Is Probiotics Supplementation an Appropriate Strategy to Modulate Inflammation in Physically Active Healthy Adults or Athletes? A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Quality Assessment
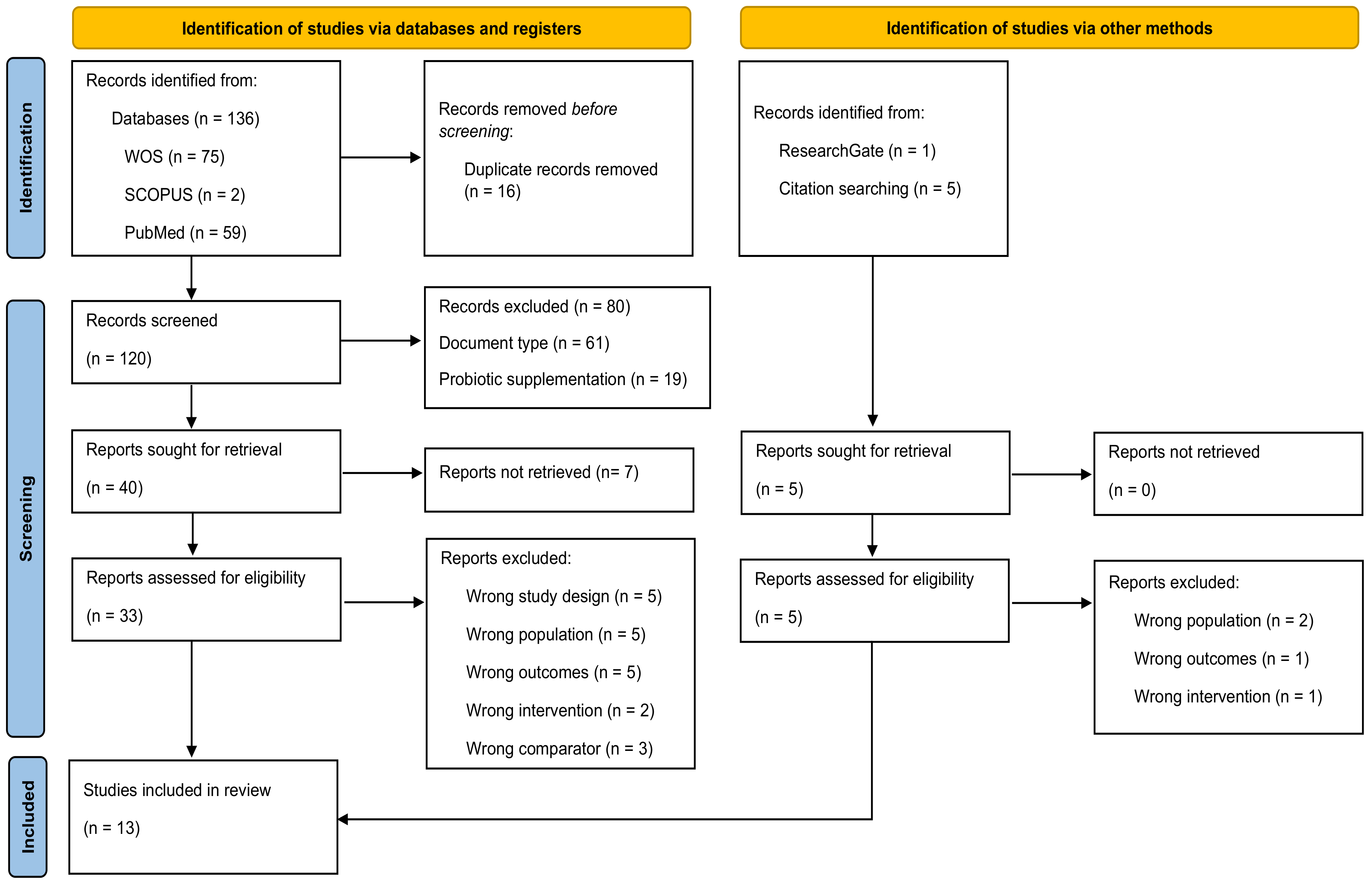
3. Results
3.1. Study Selection
3.2. Quality Assessment
3.3. Characteristics of the Participants and the Intervention
3.4. Outcome Measures
3.5. Anti-Inflammatory Cytokines
3.6. Pro-Inflammatory Cytokines
4. Discussion
4.1. Probiotics Supplementation
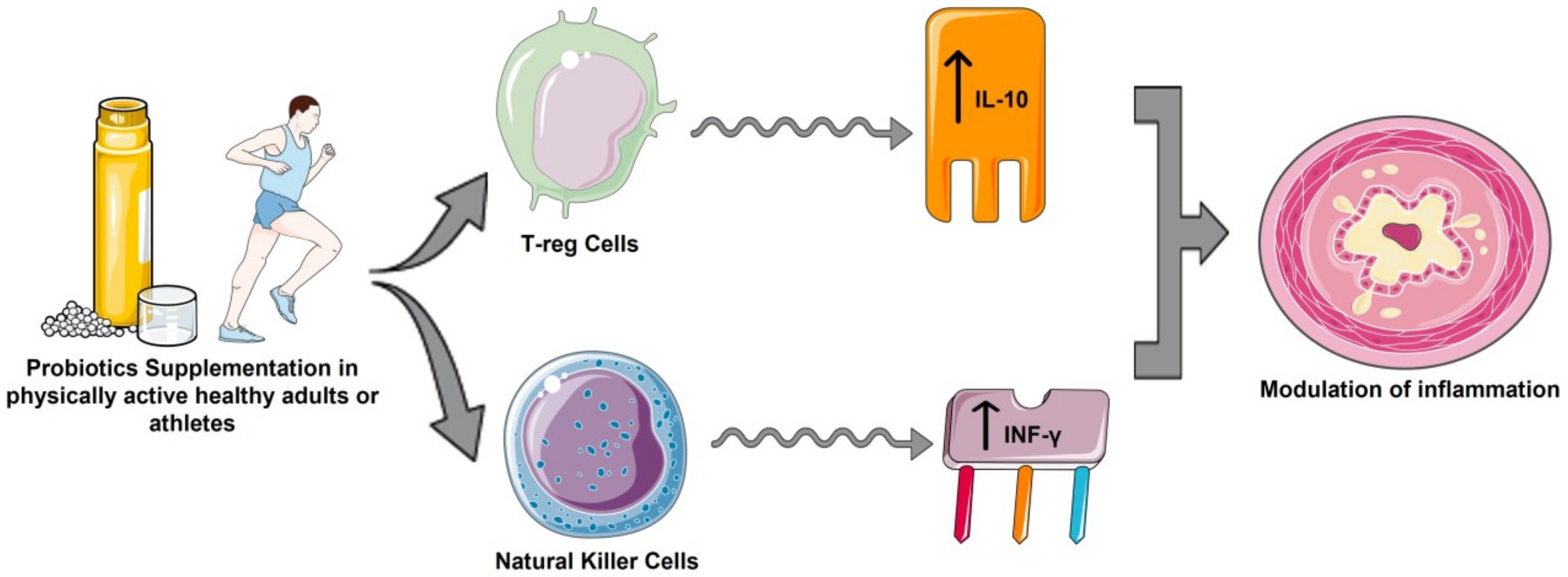
4.2. Anti-Inflammatory Cytokines
4.3. Pro-Inflammatory Cytokines
5. Future Scenarios
6. Strengths and Limitations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony Forming Units |
| EIMD | Exercise-Induced Muscle Damage |
| GIT | Gastrointestinal Tract |
| ICO | International Olympic Committee |
| ILs | Interleukins |
| JAK | Janus Kinase |
| MAPKs | Mitogen-Activated Protein Kinases |
| MeSH | Medical Subject Headings |
| MIP-1 | Macrophage Inflammatory Protein-1 |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyzes |
| SCFA | Short-Chain Fatty Acids |
| TNF-α | Tumor Necrosis Factor-alpha |
| VO2max | Maximum Volume of Oxygen |
| WOS | Web of Science |
References
- Eloe-Fadrosh, E.A.; Rasko, D.A. The Human Microbiome: From Symbiosis to Pathogenesis. Annu. Rev. Med. 2013, 64, 145–163. [Google Scholar] [CrossRef] [Green Version]
- Moles, L.; Otaegui, D. The Impact of Diet on Microbiota Evolution and Human Health. Is Diet an Adequate Tool for Microbiota Modulation? Nutrients 2020, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Del Campo-Moreno, R.; Alarcón-Cavero, T.; D’Auria, G.; Delgado-Palacio, S.; Ferrer-Martínez, M. Microbiota in human health: Characterization and transfer techniques. Enferm. Infecc. Microbiol. Clin. 2018, 36, 241–245. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; González-Bernal, J.J.; Sánchez-Serrano, N.; Navascués, L.J.; Del Río, A.A.; Mielgo-Ayuso, J. Physical Exercise as a Multimodal Tool for COVID-19: Could It Be Used as a Preventive Strategy? Int. J. Environ. Res. Public Health 2020, 17, 8496. [Google Scholar] [CrossRef] [PubMed]
- Kreher, J.B.; Schwartz, J.B. Overtraining Syndrome: A Practical Guide. Sports Health 2012, 4, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.L. Cytokine hypothesis of overtraining: A physiological adaptation to excessive stress? Med. Sci. Sports Exerc. 2000, 32, 317–331. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Navascués, L.J.; Martínez, A.C.; Seco-Calvo, J. The Role of Selenium Mineral Trace Element in Exercise: Antioxidant Defense System, Muscle Performance, Hormone Response, and Athletic Performance. A Systematic Review. Nutrients 2020, 12, 1790. [Google Scholar] [CrossRef]
- Stožer, A.; Vodopivc, P.; Bombek, L.K. Pathophysiology of Exercise-Induced Muscle Damage and Its Structural, Functional, Metabolic, and Clinical Consequences. Physiol. Res. 2020, 69, 565–598. [Google Scholar] [CrossRef]
- Malm, C. Exercise-induced muscle damage and inflammation: Fact or fiction? Acta Physiol. Scand. 2001, 171, 233–239. [Google Scholar] [CrossRef]
- Zuhl, M.; Schneider, S.; Lanphere, K.; Conn, C.; Dokladny, K.; Moseley, P. Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 2014, 48, 980–986. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Hernández-Burgos, N.; Cobreros Mielgo, R.; García-Lázaro, S. Evaluation of physical activity as a therapeutic adjuvant for patients with inflammatory bowel disease: A review. Investig. Clin. 2022, 63, 304–322. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Mika, A.S.; McCubbin, A.J. The impact of exercise modality on exercise-induced gastrointestinal syndrome and associated gastrointestinal symptoms. J. Sci. Med. Sport. 2022, 25, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Lee, E.C.; Armstrong, E.M. Interactions of Gut Microbiota, Endotoxemia, Immune Function, and Diet in Exertional Heatstroke. J. Sports Med. 2018, 2018, 5724575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Calvo, J.S.; Martínez, A.C.; García, A.C.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef] [Green Version]
- Miles, M.P. Probiotics in sports nutrition. Probiotics Adv. Food Health Appl. 2022, 1, 277–295. [Google Scholar]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef] [Green Version]
- Theodorakopoulou, M.; Perros, E.; Giamarellos-Bourboulis, E.J.; Dimopoulos, G. Controversies in the management of the critically ill: The role of probiotics. Int. J. Antimicrob. Agents 2013, 42, S41–S44. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Robles-Sánchez, C.; Abadía-Molina, F.; Morón-Calvente, V.; Sáez-Lara, M.J.; Ruiz-Bravo, A.; Jiménez-Valera, M.; Gil, Á.; Gómez-Llorente, C.; Fontana, L. Adamdec1, Ednrb and Ptgs1/Cox1, inflammation genes upregulated in the intestinal mucosa of obese rats, are downregulated by three probiotic strains. Sci. Rep. 2017, 7, 1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, A.E.; Basile, A.J.; Crawford, M.S.; Sweazea, K.L.; Carpenter, K.C. Probiotic Supplementation Has a Limited Effect on Circulating Immune and Inflammatory Markers in Healthy Adults: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2020, 120, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Calero, C.D.Q.; Rincón, E.O.; Marqueta, P.M. Probiotics, prebiotics and synbiotics: Useful for athletes and active individuals? A systematic review. Benef. Microbes 2020, 27, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.T.; Peng, Y.C.; Yen, H.Y.; Wu, J.C.; Hou, W.H. Effects of Probiotic Supplementation on Immune and Inflammatory Markers in Athletes: A Meta-Analysis of Randomized Clinical Trials. Medicina 2022, 58, 1188. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Law, M.; Stewart, C.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998; pp. 1–9. [Google Scholar]
- Axelrod, C.L.; Brennan, C.J.; Cresci, G.; Paul, D.; Hull, M.; Fealy, C.E.; Kirwan, J.P. UCC118 supplementation reduces exercise-induced gastrointestinal permeability and remodels the gut microbiome in healthy humans. Physiol. Rep. 2019, 7, e14276. [Google Scholar] [CrossRef] [Green Version]
- Batatinha, H.; Tavares-Silva, E.; Leite, G.S.F.; Resende, A.S.; Albuquerque, J.A.T.; Arslanian, C.; Fock, R.A.; Lancha AHJr Lira, F.S.; Krüger, K.; Thomatieli-Santos, R.; et al. Probiotic supplementation in marathonists and its impact on lymphocyte population and function after a marathon: A randomized placebo-controlled double-blind study. Sci. Rep. 2020, 10, 18777. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef]
- Tavares-Silva, E.; Caris, A.V.; Santos, S.A.; Ravacci, G.R.; Thomatieli-Santos, R.V. Effect of Multi-Strain Probiotic Supplementation on URTI Symptoms and Cytokine Production by Monocytes after a Marathon Race: A Randomized, Double-Blind, Placebo Study. Nutrients 2021, 13, 1478. [Google Scholar] [CrossRef]
- Aisberg, M.; Paixão, V.; Almeida, E.B.; Santos, J.M.B.; Foster, R.; Rossi, M.; Pithon-Curi, T.C.; Gorjão, R.; Momesso, C.M.; Andrade, M.S.; et al. Daily Intake of Fermented Milk Containing Lactobacillus casei Shirota (Lcs) Modulates Systemic and Upper Airways Immune/Inflammatory Responses in Marathon Runners. Nutrients 2019, 11, 1678. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.K.; Allerton, D.M.; Ansley-Robson, P.; Hemmings, K.; Cox, M.; Costa, R.J.S. Does Short-Term High Dose Probiotic Supplementation Containing Lactobacillus casei Attenuate Exertional-Heat Stress Induced Endotoxaemia and Cytokinaemia? Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Hoffman, M.W.; Zelicha, H.; Gepner, Y.; Willoughby, D.S.; Feinstein, U.; Ostfeld, I. The Effect of 2 Weeks of Inactivated Probiotic Bacillus coagulans on Endocrine, Inflammatory, and Performance Responses during Self-Defense Training in Soldiers. J. Strength Cond. Res. 2019, 33, 2330–2337. [Google Scholar] [CrossRef]
- Huang, W.C.; Wei, C.C.; Huang, C.C.; Chen, W.L.; Huang, H.Y. The Beneficial Effects of Lactobacillus plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, R.; Purpura, M.; Stone, J.D.; Turner, S.M.; Anzalone, A.J.; Eimerbrink, M.J.; Pane, M.; Amoruso, A.; Rowlands, D.S.; Oliver, J.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 Supplementation Attenuates Performance and Range-of-Motion Decrements Following Muscle Damaging Exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef]
- Lamprecht, M.; Bogner, S.; Schipper, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, J.N.; Sparks, A.S.; Doran, D.A.; Fleming, S.C.; Langan-Evans, C.; Kirk, B.; Fearn, R.; Morton, J.P.; Close, G.L. Four weeks of probiotic supplementation reduces GI symptoms during a marathon race. Eur. J. Appl. Physiol. 2019, 119, 1491–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, J.N.; Wagenmakers, A.J.M.; Doran, D.A.; Fleming, S.C.; Fielding, B.A.; Morton, J.P.; Close, G.L. Probiotic supplementation increases carbohydrate metabolism in trained male cyclists: A randomized, double-blind, placebo-controlled crossover trial. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E504–E513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, C.; Tamir, S.; Golan, R.; Weinstein, A.; Weinstein, Y. The effect of probiotic supplementation on performance, inflammatory markers and gastro-intestinal symptoms in elite road cyclists. J. Int. Soc. Sports Nutr. 2021, 18, 36. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sport Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Pugh, J.; O’Sullivan, O.; Black, K.; Townsend, J.R.; Pyne, D.B.; Wardenaar, F.C.; West, N.P.; Whisner, C.M.; McFarland, L.V. Best Practices for Probiotic Research in Athletic and Physically Active Populations: Guidance for Future Randomized Controlled Trials. Front. Nutr. 2022, 9, 809983. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S129–S134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory Effects of Probiotics During Pathogenic Infections with Emphasis on Immune Regulation. Front. Immunol. 2021, 12, 571. [Google Scholar] [CrossRef]
- Shehzad, A.; Rabail, R.; Munir, S.; Jan, H.; Fernández-Lázaro, D.; Aadil, R.M. Impact of Oats on Appetite Hormones and Body Weight Management: A Review. Curr. Nutr. Rep. 2023. online ahead of print. [Google Scholar] [CrossRef]
| Study | Item | Total | % | Quality Score | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| Axelrod et al., 2019 [27] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 15 | 93.8 | E |
| Batatinha et al., 2020 [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | 100 | E |
| Gill et al., 2016 [32] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 13 | 81.25 | VG |
| Hoffman et al., 2019 [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 14 | 87.5 | VG |
| Huang et al., 2019 [34] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 13 | 81.25 | VG |
| Jager et al., 2016 [35] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Lamprecht et al., 2021 [36] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 | 87.5 | VG |
| Pugh et al., 2019 [37] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Pugh et al., 2020 [38] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 14 | 87.5 | VG |
| Schreiber et al., 2021 [39] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | 100 | E |
| Shing et al., 2014 [29] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Tavares-Silva et al., 2021 [30] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | 100 | E |
| Vaisberg et al., 2019 [31] | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Characteristics | Types | Study |
|---|---|---|
| Participants | Endurance training | [27,28,29,30,31,32,34,36,37,38,39] |
| Strength training | [33,35] | |
| Supplementation product | Manufactured | [27,30,31,34,35,39] |
| Registered product® | [29,32,33,36,37,38] | |
| Not reported | [28] | |
| Strain formulation | Single | [27,31,32,33,34] |
| Mixed | [28,29,30,35,36,37,38,39] | |
| Total dose (108 CFU) × day−1 | 0–50 Low dose | [27,30,33,36,37] |
| 51–500 Medium dose | [28,29,31,34,35] | |
| >501 High dose | [32,38,39] | |
| Duration | 1 week | [32] |
| 2 weeks | [33] | |
| 3 weeks | [35] | |
| 4 weeks | [27,29,34,37,38] | |
| 30 days | [28,30,31] | |
| 90 days | [39] | |
| 14 weeks | [36] | |
| Dose schedule | After breakfast | [33,37] |
| Post-exercise | [38] | |
| After dinner | [28] | |
| Twice daily | [32,34,36] | |
| Once a day | [27,29,30,31,35,39] | |
| Pharmaceutical form | Capsules | [27,29,30,34,35,37,38,39] |
| Powdered | [28,33,36] | |
| Drink | [31,32] |
| First Author, Year of Publication, and Country | Study Design | Participants (Baseline Sample Size and Characteristics, Withdrawals, and Final Group Sample Size) | Intervention | Outcomes | Results |
|---|---|---|---|---|---|
| Axelrod et al., 2019, USA [27] | Randomized, double-blind, placebo-controlled crossover trial | 7 endurance athletes Age (mean ± SD): 31 ± 2.3 y BMI (mean ± SD): 24.3 ± 3.4 kg/m2 Body Fat (mean ± SD): 20.1 ± 5.9% VO2max (mean ± SD): 57.9 ± 4.5 mL/kg/min No withdrawals reported | 2 × 108 CFU 1 capsule (200 mg)/day Lactobacillus salivarius UCC118 4 weeks | IL-6 | IG vs. CG ↓IL-6 IG: Change from baseline ↓IL-6 |
| Batatinha et al., 2020 Brazil [28] | Randomized, double-blind, placebo-controlled trial | 40 ♂ marathon athletes Age (mean ± SD) IG: 35.9 ± 5.8 y CG: 40.4 ± 7.8 y Weight (mean ± SD) IG: 79.3 ± 10.9 kg CG: 72.6 ± 10.2 kg Height (mean ± SD) IG: 1.75 ± 0.06 m CG: 1.75 ± 0.09 m Fat mass (mean ± SD) IG: 16.9 ± 5.8% CG: 11.32 ± 4.4% 13 withdrawals/lost to follow-up 27 participants completed the study 13 participants CG 14 participants IG | Bifidobacterium animalis subsp. Lactis 10 × 109 CFU + Lactobacillus acidophilus 10 × 109 CFU Powdered Sachets dissolved in water before sleeping 30 days | IL-1β IL-2 IL-4 IL-6 IL-8 IL-10 IL-15 TNF-α INF-γ | IG: Change from baseline ↓IL-1β ↓IL-2 ↑IL-4 ↑IL-6 ↑IL-8 ↑IL-10 ↓IL-15 ↑TNF-α ↓INF-γ |
| Gill et al., 2016. United Kingdom [32] | Randomized, double-blind, placebo-controlled crossover trial | 8 ♂ endurance athletes (triathlon, road, trail running, ultra-endurance running) Age (mean ± SD): 26 ± 6 y Nude body mass (mean ± SD): 70.2 ± 8.8 kg Height (mean ± SD): 1.75 ± 0.05 m VO2max (mean ± SD): 59 ± 5 mL/kg/min No withdrawals reported | 1 × 1011 CFU of Lactobacillus casei. Drink 500 mL in the morning (8:00–9:00 a.m.) and Drink 500 mL in the afternoon (4:00–5:00 p.m.) 7 days | IL-1β IL-6 IL-8 IL-10 TNF-α INF-γ | GI vs. CG ↔IL-1β ↔IL-6 ↔IL-8 ↔IL-10 ↑TNF-α ↔INF-γ IG: Change from baseline ↑IL-1β *↑IL-6 *↑IL-8 *↑IL-10 *↑TNF-α ↔INF-γ |
| Hoffman et al., 2019, Israel [33] | Randomized, double-blind, placebo-controlled trial | 16 ♂ soldiers in combat training Age (mean ± SD) IG: 20.0 ± 0.6 y CG: 20.2 ± 0.6 y Weight (mean ± SD) IG: 72.0 ± 6.5 kg CG: 76.1 ± 8.2 kg Height (mean ± SD) IG: 1.76 ± 0.063 m CG: 1.80 ± 0.096 m 1 withdrawals/lost to follow-up 15 participants completed the study 7 participants CG 8 participants IG | 1 × 109 CFU Inactivated Bacillus coagulans. Powdered Sachets dissolved in 250 mL of water 14 days | IL-1β IL-6 IL-8 IL-10 TNF-α INF-γ | GI vs. CG ↔IL-1β ↔IL-6 ↔IL-8 ↔IL-10 ↔TNF-α ↔INF-γ IG: Change from baseline ↑IL-1β ↓IL-6 ↓IL-8 ↑IL-10 ↓TNF-α ↔INF-γ |
| Huang et al., 2019 Taiwan [34] | Randomized, double-blind controlled trial | 18 triathletes Age (median ± SEM) IG: 20.2 ± 0.67 y CG: 21.1 ± 1.5 y Weight (mean ± SD) IG: 63.5 ± 8.5 kg CG: 64.8 ± 5.7 kg Height (mean ± SEM) IG: 168 ± 0.08 m CG: 1.71 ± 0.05 m BMI (mean ± SEM): IG: 22.5 ± 1.2 kg/m2 CG: 22.1 ± 1.3 kg/m2 No withdrawals reported CG: 9 participants IG: 9 participants | 3 × 1010 CFU 2 capsules/day L- Plantarum PS128 4 weeks | IL-4 IL-6 IL-8 IL-10 TNF-α INF-γ | GI vs. CG ↓IL-4 *↓IL-6 *↓IL-8 ↓IL-10 *↓TNF-α *↑INF-γ IG: Change from baseline *↑IL-4 ↑IL-6 ↔IL-8 *↑IL-10 ↑TNF-α ↑INF-γ |
| Jäger et al., 2016 USA [35] | Randomized, double-blind, placebo-controlled trial | 15 ♂ healthy strength training Age (median ± SD): 25 ± 4 y Weight (mean ± SD): 81.1 ± 10.3 kg Height (mean ± SD): 1.77 ± 0.08 m No withdrawals reported | 1 × 1010 CFU S. thermophilus FP4 & B. brevis BR03 Capsules 21 days | IL-6 | GI vs. CG ↓IL-6 IG: Change from baseline ↔IL-6 |
| Lamprecht et al., 2012 Austria [36] | Randomized, double-blind, placebo-controlled trial | 24 ♂ resistance trained (triathlon, running, cyclists) Age (median ± SD) IG: 37.6 ± 4.7 y CG: 38.2 ± 4.4 y Weight (mean ± SD) IG: 80.2 ± 7.9 kg CG: 81.6 ± 6.3 kg BMI (mean ± SD): IG: 23.7 ± 2.2 kg/m2 CG: 23.9 ± 3.1 kg/m2 VO2max (mean ± SD): IG: 51.2 ± 4.1 mL/kg/min CG: 50.3 ± 3.6 mL/kg/min 1 withdrawals/lost to follow-up 23 participants completed the study 12 participants CG 11 participants IG | 10 × 1010 CFU Powdered Sachets mix: -Bifidobacterium bifidum CU23 -Bifidobacterium lactis CU51 -Lactobacillus brevis CU63 -Enterococcus faecium CU54 -Lactobacillus acidophilus CU22 -Lactococcus lactis CU58 14 weeks | IL-6 TNF-α | GI vs. CG ↔IL-6 ↓TNF-α IG: Change from baseline *↑IL-6 ↑TNF-α |
| Pugh et al., 2019 United Kingdom [37] | Randomized, double-blind controlled trial | 24 runners (20 ♂; 4♀) Age (median ± SD) IG: 34.8 ± 6.9 y CG: 36.1 ± 7.5 y Height (mean ± SD) IG: 1.79 ± 0.06 m CG: 1.75 ± 0.11 m Body mass (mean ± SD) IG: 76.5 ± 9.4 kg CG: 73.5 ± 11.3 kg VO2max (mean ± SD) IG: 57.6 ± 8.0 mL/kg/min CG: 56.4 ± 8.6 mL/kg/min 4 withdrawals/lost to follow-up 23 participants completed the study 9 participants CG 11 participants IG | 2.5 × 1010 CFU 1 capsule/day -Lactobacillus acidophilus CUL60 -L. acidophilus CUL21 -Bifidobacterium bifidum CUL20 -Bifidobacterium animalis subsp lactis CUL34 28 days | IL-6 IL-8 IL-10 | GI vs. CG ↔IL-6 ↔IL-8 ↔IL-10 IG: Change from baseline *↑IL-6 *↑IL-8 *↑IL-10 |
| Pugh et al., 2020 United Kingdom [38] | Randomized, double-blind, placebo-controlled trial | 7 trained cyclists Age (median ± SD): 23.4 ± 4.0 y Body mass (mean ± SD): 73.4 ± 7.1 kg VO2max (mean ± SD): 64.0 ± 2.2 mL/kg/min | 2.5 × 1013 CFU 1 capsule/day -Lactobacillus acidophilus CUL60 -Lactobacillus acidophilus CUL21 -Bifidobacterium bifidum CUL20 -Bifidobacterium animalis subsp lactis CUL34 28 days | IL-1α IL-6 IL-8 IL-10 | GI vs. CG ↓IL-1α ↓*IL-6 ↓IL -8 ↑IL -10 IG: Change from baseline ↑IL-1α ↑IL-6 ↑IL-8 ↑IL-10 |
| Schreiber et al., 2021 Israel [39] | Randomized, double-blind, placebo-controlled crossover trial | 27 ♂ elite cyclists Age (median ± SD) IG: 25.9 ± 4.6 y CG: 29.5 ± 6.2 y Height (mean ± SD) IG: 1.78 ± 0.05 m CG: 1.75 ± 0.04 m Weight (mean ± SD) IG: 71.3 ± 8.9 kg CG: 72.0 ± 6.2 kg BMI (mean ± SD) IG: 22.6 ± 2.7 kg/m2 CG: 23.5 ± 1.9 kg/m2 VO2max (mean ± SD) IG: 66.9 ± 6.4 mL/kg/min CG: 63.2 ± 5.0 mL/kg/min No withdrawals reported 16 participants CG 11 participants IG | 1.5 × 1010 CFU 1 capsule/day -Lactobacillus Helveticus Lafti L10 -Bifidobacterium animalis Lafti B94 -Enterococcus Faecium R0026 -Bifidobacterium Longum R0175 -Bacillus Subtilis R0179 90 days | IL-6 TNF-α | GI vs. CG ↔IL-6 ↔TNF-α IG: Change from baseline ↑IL-6 ↓TNF-α |
| Shing et al., 2013 Australia [29] | Double-blind, placebo-controlled cross-over trial | 10 ♂ trained runners Age (median ± SE): 27 ± 2 y CG: 29.5 ± 6.2 y Height (mean ± SE): 1.77 ± 0.02 m Body mass (mean ± SE): 71.5 ± 2.3 kg VO2max (mean ± SD) 62.6 ± 2.1 mL/kg/min No withdrawals reported | 4.5 × 1010 CFU 1 capsule/day -Lactobacillus acidophilus -L. rhamnosus -L. casey -B. brevis -Streptococcus thermophilus -L. plantarum -L. fermentum -Bifidobacterium lactis -B. bifidus 4 weeks | IL-1Ra IL-6 IL-10 TNF-α | GI vs. CG ↓IL-1Ra *↓IL-6 *↓IL-10 *↓TNF-α IG: Change from baseline ↑IL-1Ra ↑IL-6 ↑IL-10 ↑TNF-α |
| Tavares-Silva et al., 2021 Brazil [30] | Randomized, double-blind, placebo-controlled trial | 14 ♂ marathon runners Age (median ± SD) IG: 41.57 ± 3.20 y CG: 38.28 ± 3.09 y Height (mean ± SD) IG: 1.75 ± 0.030 m CG: 1.79 ± 0.052 m BMI (mean ± SD) IG: 23.08 ± 1.83 kg/m2 CG: 24.90 ± 1.81 kg/m2 VO2max (mean ± SD) IG: 56.92 ± 8.35 mL/kg/min CG: 54.53 ± 6.88 mL/kg/min No withdrawals reported 7 participants CG 7 participants IG | 5 × 109 CFU 2 g/day Capsules -Lactobacillus acidophilus LBG80 -Lactobacillus paracasei LPCG110 -Lactobacillus subp. lactis LLL-G25 -Bifidobacterium animalis subp lactis BL-G101 -Bifidobacterium bifidum BB-G90 90 days | IL-1β IL-2 IL-4 IL-6 IL-10 TNF-α | GI vs. CG ↔IL-1β ↔IL-2 ↔IL-4 ↔IL-6 *↑IL-10 ↔TNF-α IG: Change from baseline ↑IL-1β ↓IL-2 ↓IL-4 ↑IL-6 ↑IL-10 ↓TNF-α |
| Vaisbegr et al., 2019 Brazil [31] | Randomized, double-blind, placebo-controlled trial | 42 ♂ amateur marathoners Age (median ± SD) IG: 39.6 ± 8.8 y CG: 40.1 ± 10.3 y Height (mean ± SD) IG: 1.73 ± 0.06 m CG: 1.77 ± 0.07 m Weight (mean ± SD) IG: 72.4 ± 7.8 kg CG: 76.5 ± 10.4 kg BMI (mean ± SD) IG: 23.4 ± 2.4 kg/m2 CG: 24.4 ± 2.2 kg/m2 Body fat (mean ± SD) IG: 16.5 ± 6.6% CG: 18.6 ± 7.5% VO2max (mean ± SD) IG: 57.86 ± 6.85 mL/kg/min CG: 57.64 ± 6.89 mL/kg/min 14 withdrawals/lost to follow-up 42 participants completed the study 22 participants CG 20 participants IG | 40 × 109 CFU Drink (80 g) fermented milk 1 bottle/day Lactobacillus casei shirota (Lcs) 30 days | IL-1β IL-1Ra IL-4 IL-6 IL-10 TNF-α | GI vs. CG ↔IL-1β ↔IL-1Ra ↔IL-4 ↔IL-6 *↑IL-10 ↔TNF-α IG: Change from baseline *↑IL-1β ↓IL-1Ra ↑IL-4 *↑IL-6 *↑IL-10 *↑TNF-α |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Sánchez-Serrano, N.; Rabail, R.; Aadil, R.M.; Mielgo-Ayuso, J.; Radesca Fabiano, K.; Garrosa, E. Is Probiotics Supplementation an Appropriate Strategy to Modulate Inflammation in Physically Active Healthy Adults or Athletes? A Systematic Review. Appl. Sci. 2023, 13, 3448. https://doi.org/10.3390/app13063448
Fernández-Lázaro D, Sánchez-Serrano N, Rabail R, Aadil RM, Mielgo-Ayuso J, Radesca Fabiano K, Garrosa E. Is Probiotics Supplementation an Appropriate Strategy to Modulate Inflammation in Physically Active Healthy Adults or Athletes? A Systematic Review. Applied Sciences. 2023; 13(6):3448. https://doi.org/10.3390/app13063448
Chicago/Turabian StyleFernández-Lázaro, Diego, Nerea Sánchez-Serrano, Roshina Rabail, Rana Muhammad Aadil, Juan Mielgo-Ayuso, Krizia Radesca Fabiano, and Evelina Garrosa. 2023. "Is Probiotics Supplementation an Appropriate Strategy to Modulate Inflammation in Physically Active Healthy Adults or Athletes? A Systematic Review" Applied Sciences 13, no. 6: 3448. https://doi.org/10.3390/app13063448
APA StyleFernández-Lázaro, D., Sánchez-Serrano, N., Rabail, R., Aadil, R. M., Mielgo-Ayuso, J., Radesca Fabiano, K., & Garrosa, E. (2023). Is Probiotics Supplementation an Appropriate Strategy to Modulate Inflammation in Physically Active Healthy Adults or Athletes? A Systematic Review. Applied Sciences, 13(6), 3448. https://doi.org/10.3390/app13063448









