [Google Scholar] [CrossRef]
- Fedel, M.; Olivier, M.; Poelman, M.; Deflorian, F.; Rossi, S.; Druart, M.E. Corrosion protection properties of silane pre-treated powder coated galvanized steel. Prog. Org. Coat 2009, 66, 118–128. [Google Scholar] [CrossRef]
- Edzatty, A.N.; Norzilah, A.H.; Jamaludin, S.B. Preliminary study: Direct growth carbon nanomaterials on metal substrate to improve corrosion resistance. Mater. Sci. Forum 2015, 819, 81–86. [Google Scholar] [CrossRef]
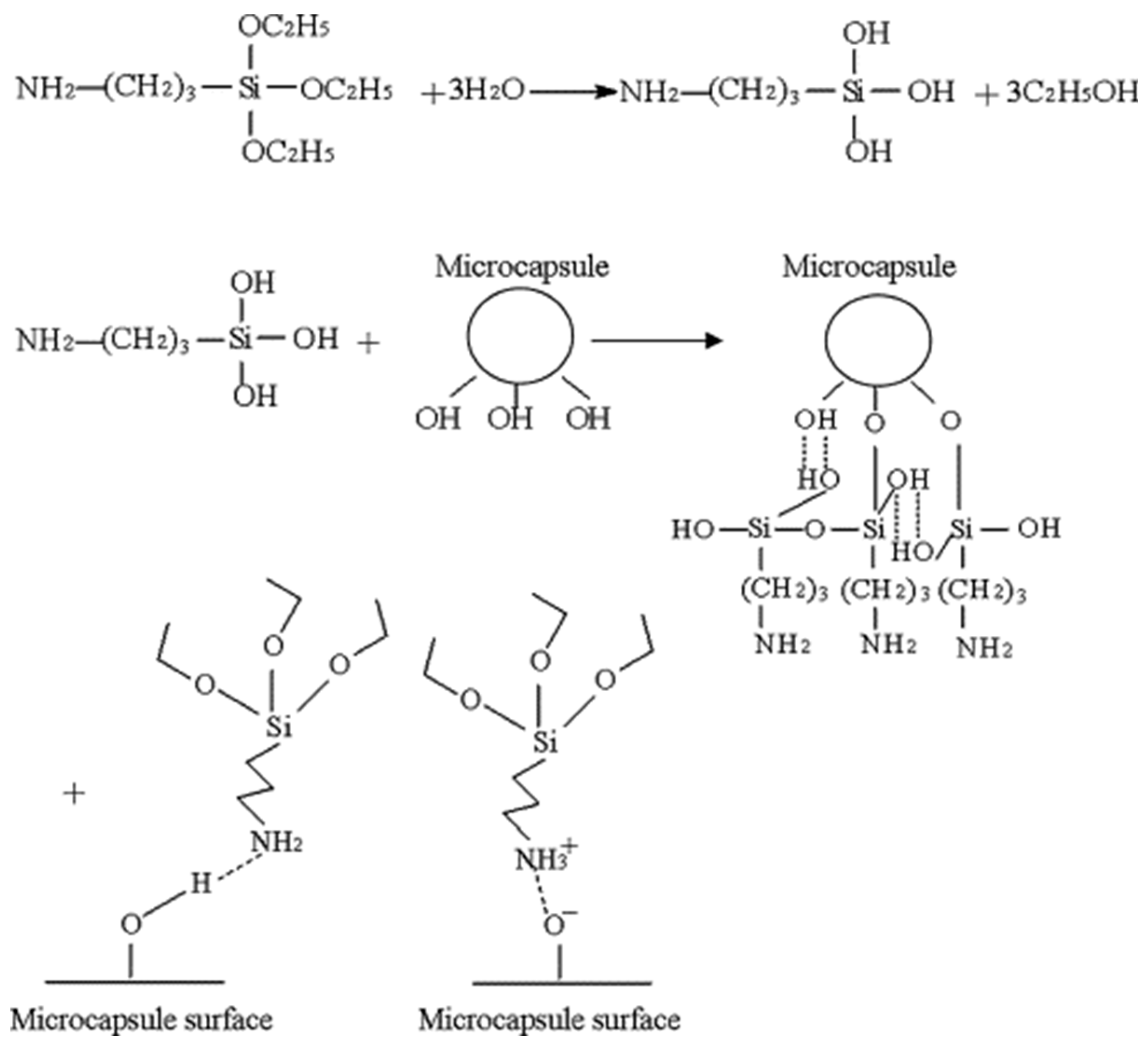
- Li, H.; Wang, R.; Hu, H.; Liu, W. Surface modification of self-healing poly(urea-formaldehyde) microcapsules using silane-coupling agent. Appl. Surf. Sci. 2008, 255, 1894–1900. [Google Scholar] [CrossRef]
- Ma, Z.; Zhu, F.; Zhao, T. Effects of surface modification of silane coupling agent on the properties of concrete with freeze-thaw damage. KSCE J. Civ. Eng. 2017, 22, 657–669. [Google Scholar] [CrossRef]
- Rashno, A.; Mohebi Damabi, R.; Rezaee Niaraki, P.; Ahmadi, S. A new amino silane coupling agent for old corrugated container fibers/high density polyethylene composites. Polym. Compos. 2018, 39, 2054–2064. [Google Scholar] [CrossRef]
- Ge, M.; Wang, X.; Du, M.; Liang, G.; Hu, G.; SM, J.A. Effects on the Mechanical Properties of Nacre-Like Bio-Hybrid Membranes with Inter-Penetrating Petal Structure Based on Magadiite. Materials 2019, 12, 173. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, Q. Study on silane treatment technology in the painting pretreatment. Adv. Mat. Res. 2012, 482–484, 1192–1195. [Google Scholar] [CrossRef]
- Wessling, B. Corrosion prevention with an organic metal (polyaniline): Surface ennobling, passivation, corrosion test results. Mater. Corros. 1996, 47, 439–445. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mele, G. Stainless steel bipolar plate coated with polyaniline/Zn-Porphyrin composites coatings for proton exchange membrane fuel cell. Sci. Rep. 2020, 10, 3277. [Google Scholar] [CrossRef]
- Cooper, L.; El-Kharouf, A. Titanium Nitride Polyaniline Bilayer Coating for Metallic Bipolar Plates used in Polymer Electrolyte Fuel Cells. Fuel Cells 2020, 20, 453–460. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Lu, H.; Meng, X. In-situ electrodeposition of conductive polypyrrole-graphene oxide composite coating for corrosion protection of 304SS bipolar plates. J. Alloys Compd. 2019, 770, 35–47. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Makhlouf, A.S.H. Recent advances in polyaniline (PANI)-based organic coatings for corrosion protection. Handb. Smart Coat. Mater. Prot. 2014, 2014, 459–486. [Google Scholar] [CrossRef]
- MA, C.; SG, P.; PR, G.; Shashwati, S.; VB, P. Synthesis and Characterization of Polypyrrole (PPy) Thin Films. Soft Nanosci. Lett. 2011, 1, 6–10. [Google Scholar]
- Liu, S.; Pan, T.J.; Wang, R.F.; Yue, Y.; Shen, J. Anti-corrosion and conductivity of the electrodeposited graphene/polypyrrole composite coating for metallic bipolar plates. Prog. Org. Coat 2019, 136, 105237. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Gao, Y.; Zhang, Q.; Zhao, J.; Lu, H.; Meng, X. Electropolymerization of camphorsulfonic acid doped conductive polypyrrole anti-corrosive coating for 304SS bipolar plates. Appl. Surf. Sci. 2017, 426, 87–98. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, C.; Cui, J.; Shen, S.; Qiu, H.; Li, J. Electrodeposition of polypyrrole coatings doped by benzenesulfonic acid-modified graphene oxide on metallic bipolar plates. Prog. Org. Coat 2022, 170, 106995. [Google Scholar] [CrossRef]
- Yan, S.; Song, H.; Li, Y.; Yang, J.; Jia, X.; Wang, S.; Yang, X. Integrated reduced graphene oxide/polypyrrole hybrid aerogels for simultaneous photocatalytic decontamination and water evaporation. Appl. Catal. B 2022, 301, 120820. [Google Scholar] [CrossRef]
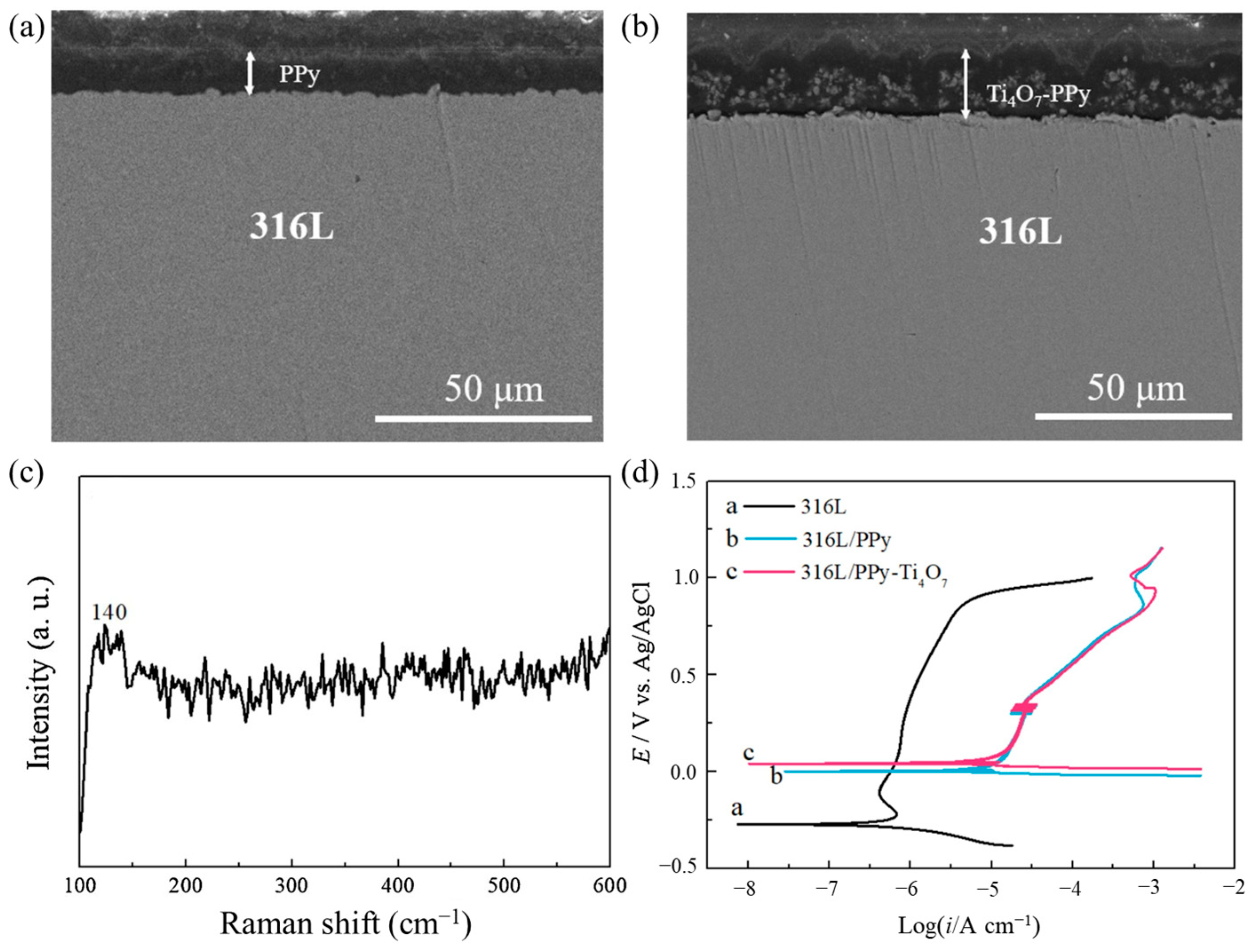
- Tan, Q.; Wang, Y. Preparation and performances of modified Ti4O7 doped polypyrrole coating for metallic bipolar plates. Corros. Sci. 2021, 190, 109703. [Google Scholar] [CrossRef]
- Akula, S.; Kalaiselvi, P.; Sahu, A.K.; Chellammal, S. Electrodeposition of conductive PAMT/PPY bilayer composite coatings on 316L stainless steel plate for PEMFC application. Int. J. Hydrogen Energy 2021, 46, 17909–17921. [Google Scholar] [CrossRef]
- He, J.; Zeng, W.; Shi, M.; Lv, X.; Fan, H.; Lei, Z. Influence of expandable graphite on flame retardancy and thermal stability property of unsaturated polyester resins/organic magnesium hydroxide composites. J. Appl. Polym. Sci. 2020, 137, 47881. [Google Scholar] [CrossRef]
- Oleksy, M.; Oliwa, R.; Heneczkowski, M.; Mossety-Leszczak, B.; Galina, H.; Budzik, G. Composites of epoxy resin with modified bentonites for aviation industry. Polimery 2012, 57, 228–235. [Google Scholar] [CrossRef]
- Luo, B.; Wang, X.; Zhao, Q.; Li, L. Synthesis, characterization and dielectric properties of surface functionalized ferroelectric ceramic/epoxy resin composites with high dielectric permittivity. Compos. Sci. Technol. 2015, 112, 1–7. [Google Scholar] [CrossRef]
- Abdullah, S.I.; Ansari, M.N.M. Mechanical properties of graphene oxide (GO)/epoxy composites. HBRC J. 2015, 11, 151–156. [Google Scholar] [CrossRef]
- Luo, B.; Wang, X.; Zhao, Q.; Li, L. A study of the effects of the matrix epoxy resin and graphene oxide (GO) manufacturing process on the tensile behaviour of GO-epoxy nanocomposites. Plast. Rubber Compos. 2017, 46, 405–412. [Google Scholar]
- Luo, B.; Wang, X.; Tian, E.; Song, H.; Li, L. Interfacial electronic and structural properties of SiO2(010)/BaTiO3(001) from first-principles calculations. Ceramics International. 2017, 43, 12988–12991. [Google Scholar] [CrossRef]
- Sun, M.Y.; Jiang, H.T.; Shi, B.; Zhou, G.Y.; Inyang, H.I.; Feng, C.X. Development of FBG salinity sensor coated with lamellar polyimide and experimental study on salinity measurement of gravel aquifer. Measurement 2019, 140, 526–537. [Google Scholar] [CrossRef]
- Liu, B.D.; Li, D.S.; Yang, J.H. The Research on Sacrificial Layer in the Fabrication of Micro Electromagnetic Relay. AMR. 2009, 60–61, 160–164. [Google Scholar] [CrossRef]
- Zhu, G.; Hou, J.; Zhu, H.; Qiu, R.; Xu, J. Electrochemical synthesis of poly(3,4-ethylenedioxythiophene) on stainless steel and its corrosion inhibition performance. J. Coat Technol. Res. 2013, 10, 659–668. [Google Scholar] [CrossRef]
- Jia, Y.K.; Chen, P.; Zhang, Q.H.; Sun, J. Thermal Reduced Graphene Oxide/Polyimide Nanocomposite Coating: Fabrication and Anticorrosive Property. Wuji Cailiao Xuebao/J. Inorg. Mater. 2017, 32, 1257–1263. [Google Scholar]
- Liu, Y.; Min, L.; Zhang, W.; Wang, Y. High-performance graphene coating on titanium bipolar plates in fuel cells via cathodic electrophoretic deposition. Coatings 2021, 11, 437. [Google Scholar] [CrossRef]
- Lee, D.; Lee, E.; Yoon, J.; Keith, B.; Oh, T.; Woo, S.P.; Yoon, Y.S.; Kim, D.J. Electrophoretic Deposition of Titanium Nitride Onto 316 Stainless Steel As a Bipolar Plate for Fuel Cell Application. ECS Trans. 2017, 80, 851. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Peng, S.; Zhang, H.; Wang, L.; Wang, X. Novel Au nanoparticles-inlaid titanium paper for PEM water electrolysis with enhanced interfacial electrical conductivity. Int. J. Miner. Metall. Mater. 2022, 29, 1090–1098. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.Y.; Han, M.; Yong, R.Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]

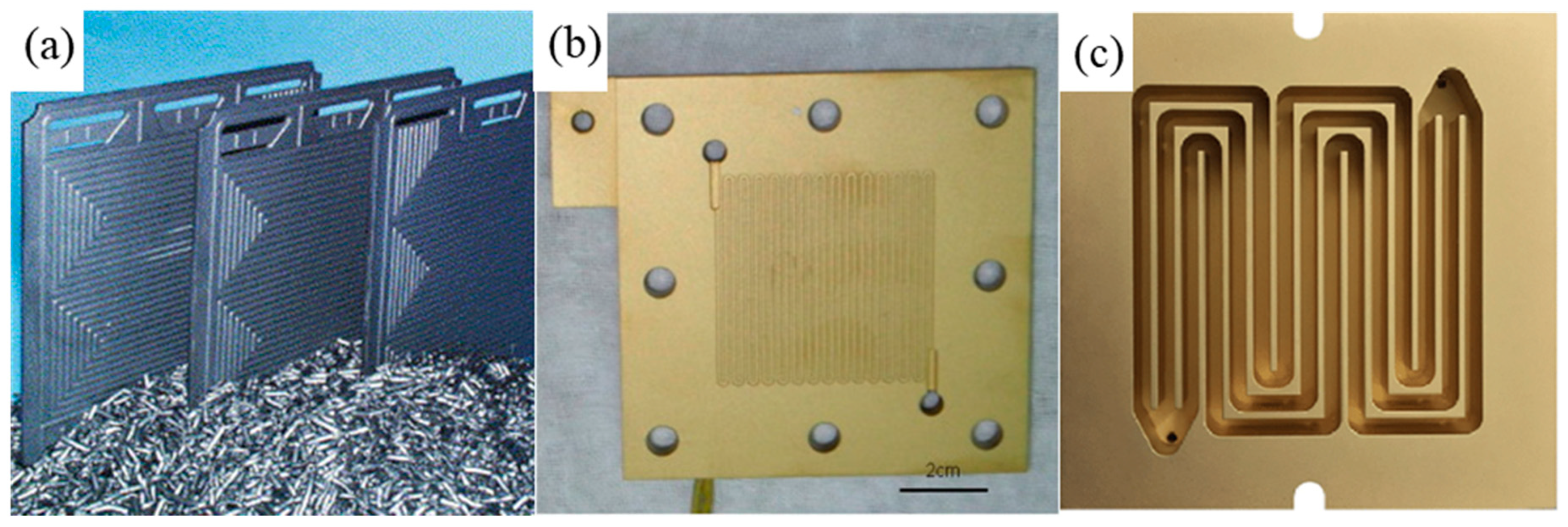
| Bipolar Plate Materials | Polymer Coatings | Nitride Coatings | Precious Metal Coatings |
|---|---|---|---|
| Coating methods | silica sol–gel, Nanocasting | Thermal nitriding process | Pulse current electrodeposition, PVD |
| Materials | Conductive polymers | Nitrides | Rare and precious metals |
| Chemical stability | good | good | good |
| Electrical conductivity | fair | fair | good |
| Thermal conductivity | good | fair | good |
| Corrosion resistance | good | good | good |
| Scratch resistance | good | fair | fair |
| Pitting corrosion resistance | good | fair | fair |
| Process complexity | easy | complex | fair |
| Cost | low | low | high |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Hou, F.; Wang, X.; Fang, B. Conductive Polymer and Nanoparticle-Promoted Polymer Hybrid Coatings for Metallic Bipolar Plates in Proton Membrane Exchange Water Electrolysis. Appl. Sci. 2023, 13, 1244. https://doi.org/10.3390/app13031244
Liu G, Hou F, Wang X, Fang B. Conductive Polymer and Nanoparticle-Promoted Polymer Hybrid Coatings for Metallic Bipolar Plates in Proton Membrane Exchange Water Electrolysis. Applied Sciences. 2023; 13(3):1244. https://doi.org/10.3390/app13031244
Chicago/Turabian StyleLiu, Gaoyang, Faguo Hou, Xindong Wang, and Baizeng Fang. 2023. "Conductive Polymer and Nanoparticle-Promoted Polymer Hybrid Coatings for Metallic Bipolar Plates in Proton Membrane Exchange Water Electrolysis" Applied Sciences 13, no. 3: 1244. https://doi.org/10.3390/app13031244
APA StyleLiu, G., Hou, F., Wang, X., & Fang, B. (2023). Conductive Polymer and Nanoparticle-Promoted Polymer Hybrid Coatings for Metallic Bipolar Plates in Proton Membrane Exchange Water Electrolysis. Applied Sciences, 13(3), 1244. https://doi.org/10.3390/app13031244








