Impact of Vanadium Complexes with Tetradentate Schiff Base Ligands on the DPPC and EYL Liposome Membranes: EPR Studies
Abstract
:1. Introduction
2. Materials and Methods
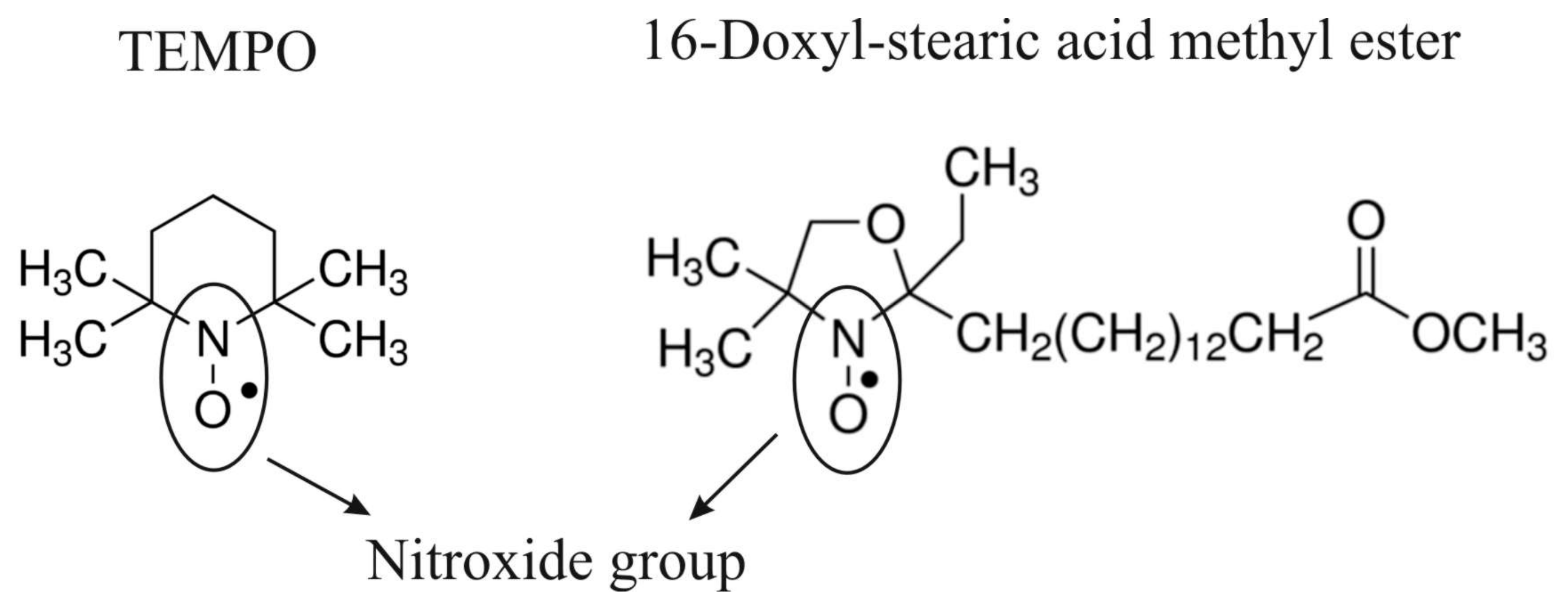
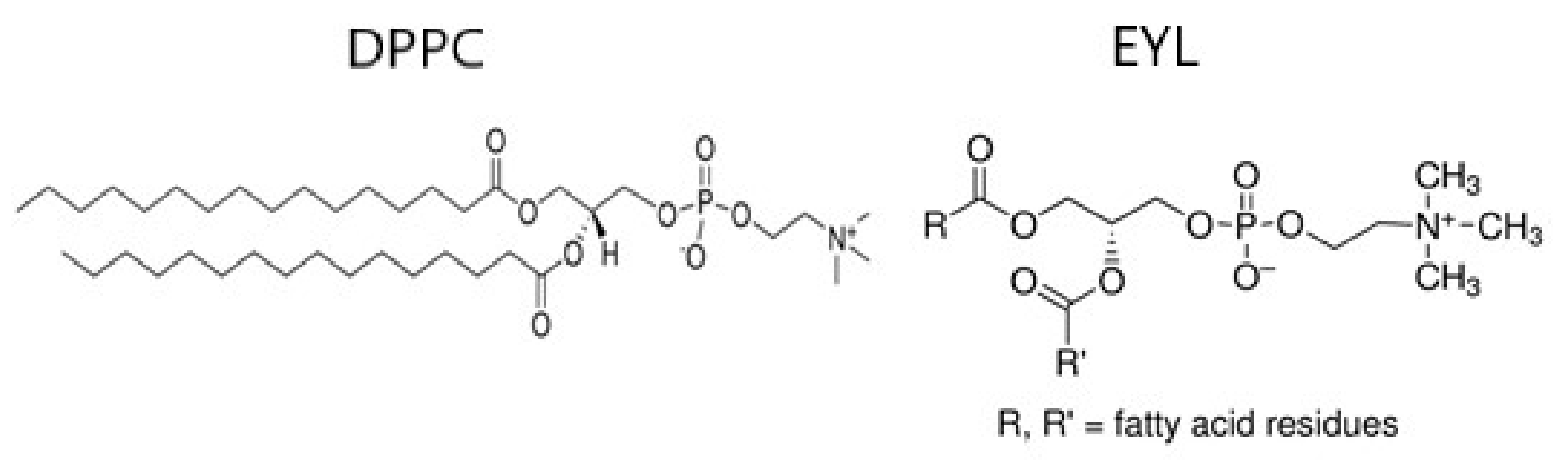
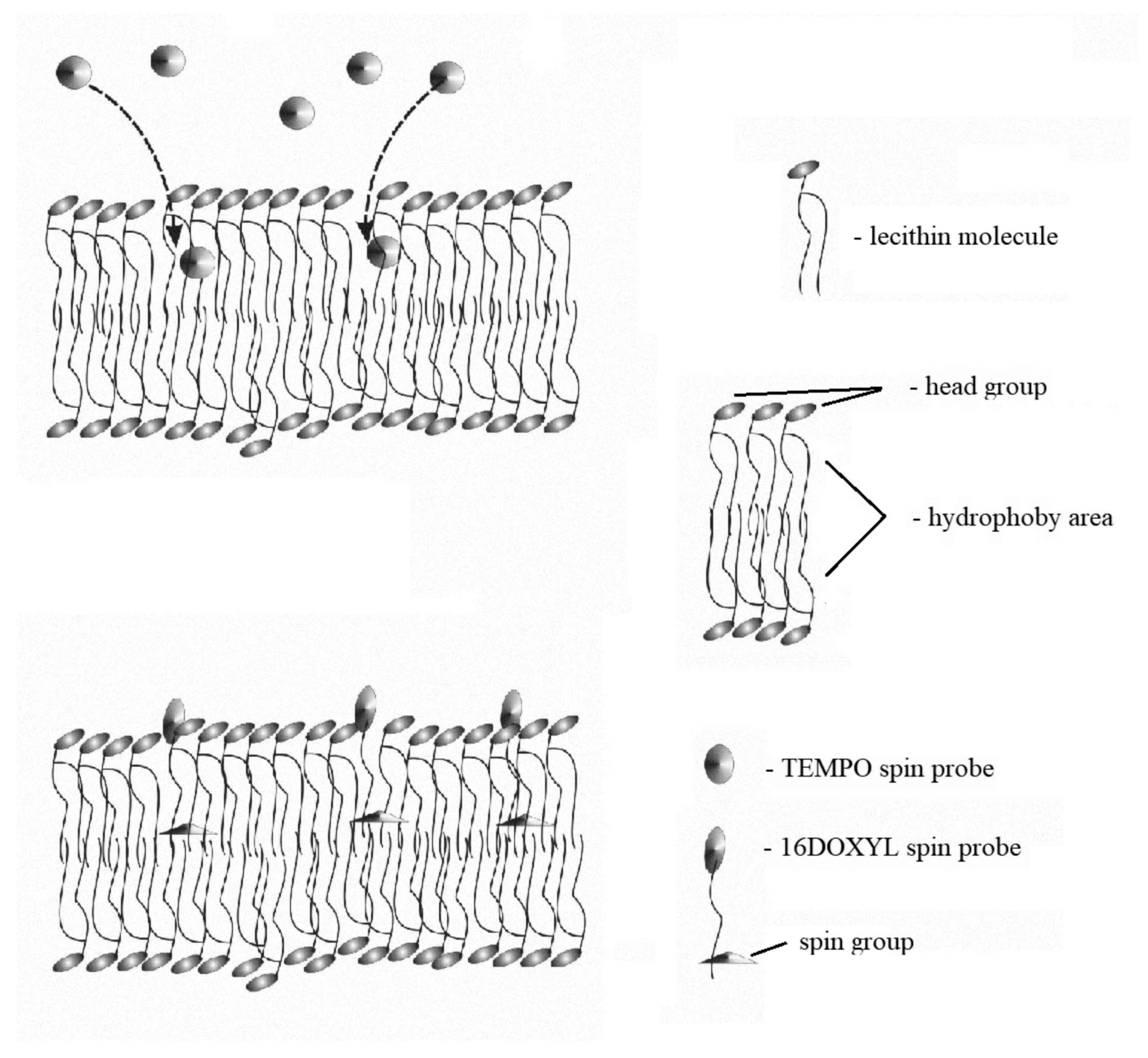
2.1. Spin Probes and Lecithin
2.2. Vanadium Complexes
2.3. Liposom Membranes
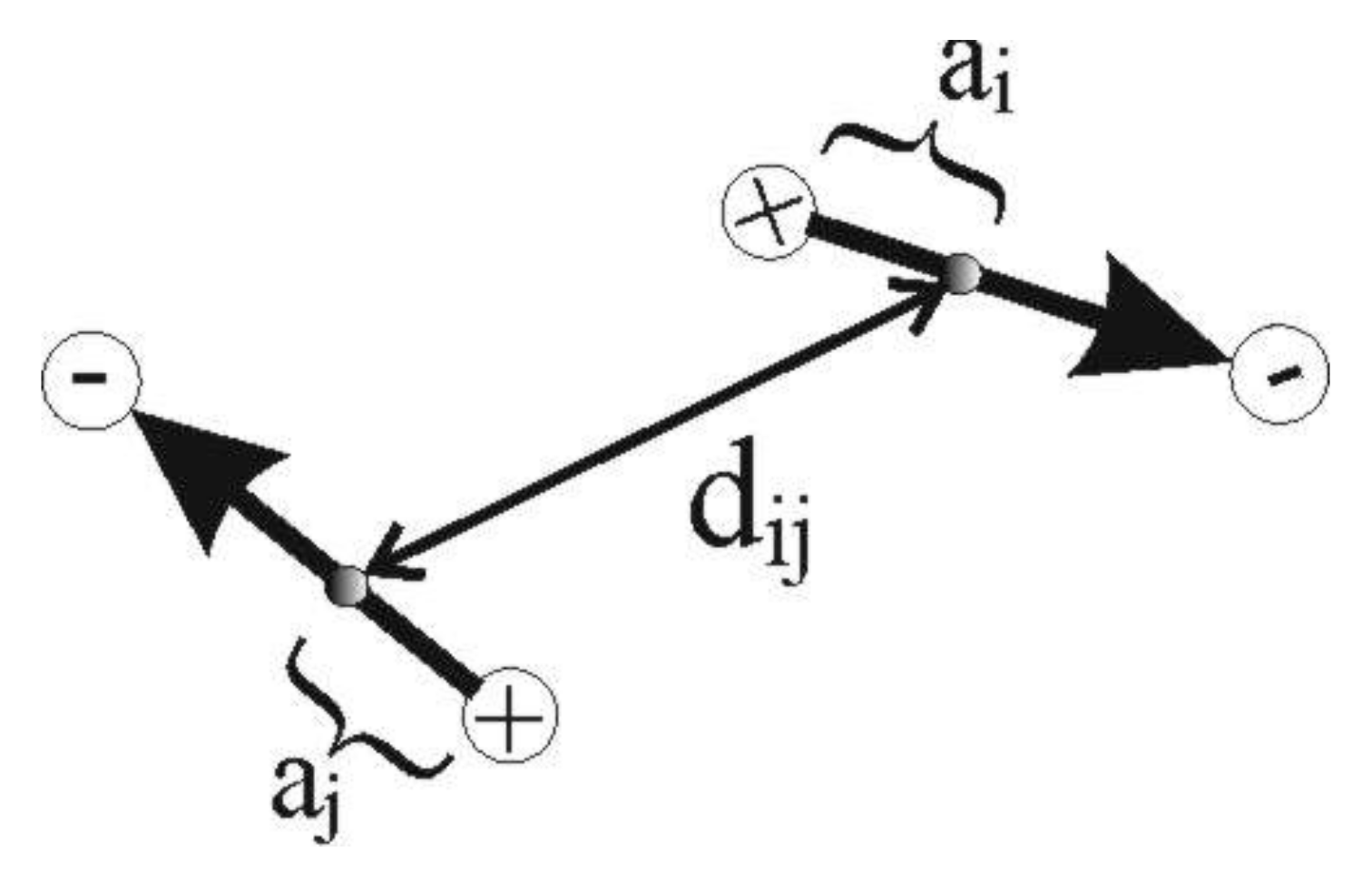
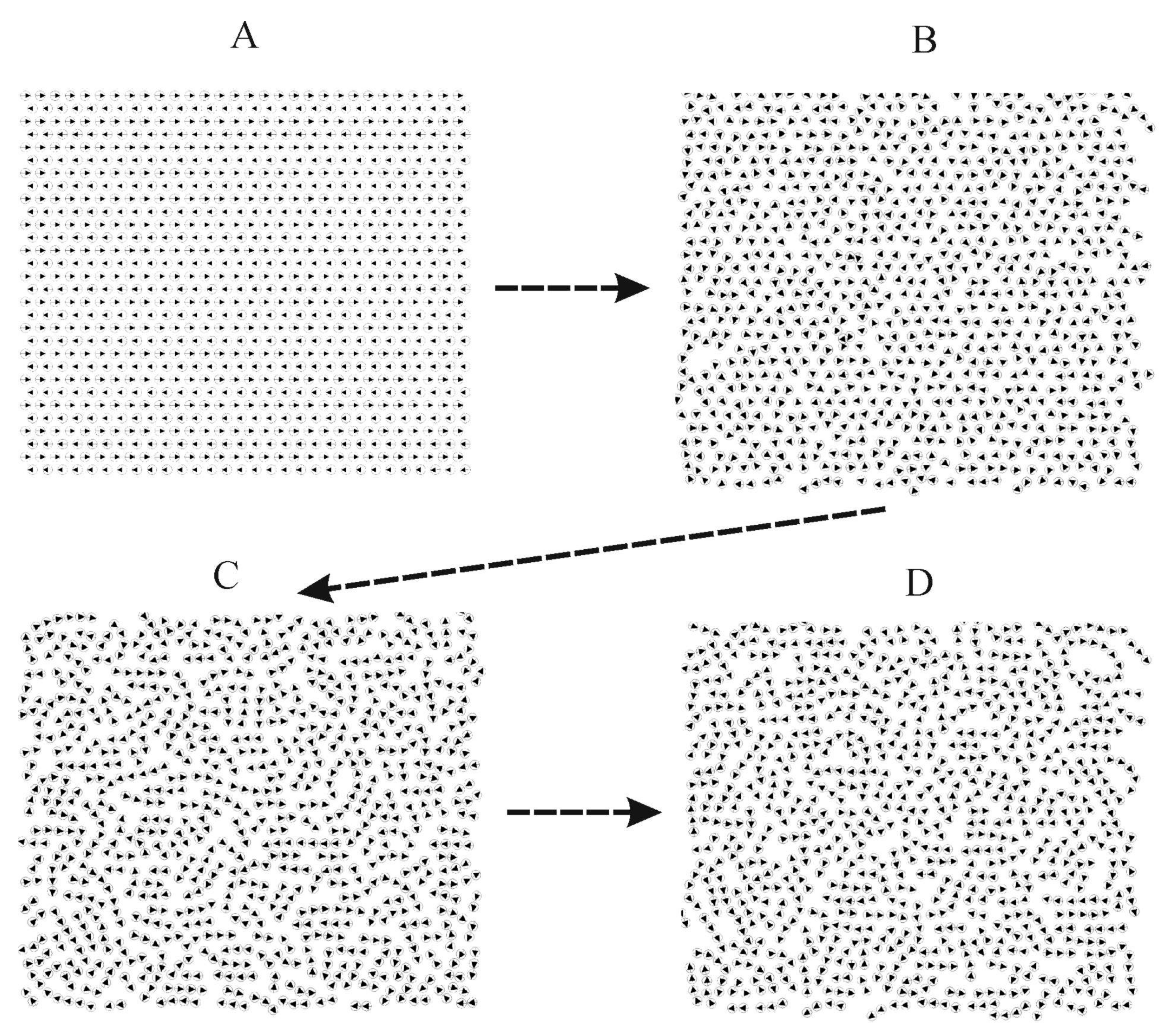
2.4. Computer-Based Model
3. Results
3.1. EPR Spectra of Vanadium Complexes
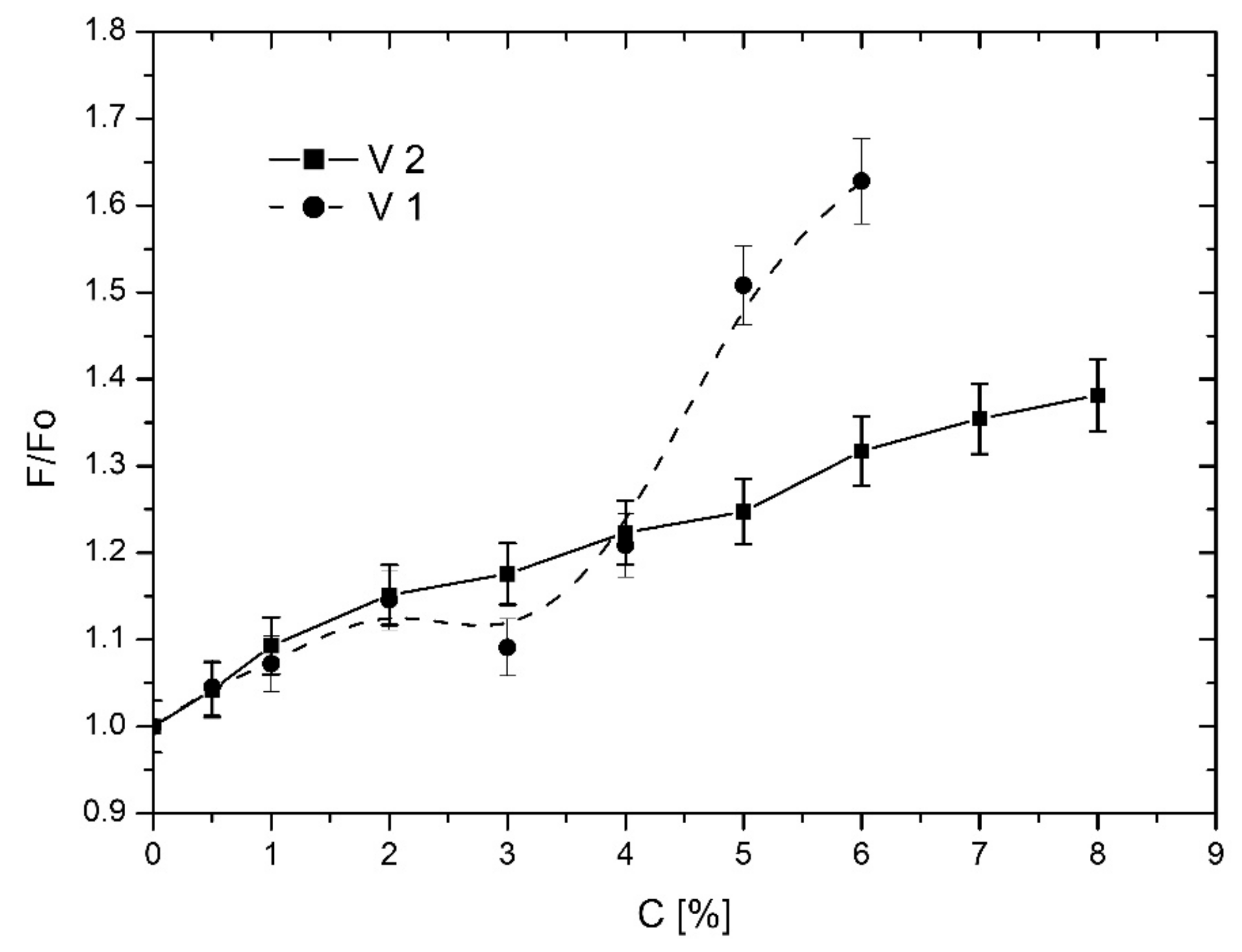
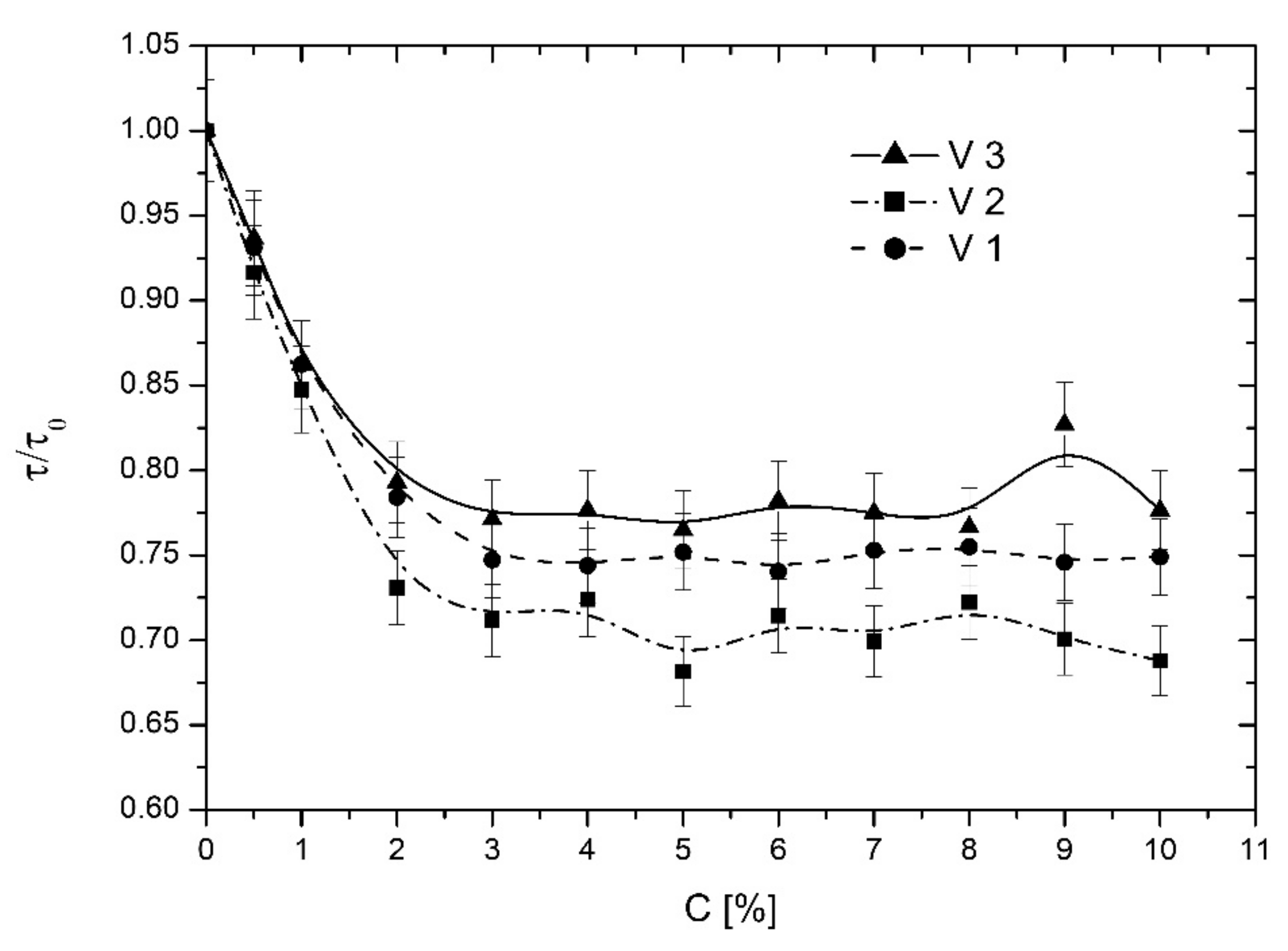
3.2. Effect of Vanadium Complexes on EYL Liposome Membranes
3.2.1. TEMPO Probe
3.2.2. 16DOXYL Probe
3.3. Effect of Vanadium Complexes on DPPC Liposome Membranes
3.3.1. TEMPO Probe
3.3.2. 16DOXYL Probe
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bihun-Kisiel, A.; Ochędzan-Siodłak, W. Vanadium catalysts for ethylene-norbornene. Copolym. Polim. 2020, 65, 757–770. [Google Scholar] [CrossRef]
- Ochędzan-Siodłak, W.; Bihun, A.; Olszowy, A.; Rajfur, M.; Jesionowski, T.; Siwińska-Stefańska, K. Ethylene polymerization using vanadium catalyst supported on silica modified by pyridinium ionic liquid. Polym. Int. 2016, 65, 1089–1097. [Google Scholar] [CrossRef]
- Mirchev, N.; Lazarova, D.; Georgieva, M.; Petrova, M.; Tachev, D.; Avdeev, G. Preparation of Cu/ZrW2O8 structures by chemical deposition from formaldehyde-free solution. Trans. IMF 2020, 100, 18–24. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K.; Białek, M.; Man, D. Tri-alkenyl polyhedral oligomeric silsesquioxanes as comonomers and active center modifiers in ethylene copolymerization catalyzed by bis(phenoxyimine) Ti, Zr, V and V salen-type complexes. Appl. Catal. A Gen. 2018, 567, 122–131. [Google Scholar] [CrossRef]
- Ishikura, H.; Neven, R.; Lange, T.; Galetova, A.; Blom, B.; Romano, D. Developments in vanadium-catalysed polymerisation reactions: A review. Inorg. Chim. Acta 2021, 515, 120047. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of Vanadium Complex Catalysts for Precise Olefin Polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal–Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef]
- Liu, X.; Hamon, J.-R. Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes. Coord. Chem. Rev. 2019, 389, 94–118. [Google Scholar] [CrossRef]
- Li, L.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.; Zhang, J.; Xia, G.; Hu, J.; Graff, G.; et al. A stable vanadium redoxflow battery with high energy density for large-scale energy storage. Adv. Energy Mater. 2011, 1, 394–400. [Google Scholar] [CrossRef]
- Rahman, F.; Skyllas-Kazacos, M. Vanadium redox battery: Positive half-cell electrolyte studies. J. Power Sources 2009, 189, 1212–1219. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—A review. ChemSusChem 2016, 9, 1521–1543. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Zhang, H.; Han, X.; Luo, Q. Investigations on transfer of water and vanadium ions across nafion membrane in an operating vanadium redox flow battery. J. Power Sources 2010, 195, 890–897. [Google Scholar] [CrossRef]
- Galloni, P.; Conte, V.; Floris, B. A journey into the electrochemistry of vanadium compounds. Coord. Chem. Rev. 2015, 301–302, 240–299. [Google Scholar] [CrossRef] [Green Version]
- Goldwaser, I.; Qian, S.; Gershonov, E.; Fridkin, M.; Shechter, Y. Organic vanadium chelators potentiate vanadium-evoked glucose metabolism in vitro and in vivo: Establishing criteria for optimal chelators. Mol. Pharmacol. 2000, 58, 738–746. [Google Scholar] [CrossRef]
- Brannick, B.; Kocak, M.; Solomon, S. Vanadium in Glucose Metabolism: Past, Present and Future. J. Toxicol. Pharm. 2017, 1, 1–011. [Google Scholar]
- Heyliger, C.E.; Tahiliani, A.G.; McNeill, J.H. Effect of Vanadate on Elevated Blood Glucose and Depressed Cardiac Performance of Diabetic Rats. Science 1985, 227, 1474–1477. [Google Scholar] [CrossRef]
- Meyerovitch, J.; Backer, J.M.; Kahn, C.R. Hepatic phosphotyrosine phosphatase activity and its alterations in diabetic rats. J. Clin. Investig. 1989, 84, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.H.; Park, S.H.; Choi, G.H.; Park, I.J.; Lee, J.H.; Lee, O.H.; Kim, J.H.; Seo, Y.H.; Cho, J.H. Antidiabetic effect of an extract of nutricultured Brassica napus containing vanadium from a Jeju water concentrate. Food Sci. Biotechnol. 2018, 28, 209–214. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Mehdi, M.Z. Insulino-mimetic and anti-diabetic effects of vanadium compounds. Diabet. Med. 2005, 22, 2–13. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, B.; Feng, C.; Zhang, Z.; Lei, Z.; Shimizu, K. Human health risk of vanadium in farmland soils near various vanadium ore mining areas and bioremediation assessment. Chemosphere 2021, 263, 128246. [Google Scholar] [CrossRef]
- Evangelou, A.M. Vanadium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Basile, A.; Mazzone, A.; Morello, S.; Turco, M.C.; Pellecchia, C.J. Therapeutic potential of a pyridoxal-based vanadium(IV) complex showing selective cytotoxicity for cancer versus healthy cells. Cell Physiol. 2013, 228, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Lay, P.A. Stabilities and biological activities of vanadium drugs: What is the nature of the active species? Chem. Asian J. 2017, 12, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301, 87–105. [Google Scholar] [CrossRef]
- Levina, A.; Pires Vieira, A.; Wijetunga, A.; Kaur, R.; Koehn, J.T.; Cran, D.C.; La, P.A. A Short-Lived but Highly Cytotoxic Vanadium (V) Complex as a Potential Drug Lead for Brain Cancer Treatment by Intratumoral Injections. Angew. Chem. Int. Ed. 2020, 59, 15834–15838. [Google Scholar] [CrossRef]
- Corona-Motolinia, N.D.; Martínez-Valencia, B.; Noriega, L.; Sánchez-Gaytán, B.L.; Méndez-Rojas, M.Á.; Melendez, F.J.; González-Vergara, E. Synthesis, crystal structure, and computational methods of vanadium and copper compounds as potential drugs for cancer treatment. Molecules 2020, 25, 4679. [Google Scholar] [CrossRef]
- Crans, D.C.; Koehn, J.T.; Petry, S.M.; Glover, C.M.; Wijetunga, A.; Kaur, R.; Lay, P.A. Hydrophobicity may enhance membrane affinity and anti-cancer effects of Schiff base vanadium (v) catecholate complexes. Dalton Trans. 2019, 48, 6383–6395. [Google Scholar] [CrossRef]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Kanna, P.S.; Mahendrakumar, C.B.; Indira, B.N.; Srivastawa, S.; Kalaiselvi, K.; Elayaraja, T.; Chatterjee, M. Chemopreventive effects of vanadium toward 1,2-dimethylhydrazine-induced genotoxicity and preneoplastic lesions in rat colon. Environ. Mol. Mutagen. 2004, 44, 113–118. [Google Scholar] [CrossRef]
- Anupam, B.; Abhijeet, W.; Mehool, A.P.; Malay, C. Vanadium in the detection, prevention and treatment of cancer: The in vivo evidence. Cancer Lett. 2010, 294, 1–12. [Google Scholar] [CrossRef]
- Liasko, R.; Karkabounas, S.; Kabanos, T. Antitumor effects of a Vanadium complex with cysteine on malignant cell lines and tumorbearing Wistar rats. Met. Ions Biol. Med. 2000, 6, 577–579. [Google Scholar]
- Man, D.; Pisarek, I.; Braczkowski, M.; Pytel, B.; Olchawa, R. The impact of humic and fulvic acids on the dynamic properties of liposome membranes: The ESR method. J. Liposome Res. 2014, 24, 106–112. [Google Scholar] [CrossRef]
- Man, D.; Olchawa, R. Two-step impact of Amphotericin B AmB on lipid membranes. ESR experiment and computer simulations. J. Liposome Res. 2013, 23, 327–335. [Google Scholar] [CrossRef]
- Wałęsa, R.; Man, D.; Engel, G.; Siodłak, D.; Kupka, T.; Ptak, T.; Broda, M.A. The Impact of Model Peptides on Structural and Dynamic Properties of Egg Yolk Lecithin Liposomes—Experimental and DFT Studies. Chem. Biodivers. 2015, 12, 1007–1024. [Google Scholar] [CrossRef]
- Pytel, B.; Filipiak, A.; Pisarek, I.; Olchawa, R.; Man, D. Impact of humic acids on EYL liposome membranes: ESR method. Nukleonika 2015, 60, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Dyrda, G.; Boniewska-Bernacka, E.; Man, D.; Barchiewicz, K.; Słota, R. The effect of organic solvents on selected microorganisms and model liposome membrane. Mol. Biol. Rep. 2019, 46, 3225–3232. [Google Scholar] [CrossRef] [Green Version]
- Man, D.; Słota, R.; Kawecka, A.; Engel, G.; Dyrda, G. Liposomes modified by mono- and bis-phthalocyanines: A comprehensive EPR study. Eur. Phys. J. E 2017, 40, 63. [Google Scholar] [CrossRef] [Green Version]
- Man, D.; Olchawa, R. Dynamics of surface of lipid membranes: Theoretical considerations and the ESR experiment. Eur. Biophys. J. 2017, 46, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Man, D.; Mrówka, I.; Wójcik, A.; Pytel, B. Impact of Vanadium(IV)-Oxy Acetylacetonate and Vanadium(III) Acetylacetonatel on the DPPC Liposome Membranes: EPR Studies. Acta Phys. Pol. A 2017, 132, 52–56. [Google Scholar] [CrossRef]
- Białek, M.; Czaja, K. Dichlorovanadium (IV) Complexes with Salen-Type Ligands for Ethylene Polymerization. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6940–6949. [Google Scholar] [CrossRef]
- Białek, M.; Liboska, O. Vanadium Complex with Tetradentate [O,N,N,O] Ligand Supported on Magnesium Type Carrier for Ethylene Homopolymerization and Copolymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 471–478. [Google Scholar] [CrossRef]
- Białek, M.; Bisz, E. A comparative study on the polymerization of 1-octene promoted by vanadium and titanium complexes supported by phenoxyimine and salen type ligands. J. Polym. Res. 2013, 20, 164. [Google Scholar] [CrossRef] [Green Version]
- Shimshick, E.J.; McConnell, H.M. Lateral phase separation in phospholipid membranes. Biochemistry 1973, 12, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Hemminga, M.A. Interpretation of ESR and saturation transfer ESR spectra of spin labeled lipids and membranes. Chem. Phys. Lipids 1983, 32, 323–383. [Google Scholar] [CrossRef]
- Stigter, D.; Mingins, J.; Dill, K.A. Phospholipid interactions in model membrane systems. Biophys 1992, 61, 1616–1629. [Google Scholar] [CrossRef]
- Lawton, J.S.; Aaron, D.S.; Tang, Z.; Zawodzinski, T.A. Qualitative behavior of vanadium ions in Nafion membranes using electron spin resonance. J. Membr. Sci. 2013, 428, 38–45. [Google Scholar] [CrossRef]
- Man, D.; Olchawa, R.; Kubica, K. Membrane fluidity and the surface properties of the lipid bilayer: ESR experiment and computer simulation. J. Liposome Res. 2010, 20, 211–218. [Google Scholar] [CrossRef]
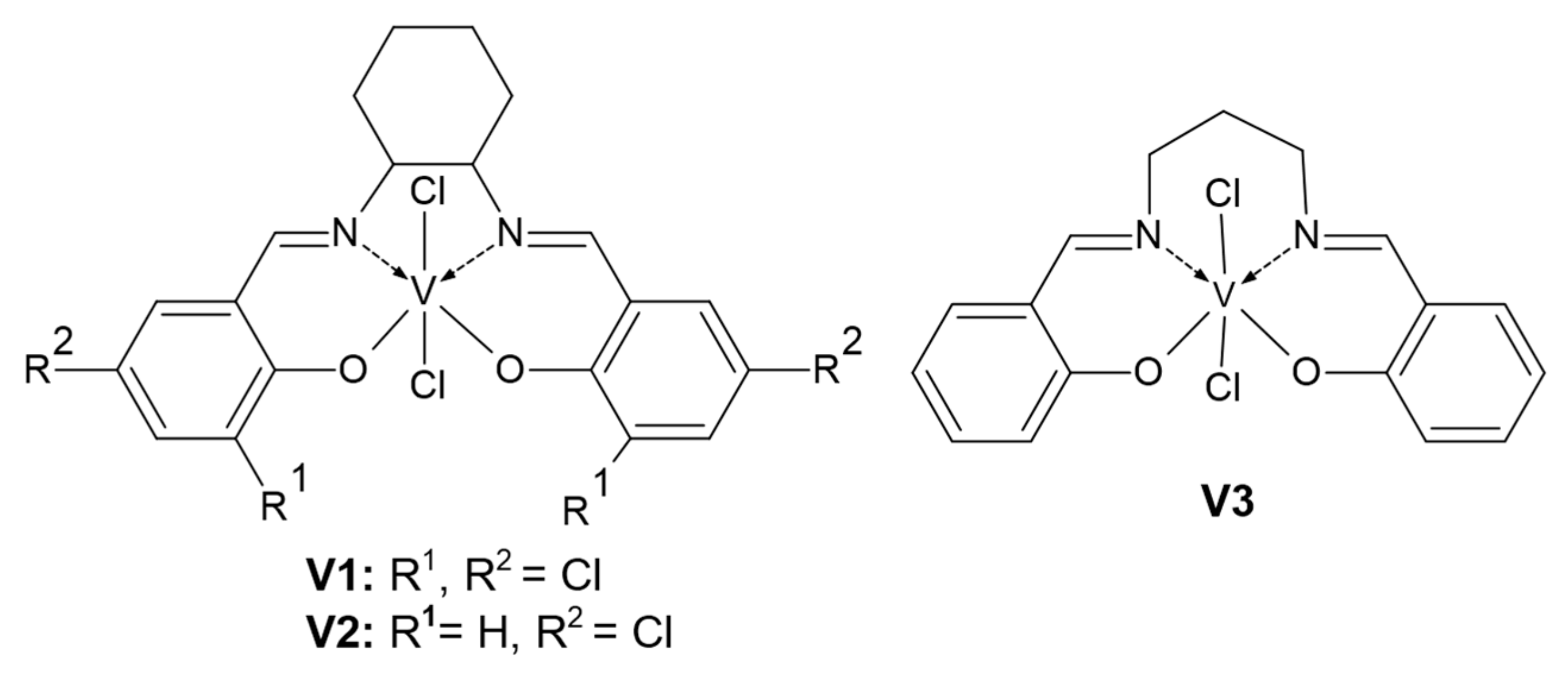
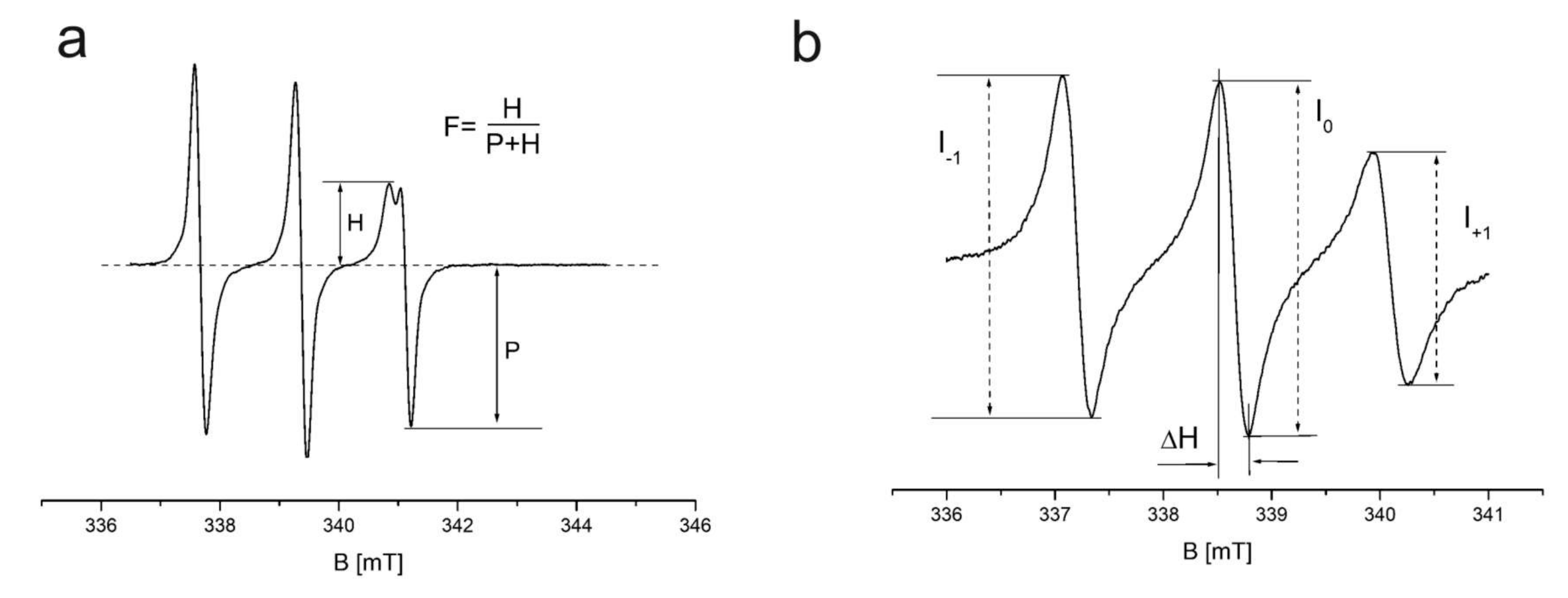
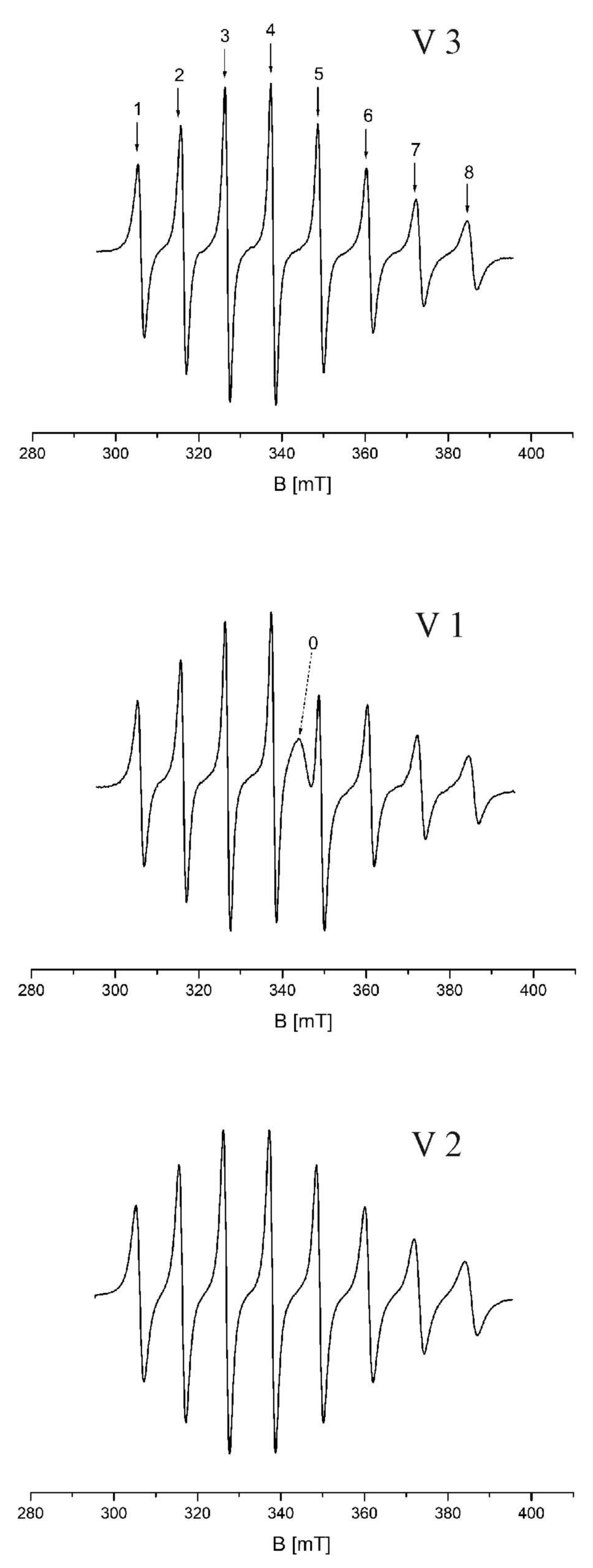


| V3 | V1 | V2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Line No. | dH [mT] | Centre [mT] | g | dH [mT] | Centre [mT] | g | dH [mT] | Centre [mT] | g |
| 1 | 1.496 | 306.146 | 2.249766 | 1.554 | 306.145 | 2.249773 | 1.902 | 306.225 | 2.249186 |
| 2 | 1.369 | 316.382 | 2.176979 | 1.353 | 316.406 | 2.176814 | 1.647 | 316.484 | 2.176277 |
| 3 | 1.211 | 326.947 | 2.106632 | 1.245 | 326.963 | 2.106529 | 1.47 | 327.022 | 2.106149 |
| 4 | 1.241 | 337.945 | 2.038074 | 1.232 | 337.97 | 2.037923 | 1.484 | 338.027 | 2.03758 |
| 5 | 1.393 | 349.364 | 1.971459 | 1.365 | 349.268 | 1.972001 | 1.669 | 349.405 | 1.971228 |
| 6 | 1.634 | 361.147 | 1.907137 | 1.633 | 361.135 | 1.907201 | 1.997 | 361.156 | 1.90709 |
| 7 | 1.925 | 373.247 | 1.845311 | 1.932 | 373.241 | 1.845341 | 2.408 | 373.186 | 1.845613 |
| 8 | 2.359 | 385.759 | 1.785459 | 2.417 | 385.736 | 1.785566 | 2.951 | 385.683 | 1.785811 |
| 0 | 2.842 | 345.424 | 1.993946 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, D.; Pytel, B.; Białek, M. Impact of Vanadium Complexes with Tetradentate Schiff Base Ligands on the DPPC and EYL Liposome Membranes: EPR Studies. Appl. Sci. 2023, 13, 3272. https://doi.org/10.3390/app13053272
Man D, Pytel B, Białek M. Impact of Vanadium Complexes with Tetradentate Schiff Base Ligands on the DPPC and EYL Liposome Membranes: EPR Studies. Applied Sciences. 2023; 13(5):3272. https://doi.org/10.3390/app13053272
Chicago/Turabian StyleMan, Dariusz, Barbara Pytel, and Marzena Białek. 2023. "Impact of Vanadium Complexes with Tetradentate Schiff Base Ligands on the DPPC and EYL Liposome Membranes: EPR Studies" Applied Sciences 13, no. 5: 3272. https://doi.org/10.3390/app13053272
APA StyleMan, D., Pytel, B., & Białek, M. (2023). Impact of Vanadium Complexes with Tetradentate Schiff Base Ligands on the DPPC and EYL Liposome Membranes: EPR Studies. Applied Sciences, 13(5), 3272. https://doi.org/10.3390/app13053272






