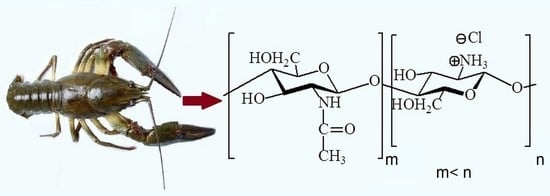
A Walkway from Crayfish to Oligochitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Matherials
2.2. Separation of Chitin
2.3. Separation of Chitosan
2.4. Preparation of Oligochitosan Hydrochloride
2.5. Protein Content Analysis
3. Results and Discussion
3.1. Crayfish Waste Treatment and Separation of Chitin
3.2. Chitin Deacetylation and Preparation of Chitosan
3.3. Preparation of Oligochitosan Hydrochloride
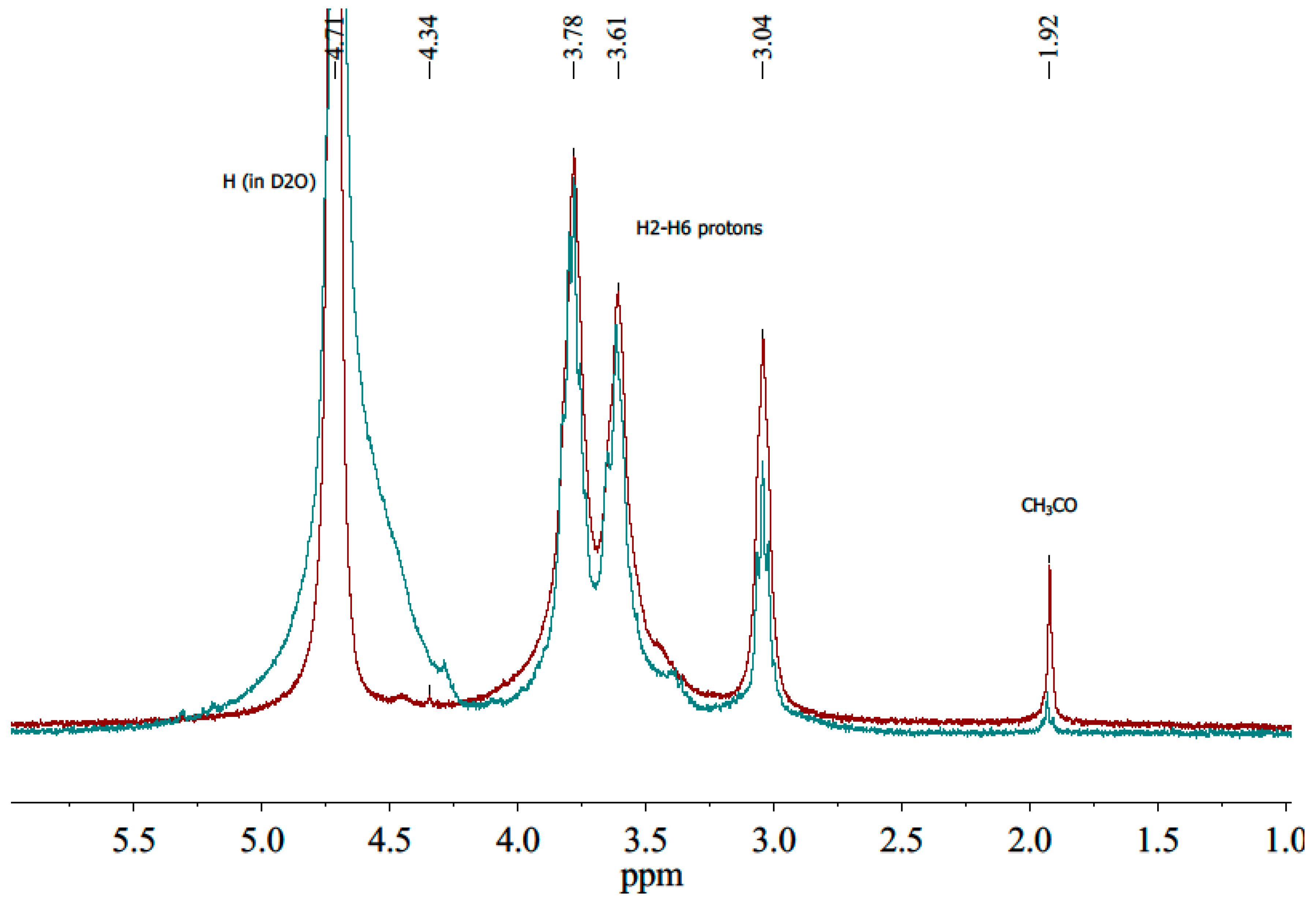
3.4. Molecular Characterization of Chitosan and Oligochitosan Hydrochloride
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, processing and modification techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical application of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of chitosan- a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef]
- Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Chitosan production by fungi: Current state of knowledge, future opportunities and constraints. Fermentation 2022, 8, 76. [Google Scholar] [CrossRef]
- “Chitosan”; USA Pharmacopoeia USP 34–NF29; Second Supplement; The United States Pharmacopeial Convention: Rockville, MD, USA, 2011; pp. 5361–5365.
- Baki, M.A.; Hossain, M.; Akter, J.; Quraishi, S.B.; Shojib, F.H.; Atique Ullah, A.K.M.; Khan, F. Concentration of heavy metals in seafood (fishes, shrimps, lobsters and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol. Environ. Saf. 2018, 159, 153–163. [Google Scholar] [CrossRef]
- Aydin, H.; Harlıoğlu, M.M.; Deniz (Bök), T. Harvest, export and economic status of freshwater crayfish (Astacus leptodactylus Esch. 1823) in Turkey. Afr. J. Agric. Res. 2012, 7, 2463–2468. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, S.; Jiang, H. A review on conversion of crayfish-shell derivatives to functional materials and their environmental applications. J. Bioresour. Bioprod. 2020, 5, 238–247. [Google Scholar] [CrossRef]
- Duman, F.; Kaya, M. Crayfish chitosan for microencapsulation of coriander (Coriandrum sativum L.) essential oil. Int. J. Biol. Macromol. 2016, 92, 125–133. [Google Scholar] [CrossRef]
- Gholampoor, M.; Mahmoodi, G.; Moshfegh, A.; Tehranifard, A. Antimicrobial properties of chitosan extracted from freshwater shrimp (Astacus leptodactylus) caught from Aras Lake. Iranian J. Aquat. Anim. Health 2020, 6, 105–117. [Google Scholar] [CrossRef]
- Riofrio, A.; Alcivar, T.; Baykara, H. Environmental and economic viability of chitosan production in Guayas-Ecuador: A robust investment and life cycle analysis. ACS Omega 2021, 6, 23038–23051. [Google Scholar] [CrossRef]
- Küçükgülmez, A. Extraction of chitin from crayfish (Astacus leptodactylus) shell waste. Alınteri J. Agric. Sci. 2018, 33, 99–104. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, L.; Qin, Y.; Li, P. Activity of chitin/chitosan/chitosan oligosaccharide against plant pathogenic nematodes and potential modes of application in agriculture: A review. Carbohydr. Polym. 2023, 306, 120592. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of chitooligosaccharides and their potential application in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef]
- Kulikov, S.; Tikhonov, V.; Blagodatskikh, I.; Bezrodnykh, E.; Lopatin, S.; Khairullin, R.; Philippova, Y.; Abramchuk, S. Molecular weight and pH aspects of the efficacy of oligochitosan against meticillin-resistant Staphylococcus aureus (MRSA). Carbohydr. Polym. 2012, 87, 545–550. [Google Scholar] [CrossRef]
- Tikhonov, V. The Terms Relating to Chitin and Chitosan. Available online: https://www.researchgate.net/profile/Vladimir_Tikhonov/contributions (accessed on 2 March 2023).
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Scherließ, R.; Buske, S.; Young, K.; Weber, B.; Rades, T.; Hook, S. In vivo evaluation of chitosan as an adjuvant in subcutaneous vaccine formulations. Vaccine 2013, 31, 4812–4819. [Google Scholar] [CrossRef]
- Rødde, R.H.; Einbu, A.; Vårum, K.M. A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohydr. Polym. 2008, 71, 388–393. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Meyers, S.P.; Lee, K.S. Isolation and characterization of chitin from crawfish shell waste. J. Agric. Food Chem. 1989, 37, 575–579. [Google Scholar] [CrossRef]
- Berezin, B.B.; Bezrodnykh, E.A.; Blagodatskikh, I.V.; Yamskov, I.A.; Tikhonov, V.E. Extraction of residual heavy metals from commercial chitosan and approach to oligochitosan hydrochloride. Carbohydr. Polym. 2019, 215, 316–321. [Google Scholar] [CrossRef]
- Ibram, A.; Ionescu, A.-M.; Cadar, E. Comparison of extraction methods of chitin and chitosan from different sources. Eur. J. Natur. Sci. Med. 2019, 2, 23–36. [Google Scholar] [CrossRef]
- Adekanmi, A.A.; Adekanmi, S.A.; Adekanmi, O.S. Different processing sequential protocols for extraction, quantification and characterization of chitosan from crayfish. IJEAIS 2020, 4, 47–61. [Google Scholar]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Palacios, J.L.; Barahona, T.; Mosi-Roa, Y.; Anguita-Barrales, F.; Bustamante, M. Synthesis of new chitosan from an endemic Chilean crayfish exoskeleton (Parastacus Pugnax): Physicochemical and biological properties. Polymers 2021, 13, 2304. [Google Scholar] [CrossRef]
- Fernandez-Kim, S.-O. Physicochemical and Functional Properties of Crawfish Chitosan as Affected by Different Processing Protocols. LSU Master’s Theses, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 2004. Available online: https://digitalcommons.lsu.edu/gradschool_theses/1338 (accessed on 2 March 2023).
- Signini, R.; Campana Filho, S.P. On the preparation and characterization of chitosan hydrochloride. Polym. Bull. 1999, 42, 159–166. [Google Scholar] [CrossRef]
- Minh, N.C.; Nguyen, V.H.; Schwarz, S.; Stevens, W.F.; Trung, T.S. Preparation of water soluble hydrochloric chitosan from low molecular weight chitosan in the solid state. Int. J. Biol. Macromol. 2019, 121, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, V.E.; Berezin, B.B.; Blagodatskikh, I.V.; Bezrodnykh, E.A. Oligochitosan hydrochloride: Preparation and characterization. INEOS Open 2021, 4, 103–106. [Google Scholar] [CrossRef]
- The European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2004; Volume 1–2, pp. 1248–1249.
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Chatterjee, S.; Adhya, M.; Guha, A.K.; Chatterjee, B.P. Chitosan from Mucor rouxii: Production and physicochemical characterization. Process Biochem. 2005, 40, 395–400. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Chitosan, The United State Pharmacopoeia, USP36-NF31, 1st ed.; United States Pharmacopeias: Rockville, MD, USA, 2013; pp. 1959–1963.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Martins de Queiroz Antonino, R.S.C.; Lia Fook, B.R.P.; Alexandre de Oliveira Lima, V.; Ícaro de Farias Rached, R.; Nascimento Lima, E.P.; José da Silva Lima, R.; Peniche Covas, C.A.; Vinícius Lia Fook, M. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeusvannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef]
- Chang, S.-H.; Lin, H.-T.V.; Wu, G.J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antimicrobial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Chae, S.Y.; Jang, M.-K.; Nah, J.-W. Influence of molecular weight on oral absorption of water soluble chitosan. J. Control Release 2005, 102, 383–394. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Sun, Y.; Xing, J.; Yamamoto, A.; Gao, Y. Absorption-improving effects of chitosan oligomers based on their mucoadhesive properties: A comparative study on the oral and pulmonary delivery of calcitonin. Drug Deliv. 2016, 23, 2419–2427. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Lisovskaya, S.A.; Zelenikhin, P.V.; Bezrodnykh, E.A.; Shakirova, D.R.; Blagodatskikh, I.V.; Tikhonov, V.E. Antifungal activity of oligochitosans (short chain chitosans) against some Candida species and clinical isolates of C. albicans: Molecular weight—Activity relationship. Eur. J. Med. Chem. 2014, 74, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Bezrodnykh, E.A.; Berezin, B.B.; Antonov, Y.A.; Zhuravleva, I.L.; Atamas, A.A.; Tsarenko, A.A.; Rogachev, A.V.; Tikhonov, V.E. A feasible approach to tune the interaction of chitosan with sodium dodecyl sulfate. Carbohydr. Polym. 2022, 292, 119642. [Google Scholar] [CrossRef]
- Allan, G.G.; Peyron, M. Molecular weight manipulation of chitosan II: Prediction and control of extent of depolymerization by nitrous acid. Carbohydr. Res. 1995, 277, 273–282. [Google Scholar] [CrossRef]
- Bezrodnykh, E.A.; Blagodatskikh, I.V.; Kulikov, S.N.; Zelenikhin, P.V.; Yamskov, I.A.; Tikhonov, V.E. Consequences of chitosan decomposition by nitrous acid: Approach to non-branched oligochitosan oxime. Carbohydr. Polym. 2018, 195, 551–557. [Google Scholar] [CrossRef]
- Ilyina, A.V.; Tikhonov, V.E.; Albulov, A.I.; Varlamov, V.P. Enzymatic preparation of acid-free-water soluble chitosan. Process Biochem. 2000, 35, 563–568. [Google Scholar] [CrossRef]
- Qin, C.Q.; Du, Y.M.; Xiao, L. Effect of hydrogen peroxide treatment on the molecular weight and structure of chitosan. Polym. Degrad. Stab. 2002, 76, 211–218. [Google Scholar] [CrossRef]
- Tian, F.; Liu, Y.; Hu, K.; Zhao, B. Study of the depolymerization behavior of chitosan by hydrogen peroxide. Carbohydr. Polym. 2004, 57, 31–37. [Google Scholar] [CrossRef]
- Tømmeraas, K.; Vårum, K.W.; Christensen, B.E.; Smidsrød, O. Preparation and characterization of oligosaccharides produced by nitrous acid depolymerization of chitosan. Carbohydr. Res. 2007, 333, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Einbu, A.; Grasdalen, H.; Vårum, K.W. Kinetics of hydrolysis of chitin/chitosan oligomers in concentrated hydrochloric acid. Carbohydr. Res. 2007, 342, 1055–1062. [Google Scholar] [CrossRef]
- Einbu, A.; Vårum, K.W. Depolymerization and de-N-acetylation of chitin oligomers in hydrochloric acid. Biomacromolecules 2007, 8, 309–314. [Google Scholar] [CrossRef]
- Vårum, K.W.; Ottøy, M.H.; Smodsrød, O. Acid hydrolysis of chitosan. Carbohydr. Polym. 2001, 46, 89–98. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors influencing the antibacterial activity of chitosan and chitosan modified by fictionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Alburquerque, C.; Bucarey, S.A.; Neira-Carrillo, A.; Urzúa, B.; Hermosilla, G.; Tapia, C.V. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med. Mycol. 2010, 48, 1018–1023. [Google Scholar] [CrossRef]
- Ballal, N.V.; Kundabala, M.; Bhat, K.S.; Acharya, S.; Ballal, M.; Kumar, R.; Prakash, P.Y. Susceptibility of Candida albicans and Enterococcus faecalis to chitosan, chlorhexidine gluconate and their combination in vitro. Aust. Endod. J. 2009, 35, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Tin, S.; Lim, C.S.; Sakharkar, M.K.; Sakharkar, K.R. Synergistic combinations of chitosans and antibiotics in Staphylococcus aureus. Lett. Drug Des. Discov. 2010, 7, 31–35. [Google Scholar] [CrossRef]
- Tin, S.; Sakharkar, K.R.; Lim, C.S.; Sakharkar, M.K. Activity of chitosans in combination with antibiotics in Pseudomonas aeruginosa. Int. J. Biol. Sci. 2009, 5, 153–160. [Google Scholar] [CrossRef] [PubMed]

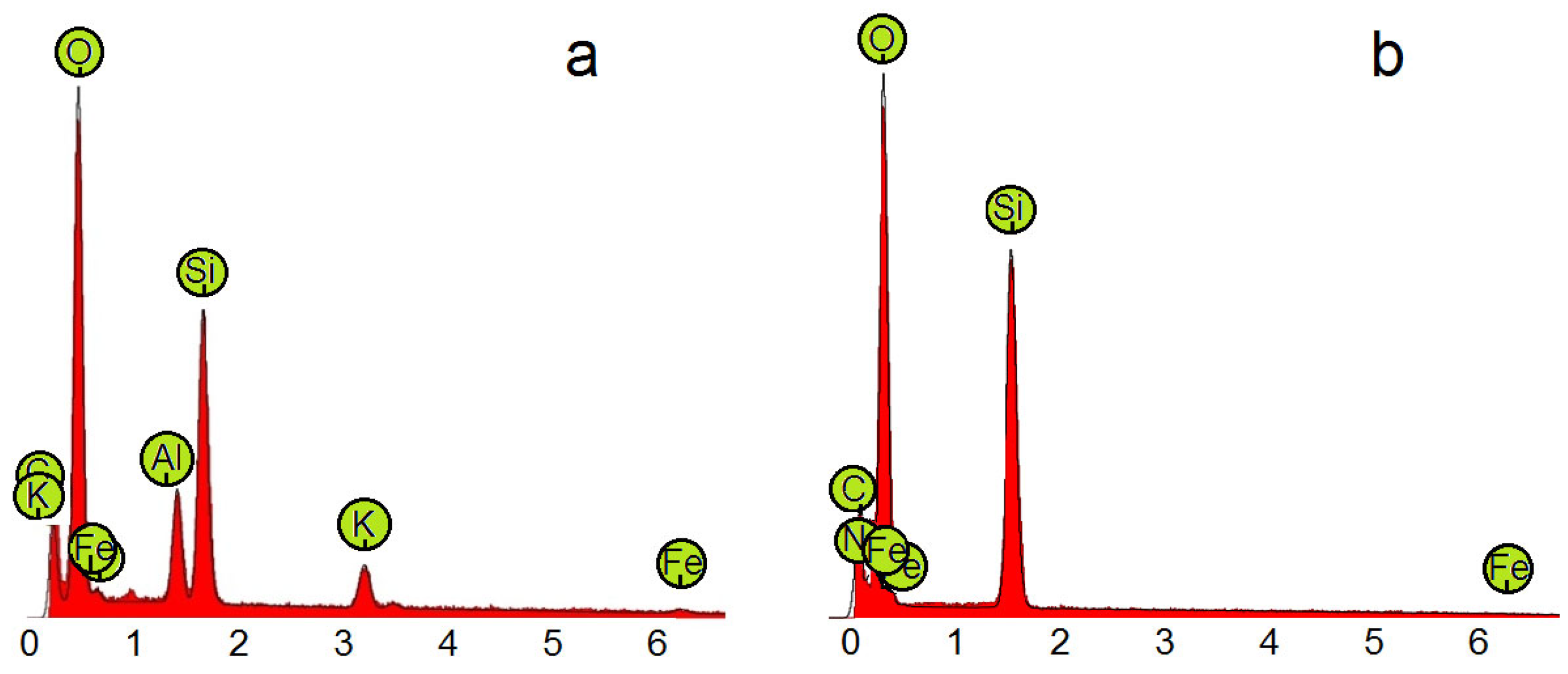


| Compound | Proteins (%) | Fe (ppm) | Cr (ppm) | Ni (ppm) |
|---|---|---|---|---|
| Crude waste | 10 ± 2 | 23.0 | 0.4 | 0.3 |
| Chitin-AlSiG | 2 ± 0.5 | 2.5 | Nf * | Nf * |
| Chitin-BSiG | 2 ± 0.5 | 3.0 | Nf * | Nf * |
| Chitosan | 0.11 ± 0.03 | 3.7 | Nf * | Nf * |
| Oligochitosan | <0.01 | 2.8 | Nf * | Nf * |
| Sample | Mw (kDa) | Mn (kDA) | Mp (kDa) | Dm |
|---|---|---|---|---|
| Chitosan | 370 | 128 | 295 | 2.88 |
| Oligochitosan | 11.3 | 6.0 | 8.6 | 1.89 |
| Characteristics | USP (2013) Acceptable Criteria for Chitosan | Factual Data for Chitosan | EP (2004) Acceptable Criteria for Chitosan Hydrochloride | Factual Data for Oligochitosan Hydrochloride |
|---|---|---|---|---|
| Appearance (in solid state) | absent | white | white or almost white | white |
| Matter insoluble in water | absent | 0.15% | ≤0.5% | 0.05% |
| Degree of deacetylation (%) | 70–95% | 96% | unstandardized value | 98% |
| Proteins (%) | ≤0.2% | 0.11 | not applied | <0.01 |
| Chlorides (%) | not applied | not found | 10–20% | 16.3% |
| Humidity (%) | ≤5% | 3.1% | ≤10% | 9.1% |
| Ash (%) | ≤1% | 0.05% | ≤1% | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezrodnykh, E.A.; Vyshivannaya, O.V.; Berezin, B.B.; Blagodatskikh, I.V.; Tikhonov, V.E. A Walkway from Crayfish to Oligochitosan. Appl. Sci. 2023, 13, 3360. https://doi.org/10.3390/app13053360
Bezrodnykh EA, Vyshivannaya OV, Berezin BB, Blagodatskikh IV, Tikhonov VE. A Walkway from Crayfish to Oligochitosan. Applied Sciences. 2023; 13(5):3360. https://doi.org/10.3390/app13053360
Chicago/Turabian StyleBezrodnykh, Evgeniya A., Oxana V. Vyshivannaya, Boris B. Berezin, Inesa V. Blagodatskikh, and Vladimir E. Tikhonov. 2023. "A Walkway from Crayfish to Oligochitosan" Applied Sciences 13, no. 5: 3360. https://doi.org/10.3390/app13053360
APA StyleBezrodnykh, E. A., Vyshivannaya, O. V., Berezin, B. B., Blagodatskikh, I. V., & Tikhonov, V. E. (2023). A Walkway from Crayfish to Oligochitosan. Applied Sciences, 13(5), 3360. https://doi.org/10.3390/app13053360