Influence of Process Parameters on the Hydrothermal Carbonization of Olive Tree Trimmings: A 13C Solid-State NMR Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Infrared Spectroscopy
2.3. 13C SSNMR Spectroscopy
3. Results
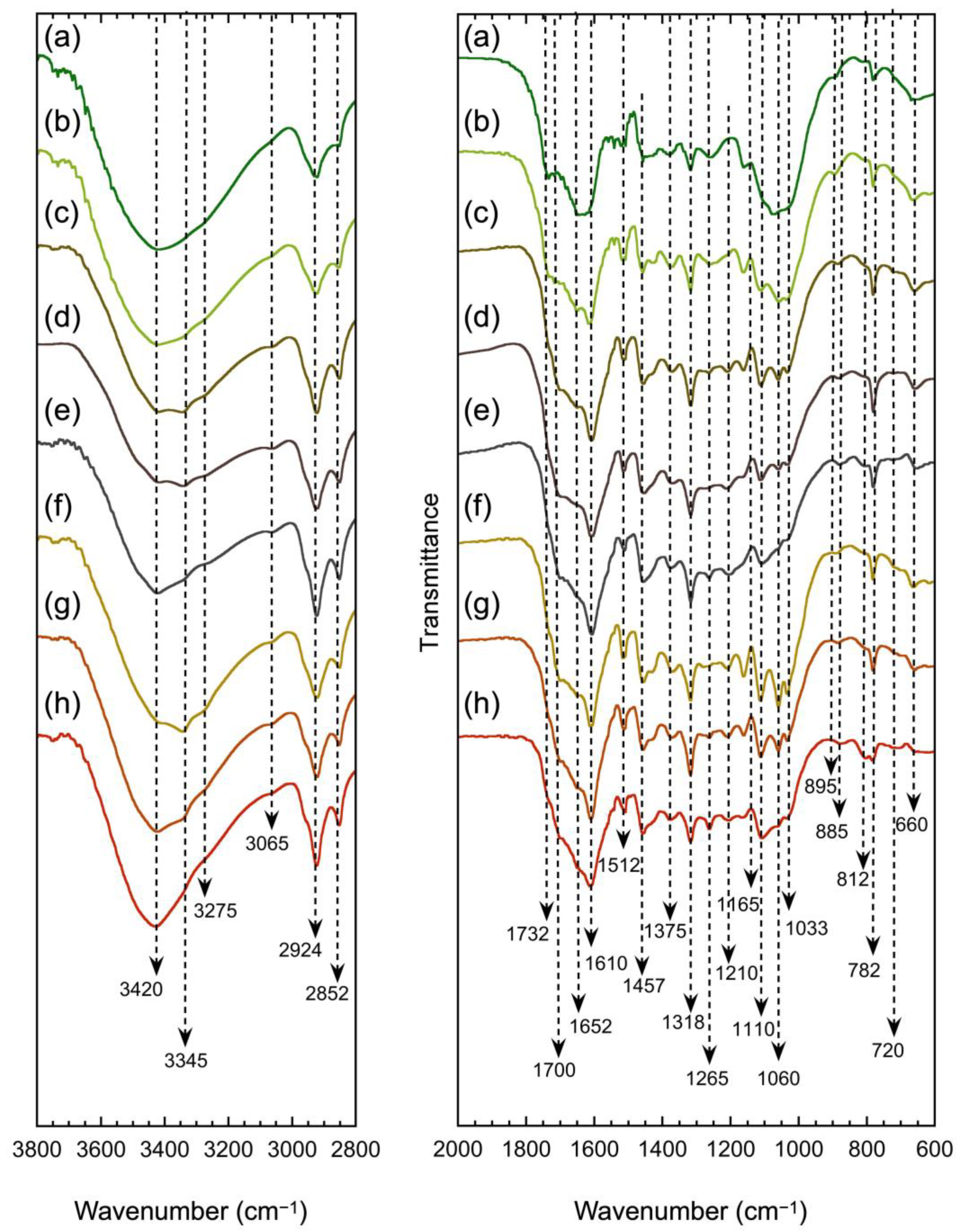
3.1. FTIR Spectroscopy
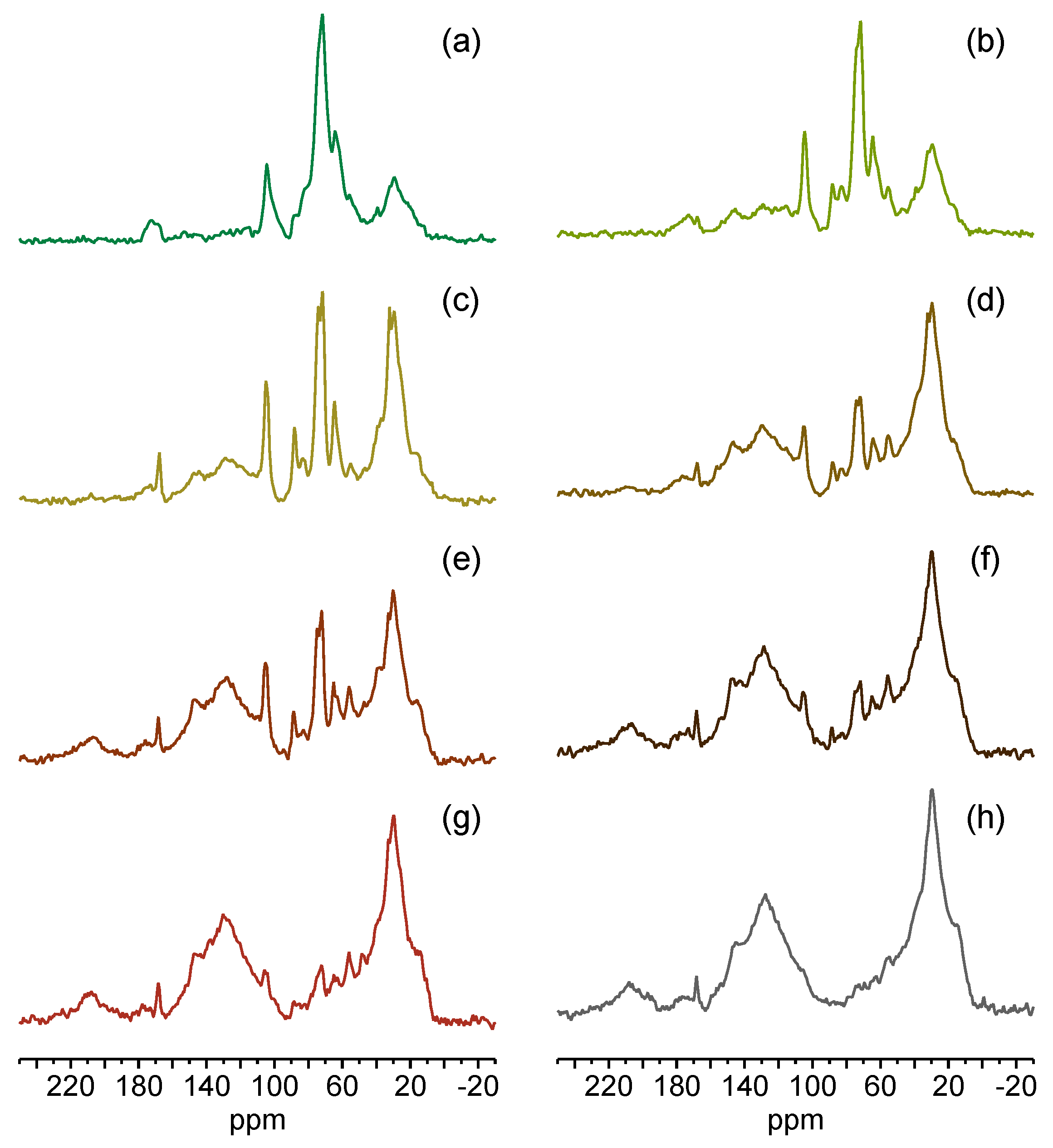
3.2. 13C SSNMR Spectroscopy
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Zhang, S.; Chen, J. Investigation on the physical and chemical properties of hydrochar and its derived pyrolysis char for their potential application: Influence of hydrothermal carbonization conditions. Energy Fuels 2015, 29, 5222–5230. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonization: The role of temperature and solid load on secondary char formation and hydrochar energy properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Ulbrich, M.; Preßl, D.; Fendt, S.; Gaderer, M.; Spliethoff, H. Impact of HTC reaction conditions on the hydrochar properties and CO2 gasification properties of spent grains. Fuel Process. Technol. 2017, 167, 663–669. [Google Scholar] [CrossRef]
- Diakité, M.; Paul, A.; Jäger, C.; Pielert, J.; Mumme, J. Chemical and morphological changes in hydrochars derived from microcrystalline cellulose and investigated by chromatographic, spectroscopic and adsorption techniques. Bioresour. Technol. 2013, 150, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal carbonization of coniferous biomass: Effect of process parameters on mass and energy yields. J. Anal. Appl. Pyrolysis 2015, 113, 551–556. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Cavali, M.; Libardi, N., Jr.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Filho, P.B.; Bayard, R.; Benbelkacem, H.; de Castilhos, A.B., Jr. A review on hydrothermal carbonization of potential biomass wastes, characterization and environmental applications of hydrochar, and biorefinery perspectives of the process. Sci. Total Environ. 2023, 857, 159627. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A. Upgrading olive trimmings residue as biofuel by hydrothermal carbonization and torrefaction: A comparative study. Chem. Eng. Trans. 2016, 50, 13–18. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Fiori, L. Hydrothermal carbonization kinetics of lignocellulosic agro-wastes: Experimental data and modeling. Energies 2019, 12, 516. [Google Scholar] [CrossRef]
- González-Arias, J.; Sánchez, M.E.; Martínez, E.J.; Covalski, C.; Alonso-Simón, A.; González, R.; Cara-Jiménez, J. Hydrothermal carbonization of olive tree pruning as a sustainable way for improving biomass energy potential. Effect of reaction parameters on fuel properties. Processes 2020, 8, 1201. [Google Scholar] [CrossRef]
- Duman, G.; Balmuk, G.; Cay, H.; Kantarli, I.C.; Yanik, J. Comparative evaluation of torrefaction and hydrothermal carbonization: Effect on fuel properties and combustion behavior of agricultural wastes. Energy Fuels 2020, 34, 11175–11185. [Google Scholar] [CrossRef]
- Gonzáles-Arias, J.; Baena-Moreno, F.M.; Sánchez, M.E.; Cara-Jiménez, J. Optimizing hydrothermal carbonization of olive tree pruning: A techno-economic analysis based on experimental results. Sci. Total Environ. 2021, 784, 147169. [Google Scholar] [CrossRef]
- Cao, X.; Pignatello, J.J.; Li, Y.; Lattao, C.; Chappell, M.A.; Chen, N.; Miller, L.F.; Mao, J. Characterization of wood chars produced at different temperatures using advanced solid-state 13C NMR spectroscopic techniques. Energy Fuels 2012, 26, 5983–5991. [Google Scholar] [CrossRef]
- Calucci, L.; Rasse, D.; Forte, C. Solid-state nuclear magnetic resonance characterization of chars obtained from hydrothermal carbonization of corncob and Miscanthus. Energy Fuels 2013, 27, 303–309. [Google Scholar] [CrossRef]
- Baccile, N.; Falco, C.; Titirici, M.-M. Characterization of biomass and its derived char using 13C-solid state nuclear magnetic resonance. Green Chem. 2014, 16, 4839–4869. [Google Scholar] [CrossRef]
- Fregolente, L.G.; dos Santos, J.V.; Vinci, G.; Piccolo, A.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C.; Spaccini, R. Insights on molecular characteristics of hydrochars by 13C-NMR and off-line TMAH-GC/MS and assessment of their potential use as plant growth promoters. Molecules 2021, 26, 1026. [Google Scholar] [CrossRef]
- Cao, X.; Ro, K.S.; Libra, J.A.; Kammann, C.I.; Lima, I.; Berge, N.; Li, L.; Li, Y.; Chen, N.; Yang, J.; et al. Effects of biomass types and carbonization conditions on the chemical characteristics of hydrochars. J. Agric. Food Chem. 2013, 61, 9401–9411. [Google Scholar] [CrossRef] [PubMed]
- Geppi, M.; Forte, C. The SPORT-NMR software: A tool for determining relaxation times in unresolved NMR spectra. J. Magn. Reson. 1999, 137, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Smernik, R.J.; Eckmeier, E.; Schmidt, M.W.I. Comparison of solid-state C-13 NMR spectra of soil organic matter from an experimental burning site acquired at two field strengths. Aust. J. Soil Res. 2008, 46, 122–127. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Structural differences between wood species: Evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. J. Appl. Polym. Sci. 2012, 126, E337–E344. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Chan, W.G.; Hajaligol, M.R. Characterization of chars from pyrolysis of lignin. Fuel 2004, 83, 1469–1482. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Sevilla, M.; Maciá-Agulló, J.A.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef]
- Lammers, K.; Arbuckle-Keil, G.; Dighton, J. FT-IR study of the changes in carbohydrate chemistry of three New Jersey pine barrens leaf litters during simulated control burning. Soil Biol. Biochem. 2009, 41, 340–347. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Hu, J.; Shen, D.; Wu, S.; Zhang, H.; Xiao, R. Effect of temperature on structure evolution in char from hydrothermal degradation of lignin. J. Anal. Appl. Pryrolysis 2014, 106, 118–124. [Google Scholar] [CrossRef]
- Budai, A.; Calucci, L.; Rasse, D.P.; Tau Strand, L.; Pengerud, A.; Wiedemeier, D.; Abiven, S.; Forte, C. Effects of pyrolysis conditions on Miscanthus and corncob chars: Characterization by IR, solid state NMR and BPCA analysis. J. Anal. Appl. Pyrolysis 2017, 128, 335–345. [Google Scholar] [CrossRef]
- Adapa, P.K.; Tabil, L.G.; Schoenau, G.J.; Canam, T.; Dumonceaux, T. Quantitative analysis of lignocellulosic components of non-treated and steam exploded barley, canola, oat and wheat straw using Fourier transform infrared spectroscopy. J. Agric. Sci. Technol. B 2011, 1, 177–188. [Google Scholar]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Liu, Y.; Sun, X.; Zhang, D.; Xue, D. First identification of primary nanoparticles in the aggregation of HMF. Nanoscale Res. Lett. 2012, 7, 38. [Google Scholar] [CrossRef]
- He, Q.; Cheng, C.; Raheem, A.; Ding, L.; Shiung Lam, S.; Yu, G. Effect of hydrothermal carbonization on woody biomass: From structure to reactivity. Fuel 2022, 330, 125586. [Google Scholar] [CrossRef]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One stage olive mill waste streams valorisation via hydrothermal carbonization. Waste Manag. 2018, 20, 224–234. [Google Scholar] [CrossRef]
- Kolodziejski, W.; Frye, J.S.; Maciel, G.E. Carbon-13 nuclear magnetic resonance spectrometry with cross polarization and magic-angle spinning for analysis of lodgepole pine wood. Anal. Chem. 1982, 54, 1419–1424. [Google Scholar] [CrossRef]
- Haw, J.F.; Maciel, G.E.; Schroeder, H.A. Carbon-13 nuclear magnetic resonance spectrometric study of wood and wood pulping with cross polarization and magic-angle spinning. Anal. Chem. 1984, 56, 1323–1329. [Google Scholar] [CrossRef]
- Bardet, M.; Emsley, L.; Vincendon, M. Two-dimensional spin-exchange solid-state NMR studies of 13C-enriched wood. Solid State Nucl. Magn. Reson. 1997, 8, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Czimczik, C.I.; Preston, C.M.; Schmidt, M.W.I.; Werner, R.A.; Schulze, E.-D. Effects of charring on mass, organic carbon, and stable carbon isotope composition of wood. Org. Geochem. 2002, 33, 1207–1223. [Google Scholar] [CrossRef]
- Atalla, R.H.; VanderHart, D.L. The role of solid state 13C NMR spectroscopy in studies of the nature of native celluloses. Solid State Nucl. Magn. Reson. 1999, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lüdemann, H.-D.; Nimz, H. Carbon-13 nuclear magnetic resonance spectra of lignins. Biochem. Biophys. Res. Commun. 1973, 52, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.; Sefcik, M.D.; Stejskal, E.O.; McKay, R.A.; Hall, P.L. Characterization of the catabolic transformation of lignin in culture by magic-angle carbon-13 nuclear magnetic resonance. Macromolecules 1981, 14, 557–559. [Google Scholar] [CrossRef]
- Hatfield, G.R.; Maciel, G.E.; Erbatur, O.; Erbatur, G. Qualitative and quantitative analysis of solid lignin samples by carbon-13 nuclear magnetic resonance spectrometry. Anal. Chem. 1987, 59, 172–179. [Google Scholar] [CrossRef]
- Hatcher, P.G. Chemical structural studies of natural lignin by dipolar dephasing solid-state 13C nuclear magnetic resonance. Org. Geochem. 1987, 11, 31–39. [Google Scholar] [CrossRef]
- Mao, J.; Holtman, K.M.; Scott, J.T.; Kadla, J.F.; Schmidt-Rohr, K. Differences between lignin in unprocessed wood, milled wood, mutant wood, and extracted lignin detected by 13C solid-state NMR. J. Agric. Food Chem. 2006, 54, 9677–9686. [Google Scholar] [CrossRef]
- Teerman, S.C.; Hwang, R.J. Evaluation of the liquid hydrocarbon potential of coal by artificial maturation techniques. Org. Geochem. 1991, 17, 749–764. [Google Scholar] [CrossRef]
- Mao, J.-D.; Schmidt-Rohr, K. Recoupled long-range C-H dipolar dephasing in solid-state NMR, and its use for spectral selection of fused aromatic rings. J. Magn. Reson. 2003, 162, 217–227. [Google Scholar] [CrossRef]
- Baccile, N.; Laurent, G.; Babonneau, F.; Fayon, F.; Titirici, M.-M.; Antonietti, M. Structural characterization of hydrothermal carbon spheres by advanced solid-state MAS 13C NMR investigations. J. Phys. Chem. C 2009, 113, 9644–9654. [Google Scholar] [CrossRef]
- Falco, C.; Caballero, F.P.; Babonneau, F.; Gervais, C.; Laurent, G.; Titirici, M.-M.; Baccile, N. Hydrothermal carbon from biomass: Structural differences between hydrothermal and pyrolyzed carbons via 13C solid state NMR. Langmuir 2011, 27, 14460–14471. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Pellechia, P.J.; Flora, J.R.V.; Berge, N.D. Influence of reaction time and temperature on product formation and characteristics associated with the hydrothermal carbonization of cellulose. Bioresour. Technol. 2013, 138, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Mok, W.S.-L.; Antal, M.J., Jr. Uncatalyzed solvolysis of whole biomass hemicellulose by hot compressed liquid water. Ind. Eng. Chem. Res. 1992, 31, 1157–1161. [Google Scholar] [CrossRef]
- Lei, Y.; Su, H.; Tianc, R. Morphology evolution, formation mechanism and adsorption properties of hydrochars prepared by hydrothermal carbonization of corn stalk. RSC Adv. 2016, 6, 107829. [Google Scholar] [CrossRef]
- Hashaikeh, R.; Fang, Z.; Butler, I.S.; Hawari, J.; Kozinski, J.A. Hydrothermal dissolution of willow in hot compressed water as a model for biomass conversion. Fuel 2007, 86, 1614–1622. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Dinjus, E.; Kruse, A.; Tröger, N. Hydrothermal carbonization—1. Influence of lignin in lignocelluloses. Chem. Eng. Technol. 2011, 34, 2037–2043. [Google Scholar] [CrossRef]
- Zawadzki, J.; Wisniewski, M. 13C NMR study of cellulose thermal treatment. J. Anal. Appl. Pyrolysis 2002, 62, 111–121. [Google Scholar] [CrossRef]
- Yu, L.; Falco, C.; Weber, J.; White, R.J.; Howe, J.Y.; Titirici, M.-M. Carbohydrate-derived hydrothermal carbons: A thorough characterization study. Langmuir 2012, 28, 12373–12383. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calucci, L.; Forte, C. Influence of Process Parameters on the Hydrothermal Carbonization of Olive Tree Trimmings: A 13C Solid-State NMR Study. Appl. Sci. 2023, 13, 1515. https://doi.org/10.3390/app13031515
Calucci L, Forte C. Influence of Process Parameters on the Hydrothermal Carbonization of Olive Tree Trimmings: A 13C Solid-State NMR Study. Applied Sciences. 2023; 13(3):1515. https://doi.org/10.3390/app13031515
Chicago/Turabian StyleCalucci, Lucia, and Claudia Forte. 2023. "Influence of Process Parameters on the Hydrothermal Carbonization of Olive Tree Trimmings: A 13C Solid-State NMR Study" Applied Sciences 13, no. 3: 1515. https://doi.org/10.3390/app13031515
APA StyleCalucci, L., & Forte, C. (2023). Influence of Process Parameters on the Hydrothermal Carbonization of Olive Tree Trimmings: A 13C Solid-State NMR Study. Applied Sciences, 13(3), 1515. https://doi.org/10.3390/app13031515






