Investigating and Compensating for Periphery-Center Effect among Commercial Near Infrared Imaging Systems Using an Indocyanine Green Phantom
Abstract
:1. Introduction
2. Methods
2.1. Phantom Preparation
2.2. NIR System Assessment
2.3. Fluorescent Assessment with the Phantom
2.4. Signal Quantification
3. Results
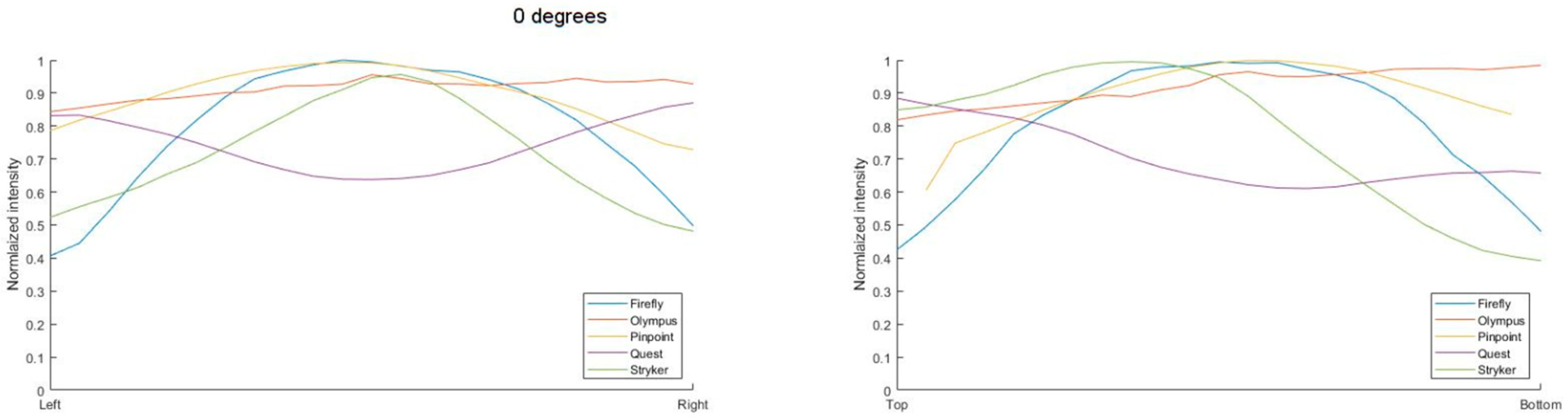
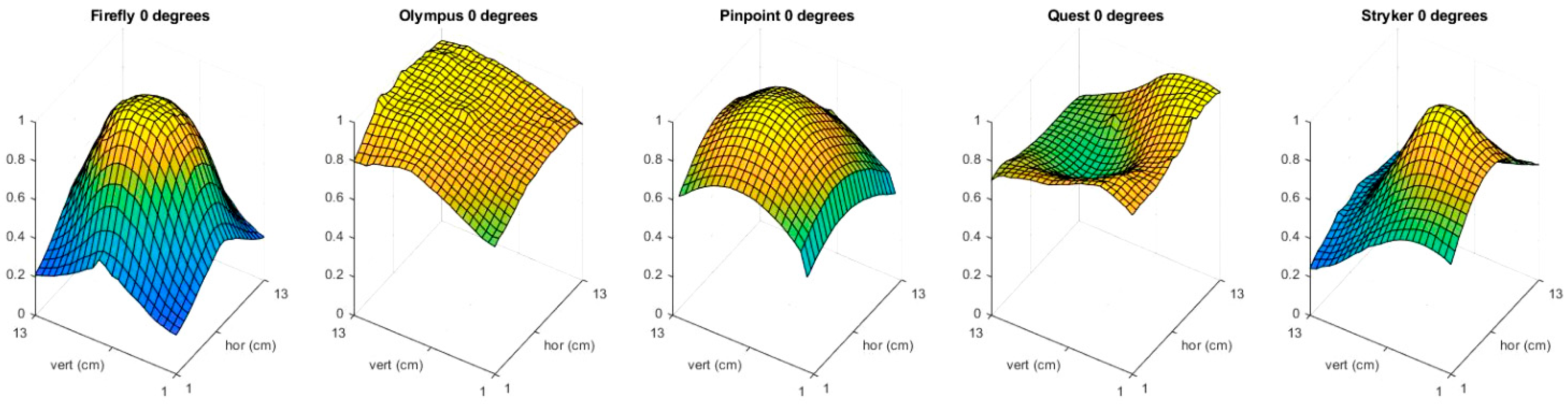
3.1. Light Distribution
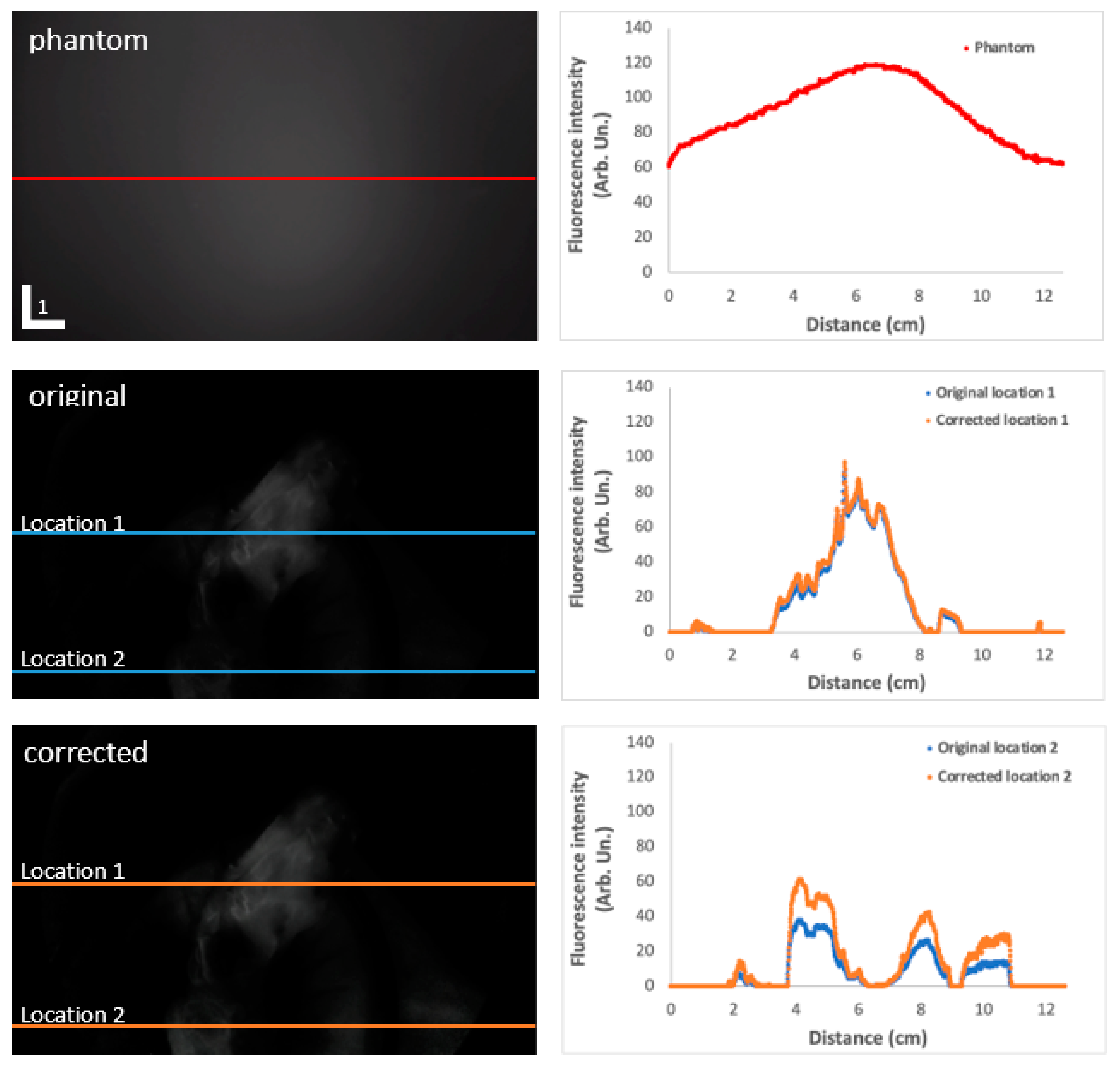
3.2. Correction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gilshtein, H.; Yellinek, S.; Wexner, S.D. The evolving role of indocyanine green fluorescence in the treatment of low rectal cancer. Ann. Laparosc. Endosc. 2018, 3, 85. [Google Scholar] [CrossRef]
- Achterberg, F.B.; Sibinga Mulder, B.G.; Meijer, R.P.J.; Bonsing, B.A.; Hartgrink, H.H.; Mieog, J.S.D.; Zlitni, A.; Park, S.M.; Farina Sarasqueta, A.; Vahrmeijer, A.L.; et al. Real-time surgical margin assessment using ICG-fluorescence during laparoscopic and robot-assisted resections of colorectal liver metastases. Ann. Transl. Med. 2020, 8, 1448. [Google Scholar] [CrossRef] [PubMed]
- van der Vorst, J.R.; Schaafsma, B.E.; Hutteman, M.; Verbeek, F.P.; Liefers, G.J.; Hartgrink, H.H.; Smit, V.T.; Lowik, C.W.; van de Velde, C.J.; Frangioni, J.V.; et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013, 119, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Landsman, M.L.; Kwant, G.; Mook, G.A.; Zijlstra, W.G. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Colino, R.; Espin-Basany, E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: A systematic review and meta-analysis. Tech. Coloproctol. 2018, 22, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Slooter, M.D.; Eshuis, W.J.; Cuesta, M.A.; Gisbertz, S.S.; van Berge Henegouwen, M.I. Fluorescent imaging using indocyanine green during esophagectomy to prevent surgical morbidity: A systematic review and meta-analysis. J. Thorac. Dis. 2019, 11, S755–S765. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, M.; Rybakov, E.; Shelygin, Y.; Chernyshov, S.; Zarodnyuk, I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: Results of the FLAG randomized trial. Color. Dis. 2020, 22, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.; Carvello, M.; Kotze, P.G.; Maroli, A.; Montroni, I.; Montorsi, M.; Buchs, N.C.; Ris, F. Ileal pouch-anal anastomosis with fluorescence angiography: A case-matched study. Color. Dis. 2019, 21, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Hardy, N.P.; Dalli, J.; Khan, M.F.; Andrejevic, P.; Neary, P.M.; Cahill, R.A. Inter-user variation in the interpretation of near infrared perfusion imaging using indocyanine green in colorectal surgery. Surg. Endosc. 2021, 35, 7074–7081. [Google Scholar] [CrossRef] [PubMed]
- Hardy, N.P.; Joosten, J.J.; Dalli, J.; Hompes, R.; Cahill, R.A.; van Berge Henegouwen, M.I. Evaluation of inter-user variability in indocyanine green fluorescence angiography to assess gastric conduit perfusion in esophageal cancer surgery. Dis. Esophagus 2022, 35, doac016. [Google Scholar] [CrossRef] [PubMed]
- Nerup, N.; Andersen, H.S.; Ambrus, R.; Strandby, R.B.; Svendsen, M.B.S.; Madsen, M.H.; Svendsen, L.B.; Achiam, M.P. Quantification of fluorescence angiography in a porcine model. Langenbeck’s Arch. Surg. 2017, 402, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Lutken, C.D.; Achiam, M.P.; Osterkamp, J.; Svendsen, M.B.; Nerup, N. Quantification of fluorescence angiography: Toward a reliable intraoperative assessment of tissue perfusion—A narrative review. Langenbeck’s Arch. Surg. 2021, 406, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Hardy, N.; Mac Aonghusa, P.G.; Epperlein, J.P.; Cantillon Murphy, P.; Cahill, R.A. Challenges in the interpretation of colorectal indocyanine green fluorescence angiography—A video vignette. Color. Dis. 2021, 23, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Gorpas, D.; Koch, M.; Anastasopoulou, M.; Bozhko, D.; Klemm, U.; Nieberler, M.; Ntziachristos, V. Multi-Parametric Standardization of Fluorescence Imaging Systems Based on a Composite Phantom. IEEE Trans. Bio-Med. Eng. 2020, 67, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.J.; Wu, M.; LaRochelle, E.P.M.; Gorpas, D.; Ntziachristos, V.; Pfefer, T.J.; Pogue, B.W. Indocyanine green matching phantom for fluorescence-guided surgery imaging system characterization and performance assessment. J. Biomed. Opt. 2020, 25, 056003. [Google Scholar] [CrossRef] [PubMed]
- Anastasopoulou, M.; Koch, M.; Gorpas, D.; Karlas, A.; Klemm, U.; Garcia-Allende, P.B.; Ntziachristos, V. Comprehensive phantom for interventional fluorescence molecular imaging. J. Biomed. Opt. 2016, 21, 091309. [Google Scholar] [CrossRef] [PubMed]
- Nadler, S.B.; Hidalgo, J.H.; Bloch, T. Prediction of blood volume in normal human adults. Surgery 1962, 51, 224–232. [Google Scholar] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Company Website. Available online: https://mologic.co.uk/our-core-markets/infection-and-infectious-disease/periplex/ (accessed on 10 October 2022).
- Nerup, N.; Knudsen, K.B.K.; Ambrus, R.; Svendsen, M.B.S.; Thymann, T.; Ifaoui, I.B.R.; Svendsen, L.B.; Achiam, M.P. Reproducibility and Reliability of Repeated Quantitative Fluorescence Angiography. Surg. Technol. Int. 2017, 31, 35–39. [Google Scholar] [PubMed]
- Dalli, J.; Gallagher, G.; Jindal, A.; Epperlein, J.; Hardy, N.; Malallah, R.e.; O’Donoghue, K.; Cantillon-Murphy, P.; Mac Aonghusa, P.; Cahill, R. Objective Interrogation of Signal Presentation from Surgical Near-Infrared Fluorescence Systems for User and Computerised Interpretation; SPIE: Bellingham, WA, USA, 2022; Volume 12146. [Google Scholar]


| Imaging Device | Highest Intensity (AU) | Lowest Intensity (AU) | FSL% |
|---|---|---|---|
| Firefly | 114 | 23 | 80 |
| Olympus | 86 | 57 | 34 |
| Pinpoint | 174 | 76 | 57 |
| Quest | 175 | 107 | 39 |
| Stryker | 107 | 26 | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joosten, J.J.; Bloemen, P.R.; van den Elzen, R.M.; Dalli, J.; Cahill, R.A.; van Berge Henegouwen, M.I.; Hompes, R.; de Bruin, D.M. Investigating and Compensating for Periphery-Center Effect among Commercial Near Infrared Imaging Systems Using an Indocyanine Green Phantom. Appl. Sci. 2023, 13, 2042. https://doi.org/10.3390/app13042042
Joosten JJ, Bloemen PR, van den Elzen RM, Dalli J, Cahill RA, van Berge Henegouwen MI, Hompes R, de Bruin DM. Investigating and Compensating for Periphery-Center Effect among Commercial Near Infrared Imaging Systems Using an Indocyanine Green Phantom. Applied Sciences. 2023; 13(4):2042. https://doi.org/10.3390/app13042042
Chicago/Turabian StyleJoosten, Johanna J., Paul R. Bloemen, Richard M. van den Elzen, Jeffrey Dalli, Ronan A. Cahill, Mark I. van Berge Henegouwen, Roel Hompes, and Daniel M. de Bruin. 2023. "Investigating and Compensating for Periphery-Center Effect among Commercial Near Infrared Imaging Systems Using an Indocyanine Green Phantom" Applied Sciences 13, no. 4: 2042. https://doi.org/10.3390/app13042042






