The Use of Phytochemicals to Improve the Efficacy of Immune Checkpoint Inhibitors: Opportunities and Challenges
Abstract
1. Introduction
2. Literature Search and Review Structure
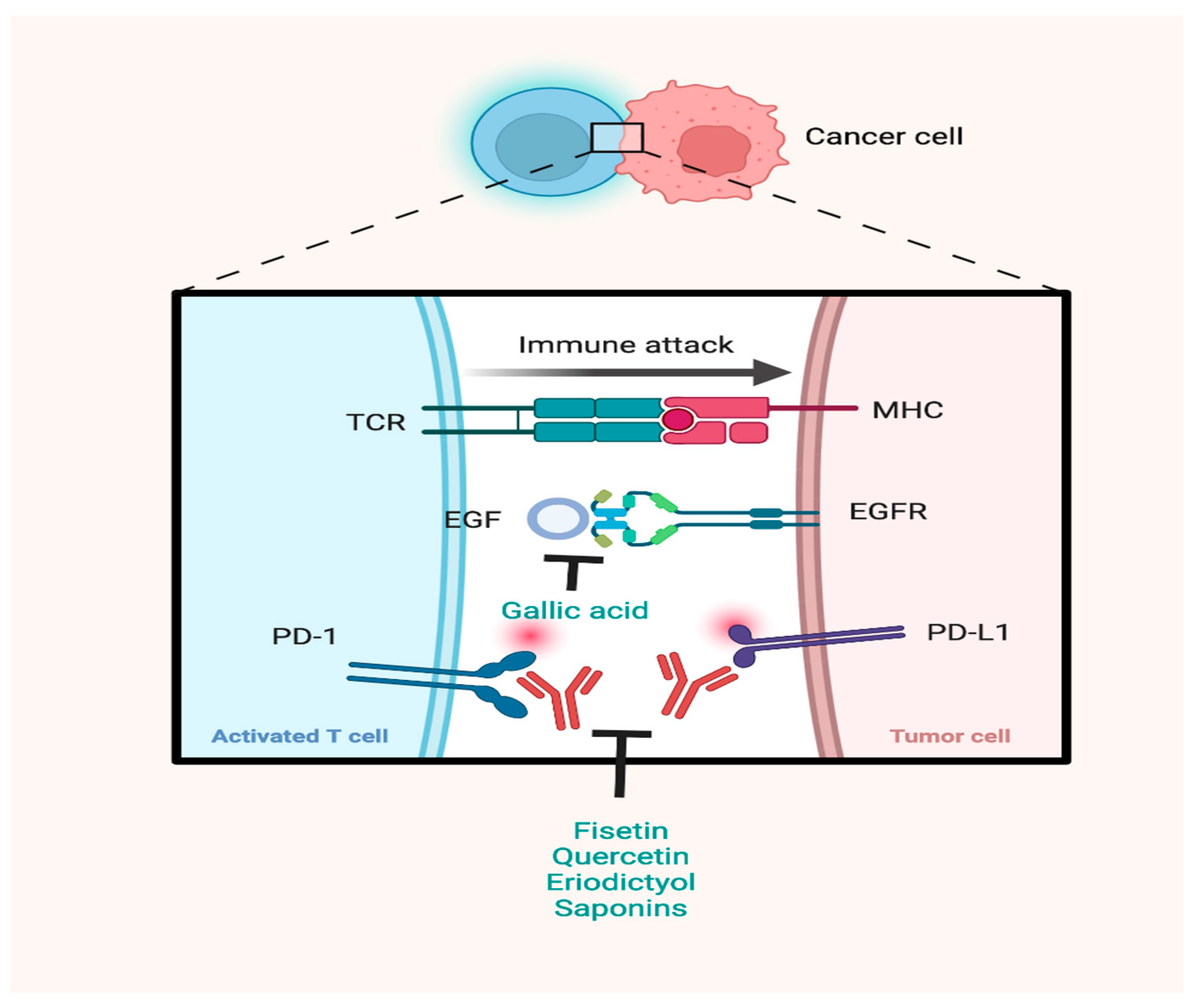
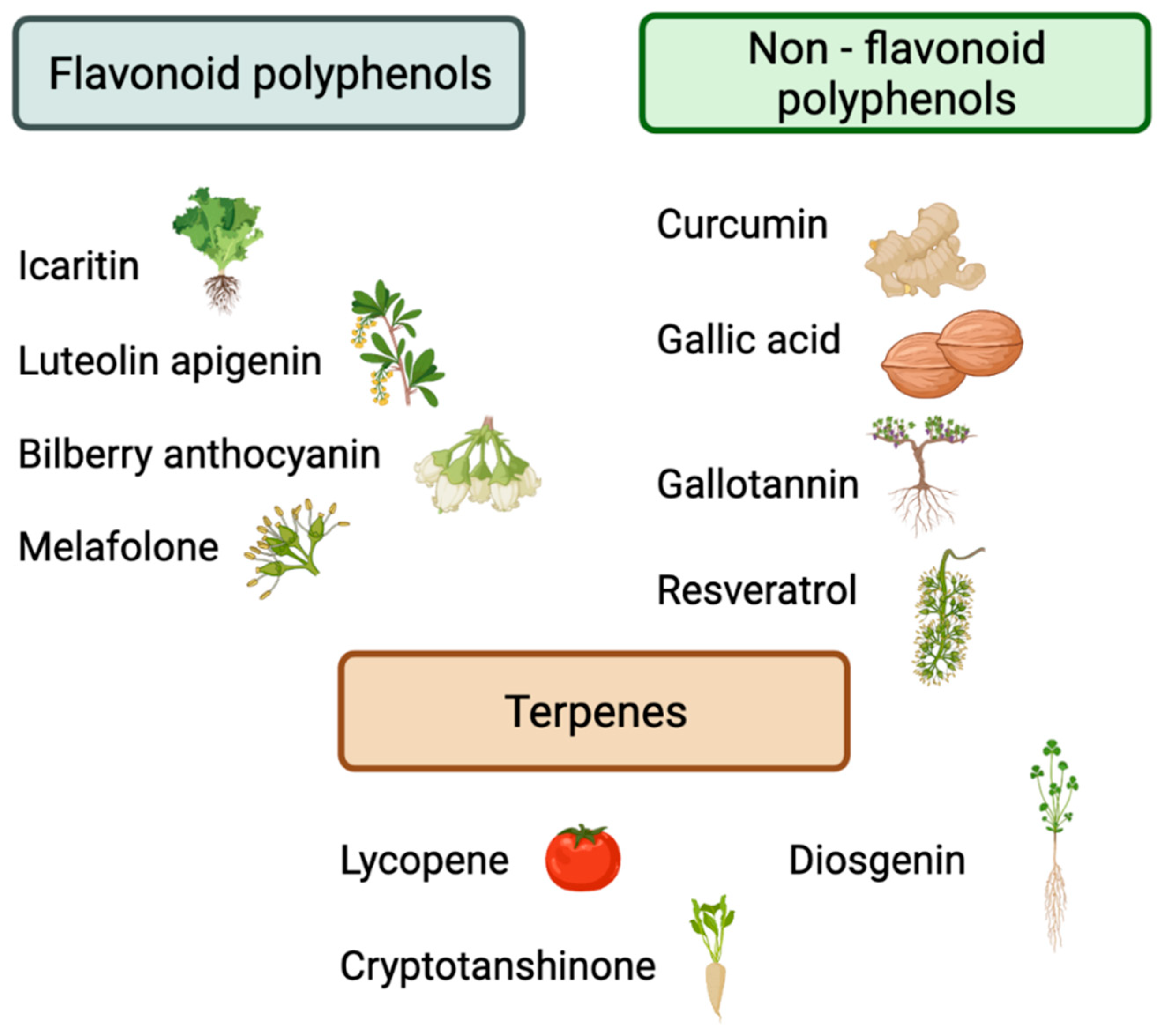
3. Preclinical Evidence Evaluating the Effects of Phytochemicals on Immune Checkpoints
| Authors | Phytochemical Group | Phytochemical Compound | Immune Checkpoint Inhibitor | Cancer Type | Cancer Model | Mechanism of Effect | Outcome |
|---|---|---|---|---|---|---|---|
| Shao et al. (2017) [27] | Non-flavonoid polyphenols | Curcumin | Anti-PD-L1 antibody (200 µg) | Bladder Cancer | MB49 bladder carcinoma tumor-bearing C57BL/6 mice | - Increase intratumoral CD8+ T-cell infiltration - Elevated the level of IFN-γ in the blood - Decrease the number of intratumoral MDSCs | Prolonged survival of intraperitoneal metastasized bladder cancer |
| Dent et al. (2020) [28] | Non-flavonoid polyphenols | Curcumin | Anti-PD-1 antibody (50 mg/kg) | CRC | CT26 colorectal tumor-bearing C57/BL6 mice | - Reduce the expression of PD-L1, PD-L2, and ODC - Elevated the level of MHCA | Reduce tumor growth |
| Guo et al. (2021) [29] | Non-flavonoid polyphenols | Curcumin | Anti-PD-1 antibody (10 mg/kg) | HCC | Hep3B hepatocellular tumor-bearing BALB/c female nude mice | - Reduce surface PD-L1 expression - Activate lymphocytes - Inhibit immune evasion - Down-regulate TGF-β1 expression | Lowered the HCC growth rate and improved tumor microenvironment |
| Gong et al. (2021) [30] | Non-flavonoid polyphenols | Curcumin | Anti-PD-1 antibody (10 mg/kg) | CRC | MC-38 colorectal tumor-bearing C57BL/6 mice | N/A | - Reduce tumor growth and tumor volume -Reduce the risk of tumor recurrence (0% vs. 50% in the combination and IO monotherapy arms) |
| Hayakawa et al. (2021) [31] | Non-flavonoid polyphenols | Curcumin | Anti-PD-1 Ab and Anti-PD-L1 Ab (200 µg/100 µL/mouse) | CRC | MC-38 colorectal tumor-bearing C57BL/6 mice CT26 colorectal tumor-bearing BALB/c mice | Inhibit STAT3 expression induced by exogenous IL-6 | Reduce tumor growth |
| Liu et al. (2021) [32] | Non-flavonoid polyphenols | Curcumin | Anti-PD-L1 antibody (10 µg/mL) | HNSCC | Human HNSCC cell lines (SNU1076, SNU1041, SCC15, and FaDu) | Reinvigorates defective T-cells through multiple (PD-1 and TIM-3) and multi-level (IC receptors and their ligands) IC axis inhibition | Reduce the tumor volume and weight |
| Kang et al. (2020) [33] | Non-flavonoid polyphenols | Gallic acid | Anti-PD-1 antibody (5 μg/mL) | NSCLC | A549 and H292 NSCLC cell lines | - Reduce expression levels of PD-L1 - Increase IFNγ levels | Decrease NSCLC cell viability |
| Lasso et al. (2020) [34] | Non-flavonoid polyphenols | Gallotallin | Anti-PD-L1 antibody (200 µg) | Melanoma | B16-F10 melanoma tumor-bearing C57BL/6 mice | - Increase IFNγ levels - Increase the number of activated CD4+ and CD8+ T-cells - Decrease the number of MDSCs - Increase PD-L1 expression | Decrease in tumor size |
| Zhang et al. (2019) ([35] | Non-flavonoid polyphenols | Resveratrol | Anti-PD-L1 antibody (100 µg) | Ovarian Cancer | Human ovarian carcinoma cell lines (SKOV3 and A2780) and murine ovarian carcinoma cell line (ID8) | Induction of tumor cell apoptosis | Reduce tumor growth |
| Jiang et al. (2021) [25] | Flavonoid | Luteolin Apigenin | Anti-PD-1 antibody (10 mg/kg) | NSCLC | KRAS-mutant human lung cell lines (H358, H460, H2122, and A549) and mice in vivo mode | Down-regulated the IFN-γ-induced PD-L1 expression by suppressing the phosphorylation of STAT3 | Reduce the tumor volume and weight |
| Liu et al. (2020) [36] | Flavonoid | Bilberry Anthocyanin | Anti-PD-L1 antibody (200 µg) | CRC | MC-38 colorectal tumor-bearing C57BL/6 mice | Modulate the gut microbiome | Tumor growth delay |
| Mo et al. (2021) [37] | Flavonoid | Icaritin | Anti-PD-1 antibody (10 mg/kg) | HCC, CRC, and melanoma | Human hepatocellular carcinoma, colorectal cancer and melanoma cell lines (HEPA1–6, MC-38, and B16F10) and mice in vivo model | Down-regulate PD-L1 expression and reduced nuclear translocation of NF-κB p6. | Reduce the tumor volume and weight |
| Tang et al. (2019) [38] | Flavonoid | Melafolone | Anti-PD-1 Ab (200 µg/100 µL/mouse) | Lung Cancer | Lewis lung carcinoma or CMT tumor-bearing C57BL/6 mice | Down-regulate VEGF, TGF-β, and PD-L1 through COX-2 and EGFR inhibition | Promoted survival Tumor growth inhibition |
| Jiang et al. (2019) [39] | Terpenes | Lycopene | Anti-PD-1 antibody (6 mg/kg) | Lung Cancer | Lewis lung carcinoma tumor-bearing C57BL/6 mice | - Increase IFNγ levels - Inhibit the expression of PD-L1 via activating JAK - Inhibit the phosphorylation of AKT | Reduce the tumor volume and weight |
| Han et al. (2019) [40] | Terpenes | Cryptotanshinone | Anti-PD-L1 antibody 10 µg) | HCC | HCC-bearing mice model | Develops long-term ani-tumor immunity and increased tumor infiltration of CD8+ T-cell | Tumor growth inhibition |
| Dong et al. (2018) [41] | Terpenes | Diosgenin | Anti-PD-1 antibody (200 µg) | Melanoma | B16-F10 melanoma tumor-bearing C57BL/6 mice | Enhances T-cell immune response by modulating intestinal microbiota and inducing T-cell infiltration | Tumor growth inhibition |
| Ye et al. (2021) [42] | Others | Agrocybe aegerita galectin | Anti-PD-1 Ab (200 µg intraperitoneal) | HCC | H22, HepG2, and RAW264.7 cell lines Male Balb/c mice | Increase CD4+ and CD8+ T-cells with combination | Tumor growth inhibition |
4. Preclinical Studies Evaluating the Efficacy of Phytochemical and Immune Checkpoint Inhibitor Combinations
| Authors/Year | Phytochemical Group | Phytochemical Compound | Source | Cancer Type | Cancer Model or Toxicity | Mechanism of Action |
|---|---|---|---|---|---|---|
| Liao et al. (2018) [21] | Non-flavonoid polyphenols | Curcumin | Turmeric | HNSCC | 4-NQO induced C57BL/6 tongue squamous cell carcinoma mice | Decrease PD-L1 and p-STAT3Y705 protein expression |
| Deng et al. (2020) [44] | Non-flavonoid polyphenols | Curcumin | Turmeric | HCC | HepG2 hepatocellular tumor-bearing BALB/c female nude mice | Decreased the protein expression of PD-L1 Inhibiting the TLR4/NF-κB signaling pathway angiogenesis. |
| Lucas et al. (2018) [43] | Non-flavonoid polyphenols | Resveratrol | Red wine, Grapes, Passion fruit | Breast cancer | Cal51 triple-negative breast cancer and SW620 colon cancer | Increase the expression level of PD-L1 via HDAC3/p300-mediated nuclear factor (NF)-κB signaling |
| Verdura et al. (2020) [45] | Non-flavonoid polyphenols | Resveratrol | Red wine, Grapes, Passion fruit | Breast Cancer | JIMT-1 and MDA-MB-231 breast cancer cells | Increased PD-L1 dysfunction |
| Yang et al. (2021) [46] | Non-flavonoid polyphenols | Resveratrol | Red wine, Grapes, Passion fruit | NSCLC | Human lung adenocarcinoma cell lines (A549 and H1299) | Activation of SirT1 deacetylase leads to disassembly of the destruction complex, thereby enhancing the binding of β-catenin/TCF to the PD-L1 promoter |
| Coomb et al. (2016) [47] | Flavonoid | Apigenin | Parsley, onions, grapefruit, oranges, | Breast Cancer | Triple-negative MDA-MB-468 BC cells, HER2(+) SK-BR-3 BC cells, and 4T1 mouse mammary carcinoma cells | Inhibit IFNγ-induced PD-L1 upregulation |
| Xu et al. (2018) [23] | Flavonoid | Apigenin | Apple, artichoke, basil, celery, cherry, grapes | Melanoma | B16-F10 melanoma tumor-bearing C57BL/6 mice | Inhibit the IFN-γ-induced activation of STAT Decreased expression levels of PD-L1 |
| Choi et al. (2020) [48] | Flavonoid | Apigenin | Salvia plebeia | CRC | hPD-L1 knock-in MC38 tumor-bearing humanized PD-1 mouse model | Blocking of PD-1/PD-L1 interaction |
| Rawangkan et al. (2018) [49] | Flavonoid | Epigallocatechin gallate (EGCG) | Green tea | NSCLC | 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone induced nonsmall-cell lung cancer A/J mice | Down-regulate IFN-γ- and EGF-induced PD-L1 expression |
| Sellam et al. (2020) [50] | Flavonoid | Silibinin | Silybum marianum | HNSCC | Nasopharyngeal carcinoma cell line | Down-regulation in PD-L1 expression by interfering with HIF-1α/LDH-A |
| Rugamba et al. (2021) [51] | Flavonoid | Silibinin | Silybum marianum | NSCLC | A549, H292, and H460 cell lines | Suppresses the mRNA expression of PD-L1 and EMT regulators via inhibition of STAT3 phosphorylation |
| Wudtiwai et al. (2021) [52] | Flavonoid | Hesperidin | Orange peel and other citrus species | Oral Cancer | Human OSCC cell lines (HN6 and HN15) | Inhibition of phosphorylation of STAT1 and STAT3 down-regulates IFN-γ -induced PD-L1 expression |
| Ke et al. (2019) [24] | Flavonoid | Baicalin | Scutellaria baicalensis | HCC | H22 hepatocellular tumor-bearing BALB/c mice or BALB/c-nu/nu mice | Decrease STAT3 activity Down-regulate IFN-γ-induced PD-L1 expression |
| Song et al. (2022) [53] | Flavonoid | Baicalin | Scutellaria baicalensis | CRC | Human colon cancer cell lines (HCT-116 and CT26) and mice in vivo model | Inhibition of NF-κB signaling pathway down-regulated PD-L1 expression and MDSC, and up-regulated CD4 + and CD8 + T-cells |
| Liu et al. (2021) [54] | Flavonoid | Licochalcone A | Glycyrrhiza glabra | CRC | Human cancer cell lines (A549, HeLa, Hep3B, and HCT116) and mice in vivo model | PD-L1 expression was down-regulated by inhibition of NF-κB and Ras/Raf/MEK signaling pathways. |
| Hao et al. (2019) [55] | Flavonoid | Icaritin | Epimedium | Melanoma | B16-F10 melanoma tumor-bearing C57BL/6 mice MC-38 colorectal tumor-bearing C57BL/6 mice | Reduce frequency of MDSCs Down-regulate PD-L1 expression |
| Mazewski et al. (2019) [56] | Flavonoid | Cyanidin-3-O-glucoside | Blueberry, raspberry, black rice, cherry | CRC | Human colorectal cancer cells | Decreased PD-1 and PD-L1 protein expression |
| Chen et al. (2022) [57] | Flavonoid | Myricetin | Cranberry, blueberry, lemon, garlic | Lung cancer | Human lung cancer cell lines (NCI-H1650, NCI-H460, and A549) | Inhibition of IFN-γ-induced PD-L1 and IDO1 expression in cancer cells by inhibiting the JAK-STAT-IRF1 axis |
| Kim et al. (2020) [58] | Flavonoid | Kaempferol | Geranii herba | N/A | PD-1 Jurkat and PD-L1/aAPC CHO-K1 cells | Inhibiting PD-1/PD-L1 Interaction |
| Sahyon et al.(2020) [20] | Flavonoid | Gallic acid | Phoenix dactylifera | N/A | Adriamycin-induced cardiotoxicity and nephrotoxicity | Increased cardiac and kidney PD-1 protein percentage |
| Xing et al. (2018) [22] | Terpenes | Fraxinellone | Dictamnus dasycarpus | Lung cancer | Human A549 lung cancer cell line | Inhibit PD-L1 expression by downregulating the STAT3 and HIF-1α signaling pathways |
| Huang et al. (2019) [59] | Terpenes | Platycodin | Platycodon grandiflorus | Lung cancer | NCI–H1975 and NCI–H358 lung cancer cell lines | Down-regulate the protein level of PD-L1 |
| Zhang et al. (2019) [60] | Terpenes | Triptolide | Tripterygium wilfordii | Glioma | Glioma cells | Down-regulated IFN-γ-induced PD-L1 expression |
| Kuo et al. (2021) [61] | Terpenes | Triptolide | Tripterygium wilfordii | HNSCC | OSCC cell line SAS (JCRB0260) and mice in vivo model | Reduces IFN-γ-related JAK2-STAT1 pathway and decreases PD-L1 expression |
| Tian et al. (2021) [17] | Phytosterol | Z-guggulsterone | Commiphora mukul tree | NSCLC | Lewis lung carcinoma tumor-bearing C57BL/6 mice | Inducing PD-L1 upregulation partly mediated by FXR, Akt, and Erk1/2 signaling pathways |
| Wang et al. (2020) [62] | Saponins | Panaxadiol | Panax ginseng | CRC | HCT116, SW620, HT29, and HEK293 colon cancer cell line and mice in vivo model | Reduces PD-L1 expression by suppressing HIF-1α and STAT3 |
| Deng et al. (2020) [44] | Saponins | Ginsenosides | Panax | HCC | HepG2 hepatocellular tumor-bearing BALB/c female nude mice | Decreased the protein expression of PD-L1 Inhibiting the TLR4/NF-κB signaling pathway angiogenesis. |
| Bedi et al. (2019) [63] | Alkaloid | Camptothecin | Camptotheca acuminita | CRC | SW620, HCT116, and RKO colon cancer cells | Reduces PD-L1 expression and upregulates the secretion of pro-tumorigenic cytokines |
| Hunakova et al. (2019) [64] | Isothiocyanates | Isothiocyanate | Broccoli, Brussels sprouts, cabbage, cauliflower, horseradish | Breast Cancer | Human triple-negative Breast Carcinoma MDA-MB-231 Cells | Decrease expression levels of PD-L1 |
| Chang et al. (2019) [65] | Astragalus membranaceus extract | Extract | Astragalus membranaceus | Breast cancer, CRC | Mouse breast cancer 4T1 and colorectal cancer CT26 | Down-regulate PD-L1 expression by suppressing the AKT signaling pathway |
| Li et al. (2019) [16] | Rhus verniciflua Stokes extract | Eriodictyol, fisetin, quercetin, liquiritigenin | Rhus verniciflua Stokes | - | N/A | Blocked both the PD-1/PD-L1 and the CTLA-4/CD80 interactions |
| Safonova et al. (2020) [66] | Tussilago farfara extract | Rhamnogalacturonane I and neutral polysaccharides complex | Tussilago farfara | Lung cancer | Lewis lung carcinoma tumor-bearing C57BL/6 mice | Reducing expression levels of PD-1 and PD-L1 Inhibiting PD-1/PD-L1 Interaction |
| Ryan et al. (2022) [67] | Black raspberry extract | Extract | Black raspberry | HNSCC | Nitroquinoline-1-oxide (4NQO) induced head and neck cancer C57BL/6 mice | Decreased levels of PD-L1 expression |
5. Clues from Microbiome Studies on the Benefit of Phytochemicals in Immunotherapy Efficacy
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, J.-J.; Xing, R.; Zeng, Y.-C. Combination therapy: Future directions of immunotherapy in small cell lung cancer. Transl. Oncol. 2021, 14, 100889. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Larkin, J. Immunotherapy Combined or Sequenced with Targeted Therapy in the Treatment of Solid Tumors: Current Perspectives. JNCI J. Natl. Cancer Inst. 2016, 108, djv414. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kulkarni, P.; Salgia, R. Combined Checkpoint Inhibition and Chemotherapy: New Era of 1st-Line Treatment for Non-Small-Cell Lung Cancer. Mol. Ther. Oncolytics 2019, 13, 1–6. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef]
- Akinleye, A.; Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019, 12, 92. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Sahin, T.K.; Bilir, B.; Kucuk, O. Modulation of inflammation by phytochemicals to enhance efficacy and reduce toxicity of cancer chemotherapy. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Power Coombs, M.R.; Hoskin, D.W. Assessing the Impact of Phytochemicals on Immune Checkpoints: Implications for Cancer Immunotherapy. Methods Mol. Biol. 2020, 2111, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kumar, K.; Brisc, C.; Rus, M.; Nistor-Cseppento, D.C.; Bustea, C.; Aron, R.A.C.; Pantis, C.; Zengin, G.; Sehgal, A.; et al. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed. Pharmacother. 2021, 133, 110959. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.Y.; Yang, N.S.; Lin, T.J. Phytochemicals Approach for Developing Cancer Immunotherapeutics. Front. Pharm. 2017, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.; Shrama, A.; Mandal, C.C. Molecular insights into phytochemicals-driven break function in tumor microenvironment. J. Food Biochem. 2021, 45, e13824. [Google Scholar] [CrossRef]
- Li, W.; Kim, T.I.; Kim, J.H.; Chung, H.-S. Immune Checkpoint PD-1/PD-L1 CTLA-4/CD80 are Blocked by Rhus verniciflua Stokes and its Active Compounds. Molecules 2019, 24, 4062. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Gui, Y.; Wei, Y.; Shang, B.; Sun, J.; Ma, S.; You, W.; Jiang, S. Z-guggulsterone induces PD-L1 upregulation partly mediated by FXR, Akt and Erk1/2 signaling pathways in non-small cell lung cancer. Int. Immunopharmacol. 2021, 93, 107395. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef]
- Luo, M.; Xia, Y.; Wang, F.; Zhang, H.; Su, D.; Su, C.; Yang, C.; Wu, S.; An, S.; Lin, S.; et al. PD0325901, an ERK inhibitor, enhances the efficacy of PD-1 inhibitor in non-small cell lung carcinoma. Acta Pharm. Sin. B 2021, 11, 3120–3133. [Google Scholar] [CrossRef]
- Sahyon, H.A.; Al-Harbi, S.A. Chemoprotective role of an extract of the heart of the Phoenix dactylifera tree on adriamycin-induced cardiotoxicity and nephrotoxicity by regulating apoptosis, oxidative stress and PD-1 suppression. Food Chem. Toxicol. 2020, 135, 111045. [Google Scholar] [CrossRef]
- Liao, F.; Liu, L.; Luo, E.; Hu, J. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Arch. Oral Biol. 2018, 92, 32–37. [Google Scholar] [CrossRef]
- Xing, Y.; Mi, C.; Wang, Z.; Zhang, Z.H.; Li, M.Y.; Zuo, H.X.; Wang, J.Y.; Jin, X.; Ma, J. Fraxinellone has anticancer activity in vivo by inhibiting programmed cell death-ligand 1 expression by reducing hypoxia-inducible factor-1α and STAT3. Pharm. Res. 2018, 135, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F.; et al. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J. Exp. Clin. Cancer Res. 2018, 37, 261. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Zhang, Z.; Xu, B.; Zhao, S.; Ding, Y.; Wu, X.; Wu, R.; Lv, Y.; Dong, J. Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. Int. Immunopharmacol. 2019, 75, 105824. [Google Scholar] [CrossRef]
- Jiang, Z.B.; Wang, W.J.; Xu, C.; Xie, Y.J.; Wang, X.R.; Zhang, Y.Z.; Huang, J.M.; Huang, M.; Xie, C.; Liu, P.; et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021, 515, 36–48. [Google Scholar] [CrossRef]
- Avalle, L.; Pensa, S.; Regis, G.; Novelli, F.; Poli, V. STAT1 and STAT3 in tumorigenesis: A matter of balance. JAK-STAT 2012, 1, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhu, W.; Da, J.; Xu, M.; Wang, Y.; Zhou, J.; Wang, Z. Bisdemethoxycurcumin in combination with α-PD-L1 antibody boosts immune response against bladder cancer. Onco Targets 2017, 10, 2675–2683. [Google Scholar] [CrossRef]
- Dent, P.; Booth, L.; Roberts, J.L.; Poklepovic, A.; Hancock, J.F. (Curcumin+sildenafil) enhances the efficacy of 5FU and anti-PD1 therapies in vivo. J. Cell. Physiol. 2020, 235, 6862–6874. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, H.; Fan, T.; Ma, Y.; Wang, L. Synergistic efficacy of curcumin and anti-programmed cell death-1 in hepatocellular carcinoma. Life Sci. 2021, 279, 119359. [Google Scholar] [CrossRef]
- Gong, F.; Ma, J.-C.; Jia, J.; Li, F.-Z.; Wu, J.-L.; Wang, S.; Teng, X.; Cui, Z.-K. Synergistic effect of the anti-PD-1 antibody with blood stable and reduction sensitive curcumin micelles on colon cancer. Drug Deliv. 2021, 28, 930–942. [Google Scholar] [CrossRef]
- Hayakawa, T.; Yaguchi, T.; Kawakami, Y. Enhanced anti-tumor effects of the PD-1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer Sci. 2020, 111, 4326–4335. [Google Scholar] [CrossRef]
- Liu, L.; Lim, M.A.; Jung, S.-N.; Oh, C.; Won, H.-R.; Jin, Y.L.; Piao, Y.; Kim, H.J.; Chang, J.W.; Koo, B.S. The effect of Curcumin on multi-level immune checkpoint blockade and T cell dysfunction in head and neck cancer. Phytomedicine 2021, 92, 153758. [Google Scholar] [CrossRef]
- Kang, D.Y.; Sp, N.; Jo, E.S.; Rugamba, A.; Hong, D.Y.; Lee, H.G.; Yoo, J.S.; Liu, Q.; Jang, K.J.; Yang, Y.M. The Inhibitory Mechanisms of Tumor PD-L1 Expression by Natural Bioactive Gallic Acid in Non-Small-Cell Lung Cancer (NSCLC) Cells. Cancers 2020, 12, 727. [Google Scholar] [CrossRef]
- Lasso, P.; Gomez-Cadena, A.; Urueña, C.; Donda, A.; Martinez-Usatorre, A.; Romero, P.; Barreto, A.; Fiorentino, S. An Immunomodulatory Gallotanin-Rich Fraction From Caesalpinia spinosa Enhances the Therapeutic Effect of Anti-PD-L1 in Melanoma. Front. Immunol. 2020, 11, 584959. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Yang, Y.; Liu, T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect. Agent Cancer 2019, 14, 27. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Jing, N.; Jiang, G.; Liu, Z. Biostimulating Gut Microbiome with Bilberry Anthocyanin Combo to Enhance Anti-PD-L1 Efficiency against Murine Colon Cancer. Microorganisms 2020, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Mo, D.; Zhu, H.; Wang, J.; Hao, H.; Guo, Y.; Wang, J.; Han, X.; Zou, L.; Li, Z.; Yao, H.; et al. Icaritin inhibits PD-L1 expression by Targeting Protein IκB Kinase α. Eur. J. Immunol. 2021, 51, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, Y.; Wang, C.; Zheng, H.; Chen, Y.; Liu, W.; Chen, X.; Zhang, J.; Chen, H.; Yang, Y.; et al. Inhibition of COX-2 and EGFR by Melafolone Improves Anti-PD-1 Therapy through Vascular Normalization and PD-L1 Downregulation in Lung Cancer. J. Pharmacol. Exp. Ther. 2019, 368, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wu, H.; Zhao, W.; Ding, X.; You, Q.; Zhu, F.; Qian, M.; Yu, P. Lycopene improves the efficiency of anti-PD-1 therapy via activating IFN signaling of lung cancer cells. Cancer Cell Int. 2019, 19, 68. [Google Scholar] [CrossRef]
- Han, Z.; Liu, S.; Lin, H.; Trivett, A.L.; Hannifin, S.; Yang, D.; Oppenheim, J.J. Inhibition of murine hepatoma tumor growth by cryptotanshinone involves TLR7-dependent activation of macrophages and induction of adaptive antitumor immune defenses. Cancer Immunol. Immunother. 2019, 68, 1073–1085. [Google Scholar] [CrossRef]
- Dong, M.; Meng, Z.; Kuerban, K.; Qi, F.; Liu, J.; Wei, Y.; Wang, Q.; Jiang, S.; Feng, M.; Ye, L. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. 2018, 9, 1039. [Google Scholar] [CrossRef]
- Ye, X.; Wang, X.; Yu, W.; Yang, Q.; Li, Y.; Jin, Y.; Su, Y.; Song, J.; Xu, B.; Sun, H. Synergistic effects of AAGL and anti-PD-1 on hepatocellular carcinoma through lymphocyte recruitment to the liver. Cancer Biol. Med. 2021, 18, 1092. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; Hsieh, T.C.; Halicka, H.D.; Darzynkiewicz, Z.; Wu, J.M. Upregulation of PD-L1 expression by resveratrol and piceatannol in breast and colorectal cancer cells occurs via HDAC3/p300-mediated NF-κB signaling. Int. J. Oncol. 2018, 53, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Xu, X.-Y.; Yunita, F.; Zhou, Q.; Wu, Y.-R.; Hu, Y.-X.; Wang, Z.-Q.; Tian, X.-F. Synergistic anti-liver cancer effects of curcumin and total ginsenosides. World J. Gastrointest. Oncol. 2020, 12, 1091–1103. [Google Scholar] [CrossRef]
- Verdura, S.; Cuyàs, E.; Cortada, E.; Brunet, J.; Lopez-Bonet, E.; Martin-Castillo, B.; Bosch-Barrera, J.; Encinar, J.A.; Menendez, J.A. Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging 2020, 12, 8. [Google Scholar] [CrossRef]
- Yang, M.; Li, Z.; Tao, J.; Hu, H.; Li, Z.; Zhang, Z.; Cheng, F.; Sun, Y.; Zhang, Y.; Yang, J.; et al. Resveratrol induces PD-L1 expression through snail-driven activation of Wnt pathway in lung cancer cells. J. Cancer Res. Clin. Oncol. 2021, 147, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Coombs, M.R.P.; Harrison, M.E.; Hoskin, D.W. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett. 2016, 380, 424–433. [Google Scholar] [CrossRef]
- Choi, J.-G.; Kim, Y.S.; Kim, J.H.; Kim, T.I.; Li, W.; Oh, T.W.; Jeon, C.H.; Kim, S.J.; Chung, H.-S. Anticancer Effect of Salvia plebeia and Its Active Compound by Improving T-Cell Activity via Blockade of PD-1/PD-L1 Interaction in Humanized PD-1 Mouse Model. Front. Immunol. 2020, 11, 8556. [Google Scholar] [CrossRef]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor that Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef]
- Sellam, L.S.; Zappasodi, R.; Chettibi, F.; Djennaoui, D.; Yahi-Ait Mesbah, N.; Amir-Tidadini, Z.C.; Touil-Boukoffa, C.; Ouahioune, W.; Merghoub, T.; Bourouba, M. Silibinin down-regulates PD-L1 expression in nasopharyngeal carcinoma by interfering with tumor cell glycolytic metabolism. Arch. Biochem. Biophys. 2020, 690, 108479. [Google Scholar] [CrossRef]
- Rugamba, A.; Kang, D.Y.; Sp, N.; Jo, E.S.; Lee, J.M.; Bae, S.W.; Jang, K.J. Silibinin Regulates Tumor Progression and Tumorsphere Formation by Suppressing PD-L1 Expression in Non-Small Cell Lung Cancer (NSCLC) Cells. Cells 2021, 10, 1632. [Google Scholar] [CrossRef]
- Wudtiwai, B.; Makeudom, A.; Krisanaprakornkit, S.; Pothacharoen, P.; Kongtawelert, P. Anticancer Activities of Hesperidin via Suppression of Up-Regulated Programmed Death-Ligand 1 Expression in Oral Cancer Cells. Molecules 2021, 26, 5345. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhu, S.; Liu, C.; Zhang, Q.; Liang, X. Baicalin triggers apoptosis, inhibits migration, and enhances anti-tumor immunity in colorectal cancer via TLR4/NF-κB signaling pathway. J. Food Biochem. 2022, 46, e13703. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xing, Y.; Li, M.; Zhang, Z.; Wang, J.; Ri, M.; Jin, C.; Xu, G.; Piao, L.; Jin, H.; et al. Licochalcone A inhibits proliferation and promotes apoptosis of colon cancer cell by targeting programmed cell death-ligand 1 via the NF-κB and Ras/Raf/MEK pathways. J. Ethnopharmacol. 2021, 273, 113989. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, Q.; Zhu, H.; Wen, Y.; Qiu, D.; Xiong, J.; Fu, X.; Wu, Y.; Meng, K.; Li, J. Icaritin promotes tumor T-cell infiltration and induces antitumor immunity in mice. Eur. J. Immunol. 2019, 49, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Mazewski, C.; Kim, M.S.; Gonzalez de Mejia, E. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci. Rep. 2019, 9, 11560. [Google Scholar] [CrossRef]
- Chen, Y.-C.; He, X.-L.; Qi, L.; Shi, W.; Yuan, L.-W.; Huang, M.-Y.; Xu, Y.-L.; Chen, X.; Gu, L.; Zhang, L.-L.; et al. Myricetin inhibits interferon-γ-induced PD-L1 and IDO1 expression in lung cancer cells. Biochem. Pharmacol. 2022, 197, 114940. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.S.; Choi, J.-G.; Li, W.; Lee, E.J.; Park, J.-W.; Song, J.; Chung, H.-S. Kaempferol and Its Glycoside, Kaempferol 7-O-Rhamnoside, Inhibit PD-1/PD-L1 Interaction In Vitro. Int. J. Mol. Sci. 2020, 21, 3239. [Google Scholar] [CrossRef]
- Huang, M.-Y.; Jiang, X.-M.; Xu, Y.-L.; Yuan, L.-W.; Chen, Y.-C.; Cui, G.; Huang, R.-Y.; Liu, B.; Wang, Y.; Chen, X.; et al. Platycodin D triggers the extracellular release of programed death Ligand-1 in lung cancer cells. Food Chem. Toxicol. 2019, 131, 110537. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J.S. Triptolide reverses helper T cell inhibition and down-regulates IFN-γ induced PD-L1 expression in glioma cell lines. J. Neurooncol. 2019, 143, 429–436. [Google Scholar] [CrossRef]
- Kuo, C.S.; Yang, C.Y.; Lin, C.K.; Lin, G.J.; Sytwu, H.K.; Chen, Y.W. Triptolide suppresses oral cancer cell PD-L1 expression in the interferon-γ-modulated microenvironment in vitro, in vivo, and in clinical patients. Biomed. Pharm. 2021, 133, 111057. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, M.Y.; Zhang, Z.H.; Zuo, H.X.; Wang, J.Y.; Xing, Y.; Ri, M.; Jin, H.L.; Jin, C.H.; Xu, G.H.; et al. Panaxadiol inhibits programmed cell death-ligand 1 expression and tumour proliferation via hypoxia-inducible factor (HIF)-1α and STAT3 in human colon cancer cells. Pharm. Res. 2020, 155, 104727. [Google Scholar] [CrossRef] [PubMed]
- Bedi, D.; Henderson, H.J.; Manne, U.; Samuel, T. Camptothecin induces PD-L1 and immunomodulatory cytokines in colon cancer cells. Medicines 2019, 6, 51. [Google Scholar] [CrossRef]
- Hunakova, L.; Horvathova, E.; Gronesova, P.; Bobal, P.; Otevrel, J.; Brtko, J. Triorganotin Isothiocyanates Affect Migration and Immune Check-point Receptors in Human Triple-negative Breast Carcinoma MDA-MB-231 Cells. Anticancer Res. 2019, 39, 4845–4851. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-L.; Kuo, Y.-H.; Wu, L.-H.; Chang, C.-M.; Cheng, K.-J.; Tyan, Y.-C.; Lee, C.-H. The extracts of Astragalus membranaceus overcome tumor immune tolerance by inhibition of tumor programmed cell death protein ligand-1 expression. Int. J. Med. Sci. 2020, 17, 939–945. [Google Scholar] [CrossRef]
- Safonova, E.A.; Lopatina, K.A.; Razina, T.G.; Zueva, E.P.; Gur’ev, A.M.; Belousov, M.V. Effects of Tussilago farfara L. Polysaccharides on the Expression of PD-1 (CD279) and PD-L1 (CD274) in Peripheral Blood and Tumor Tissue Lymphocytes in Mice with Lewis Lung Carcinoma. Bull. Exp. Biol. Med. 2020, 169, 378–382. [Google Scholar] [CrossRef]
- Ryan, N.M.; Lamenza, F.F.; Upadhaya, P.; Pracha, H.; Springer, A.; Swingler, M.; Siddiqui, A.; Oghumu, S. Black raspberry extract inhibits regulatory T-cell activity in a murine model of head and neck squamous cell carcinoma chemoprevention. Front. Immunol. 2022, 13, 2742. [Google Scholar] [CrossRef]
- Wu, H.-J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Iebba, V.; Albiges, L.; Fidelle, M.; Bonvalet, M.; Colomba, E.; Zitvogel, L.; Escudier, B.; Routy, B. Gut microbiome composition to predict resistance in renal cell carcinoma (RCC) patients on nivolumab. J. Clin. Oncol. 2018, 36, 4519. [Google Scholar] [CrossRef]
- Chung, M.W.; Kim, M.J.; Won, E.J.; Lee, Y.J.; Yun, Y.W.; Cho, S.B.; Joo, Y.E.; Hwang, J.E.; Bae, W.K.; Chung, I.J.; et al. Gut microbiome composition can predict the response to nivolumab in advanced hepatocellular carcinoma patients. World J. Gastroenterol. 2021, 27, 7340–7349. [Google Scholar] [CrossRef]
- Grenda, A.; Iwan, E.; Chmielewska, I.; Krawczyk, P.; Giza, A.; Bomba, A.; Frąk, M.; Rolska, A.; Szczyrek, M.; Kieszko, R.; et al. Presence of Akkermansiaceae in gut microbiome and immunotherapy effectiveness in patients with advanced non-small cell lung cancer. AMB Express 2022, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat. Med. 2022, 28, 704–712. [Google Scholar] [CrossRef]
- Nakajima, A.; Sasaki, T.; Itoh, K.; Kitahara, T.; Takema, Y.; Hiramatsu, K.; Ishikawa, D.; Shibuya, T.; Kobayashi, O.; Osada, T.; et al. A Soluble Fiber Diet Increases Bacteroides fragilis Group Abundance and Immunoglobulin A Production in the Gut. Appl. Environ. Microbiol. 2020, 86, e00405-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-W.; Cephas, K.D.; Holscher, H.D.; Kerr, K.R.; Mangian, H.F.; Tappenden, K.A.; Swanson, K.S. Nondigestible Fructans Alter Gastrointestinal Barrier Function, Gene Expression, Histomorphology, and the Microbiota Profiles of Diet-Induced Obese C57BL/6J Mice. J. Nutr. 2016, 146, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Shalini, R.; Abinaya, G.; Saranya, P.; Antony, U. Growth of selected probiotic bacterial strains with fructans from Nendran banana and garlic. LWT Food Sci. Technol. 2017, 83, 68–78. [Google Scholar] [CrossRef]
- Guven, D.C.; Aktas, B.Y.; Simsek, C.; Aksoy, S. Gut microbiota and cancer immunotherapy: Prognostic and therapeutic implications. Future Oncol. 2020, 16, 497–506. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, W.; Li, X.; Xin, X.; Liu, D. Daily Supplementation with Fresh Angelica keiskei Juice Alleviates High-Fat Diet-Induced Obesity in Mice by Modulating Gut Microbiota Composition. Mol. Nutr. Food Res. 2019, 63, e1900248. [Google Scholar] [CrossRef]
- González, S.; Salazar, N.; Ruiz-Saavedra, S.; Gómez-Martín, M.; de Los Reyes-Gavilán, C.G.; Gueimonde, M. Long-Term Coffee Consumption is Associated with Fecal Microbial Composition in Humans. Nutrients 2020, 12, 1287. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, B.; Zhong, C.; Guo, J.; Zhang, L.; Mu, T.; Zhang, Q.; Bi, X. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Carcinogenesis 2018, 39, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharm. 2012, 63, 497–503. [Google Scholar]
- Gu, J.; Thomas-Ahner, J.M.; Riedl, K.M.; Bailey, M.T.; Vodovotz, Y.; Schwartz, S.J.; Clinton, S.K. Dietary Black Raspberries Impact the Colonic Microbiome and Phytochemical Metabolites in Mice. Mol. Nutr. Food Res. 2019, 63, e1800636. [Google Scholar] [CrossRef]
- Ullah, M.F.; Khan, M.W. Food as medicine: Potential therapeutic tendencies of plant derived polyphenolic compounds. Asian Pac. J. Cancer Prev. 2008, 9, 187–195. [Google Scholar] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Anhê, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Jena, P.K.; Liu, H.-X.; Hu, Y.; Nagar, N.; Bronner, D.N.; Settles, M.L.; Bäumler, A.J.; Wan, Y.-J.Y. Obesity treatment by epigallocatechin-3-gallate-regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 6371–6384. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Wilson, M.; Moran, U.; Pavlick, A.C.; Izsak, A.; Wechter, T.; Weber, J.S.; Osman, I.; Ahn, J. Gut microbiome and immunotherapy response in melanoma patients. J. Clin. Oncol. 2018, 36, 9575. [Google Scholar] [CrossRef]
- Fukuoka, S.; Daisuke, M.; Togashi, Y.; Sugiyama, E.; Udagawa, H.; Kirita, K.; Kamada, T.; Kawazoe, A.; Goto, K.; Doi, T.; et al. Association of gut microbiome with immune status and clinical response in solid tumor patients who received on anti-PD-1 therapies. J. Clin. Oncol. 2018, 36, 3011. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; Cortés-Martín, A.; Loria-Kohen, V.; Ramírez-de-Molina, A.; García-Mantrana, I.; Collado, M.C.; Espín, J.C.; Selma, M.V. Deciphering the Human Gut Microbiome of Urolithin Metabotypes: Association with Enterotypes and Potential Cardiometabolic Health Implications. Mol. Nutr. Food Res. 2019, 63, e1800958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Al-Maghout, T.; Cao, H.; Pelzl, L.; Salker, M.S.; Veldhoen, M.; Cheng, A.; Lang, F.; Singh, Y. Gut Bacterial Metabolite Urolithin A (UA) Mitigates Ca2+ Entry in T Cells by Regulating miR-10a-5p. Front. Immunol. 2019, 10, 1737. [Google Scholar] [CrossRef]
- Maia, M.C.; Poroyko, V.; Won, H.; Almeida, L.; Bergerot, P.G.; Dizman, N.; Hsu, J.; Jones, J.; Salgia, R.; Pal, S.K. Association of microbiome and plasma cytokine dynamics to nivolumab response in metastatic renal cell carcinoma (mRCC). J. Clin. Oncol. 2018, 36, 656. [Google Scholar] [CrossRef]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef]
- Botticelli, A.; Vernocchi, P.; Marini, F.; Quagliariello, A.; Cerbelli, B.; Reddel, S.; Del Chierico, F.; Di Pietro, F.; Giusti, R.; Tomassini, A.; et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J. Transl. Med. 2020, 18, 49. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef]
- Buerki, R.A.; Chheda, Z.S.; Okada, H. Immunotherapy of Primary Brain Tumors: Facts and Hopes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5198–5205. [Google Scholar] [CrossRef] [PubMed]
- Birdi, H.K.; Jirovec, A.; Cortés-Kaplan, S.; Werier, J.; Nessim, C.; Diallo, J.-S.; Ardolino, M. Immunotherapy for sarcomas: New frontiers and unveiled opportunities. J. Immunother. Cancer 2021, 9, e001580. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qi, W.; Guo, J.; Sun, L.; Ding, A.; Zhao, G.; Li, H.; Qiu, W.; Lv, J. Immune checkpoint inhibitor combination therapy for gastric cancer: Research progress. Oncol. Lett. 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Xiu, Z.; Guo, J.; Wang, L.; Zhou, Y.; Jiao, Y.; Sun, M.; Cai, J. Combination of Immune Checkpoint Inhibitors with Chemotherapy in Lung Cancer. OncoTargets Ther. 2020, 13, 7229–7241. [Google Scholar] [CrossRef]
- Huang, Z.; Su, W.; Lu, T.; Wang, Y.; Dong, Y.; Qin, Y.; Liu, D.; Sun, L.; Jiao, W. First-Line Immune-Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Current Landscape and Future Progress. Front. Pharmacol. 2020, 11, 8091. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.; Dearden, H.C.; Nguyen, B.; Soon, J.A.; Smith, J.L.; Randhawa, M.; Mant, A.; Warburton, L.; Lo, S.; Meniawy, T.; et al. Combined ipilimumab and nivolumab first-line and after BRAF-targeted therapy in advanced melanoma. Pigment Cell Melanoma Res. 2020, 33, 358–365. [Google Scholar] [CrossRef]
- Garje, R.; An, J.; Greco, A.; Vaddepally, R.K.; Zakharia, Y. The Future of Immunotherapy-Based Combination Therapy in Metastatic Renal Cell Carcinoma. Cancers 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Mo, Z.; Cao, Z.; Yu, L.; Wang, Y.; Li, P.; Lin, Y.; Zhang, S. An Integrative Analysis Reveals the Potential Mechanism between Herbal Medicine Yinchen and Immunoregulation in Hepatocellular Carcinoma. BioMed Res. Int. 2020, 2020, 8886914. [Google Scholar] [CrossRef] [PubMed]
| Lead Author, Year | Target Population/Patient Number (n) | Main Findings | Candidate Phytochemical | Phytochemical Enriched Nutrient | Bacteria |
|---|---|---|---|---|---|
| Vetizou et al. (2015) [78] | Melanoma/25 | Increased levels of Bacteroides thetaiotaomicron and Bacteroides fragilis in ICI responders Improved response to CTLA-4 blockade with FMT from patients with increased fecal Bacteroides spp. levels | Polyphenol/coumarin [79] | Soluble fiber-rich diet [74]/High coffee-consumption [80] | Bacteroides thetaiotaomicron Bacteroides fragilis |
| Sivan et al. (2015) [81] | Melanoma/Mice | Increased levels of Bacteroides spp. in ICI responders Similar tumor control as PD-L1 blockade with oral supplementation of Bifidobacterium spp. | Resveratrol [82] | Grapes, wine, and peanuts [83] | Bifidobacterium spp. |
| Dubin et al. (2016) [84] | Melanoma/34 | Lower risk of colitis in Bacteroides spp. enriched patients | Polyphenol/coumarin [79] | Soluble fiber-rich diet [74]/High coffee-consumption [80] | Bacteroides spp. |
| Chaput et al. (2017) [85] | Melanoma/26 | Longer progression-free and overall survival in Faecalibacterium spp. enriched patients | Anthocyanin [86] | Black Raspberries [86] | Faecalibacterium spp. |
| Gopalakrishnan et al. (2018) [87] | Melanoma/112 | Higher alpha diversity and increased Ruminococcaceae levels in the feces of ICI responders Higher buccal and fecal levels of Bacteroidales in ICI non-responders | Polyphenols [88] (Naringenin and quercetin) Anthocyanin [89] | Onion, apple, broccoli [90] Black Raspberries [89] | Ruminococcaceae Bacteroidales |
| Matson et al. (2018) [91] | Melanoma/42 | Increased Bifidobacterium longum in the feces of ICI responders Tumor control, augmented T-cell responses, and improved efficacy of anti-PD-L1 blockade with oral supplementation of responders’ feces to germ-free mice | Resveratrol [92] | Grapes, wine, and peanuts [83] | Bifidobacterium longum |
| Routy et al. (2018) [69] | NSCLC/140 RCC/67 | Increased levels of Akkermansia muciniphila in ICI responders Restoration of the efficacy of PD-1 blockade in antibiotic pretreated mice after FMT from ICI responders | Curcumin [93]/EGCG [94] | Prebiotic nondigestible fiber-rich diet [95] | Akkermansia muciniphilia |
| Peters et al. (2018) [96] | Melanoma/26 | Lower risk of progression in patients with higher community diversity Lower risk of progression in patients enriched with Faecalibacterium prausnitzii | Anthocyanin [86] | Black Raspberries [86] | Faecalibacterium prausnitzii |
| Fukuoka et al. (2018) [97] | NSCLC/14 Gastric cancer/24 | Higher alpha diversity and Ruminococcaceae levels in ICI responders | Ellagitannins [98] | Pomegranates, nuts [99] | Ruminococcaceae |
| Derosa et al. (2018) [70] | RCC/85 | Increased abundance of Akkermansia muciniphila and Bacteroides salyersiae in non-resistant renal cell carcinoma patients | Curcumin [93]/EGCG [94] | Prebiotic nondigestible fiber-rich diet [95] | Akkermansia muciniphila |
| Restoration of the efficacy of the ICI with Akkermansia muciniphila and Bacteroides salyersiae transplantation to mice with unfavorable/dysbiotic profile | Not reported | Bacteroides salyersiae | |||
| Maia et al. (2018) [100] | RCC/20 | Increased abundance of Roseburia and Faecalibacterium spp. in ICI responders | Anthocyanin [86] Polyphenols [101] | Black Raspberries [86] Resistant Starch [101] | Faecalibacterium prausnitzii Roseburia |
| Botticelli et al. (2020) [102] | NSCLC/11 | Increased fecal Akkermansia muciniphila, Bifidobacterium longum, and Faecalibacterium prausnitzii levels in ICI responders | Curcumin [93]/EGCG [94] Resveratrol [82], Anthocyanin [103] Anthocyanin [86] | Prebiotic nondigestible fiber-rich diet [95] Grapes, wine, and peanuts [83], Chinese purple sweet potato cultivar [103] Black Raspberries [86] | Akkermansia muciniphila Bifidobacterium longum Faecalibacterium prausnitzii |
| Liu et al. (2020) [36] | CRC/mice | The addition of anthocyanins in the α-PD-L1 treatment showed an overrepresentation of Lachnospiraceae and Ruminococcaceae | Anthocyanin [86] | Black Raspberries [86] | Ruminococcaceae and Lachnospiraceae |
| Chung et al. (2021) [71] | HCC/8 | Increased fecal Akkermansia levels in ICI responders | Curcumin [93]/EGCG [94] | Prebiotic nondigestible fiber-rich diet [95] Grapes, wine, and peanuts [83] | Akkermansiaceae |
| Grenda et al. (2022) [72] | NSCLC/47 | Increased fecal Akkermansia levels in ICI responders | Curcumin [93]/EGCG [94] | Prebiotic nondigestible fiber-rich diet [95] Grapes, wine, and peanuts [83] | Akkermansiaceae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guven, D.C.; Sahin, T.K.; Rizzo, A.; Ricci, A.D.; Aksoy, S.; Sahin, K. The Use of Phytochemicals to Improve the Efficacy of Immune Checkpoint Inhibitors: Opportunities and Challenges. Appl. Sci. 2022, 12, 10548. https://doi.org/10.3390/app122010548
Guven DC, Sahin TK, Rizzo A, Ricci AD, Aksoy S, Sahin K. The Use of Phytochemicals to Improve the Efficacy of Immune Checkpoint Inhibitors: Opportunities and Challenges. Applied Sciences. 2022; 12(20):10548. https://doi.org/10.3390/app122010548
Chicago/Turabian StyleGuven, Deniz Can, Taha Koray Sahin, Alessandro Rizzo, Angela Dalia Ricci, Sercan Aksoy, and Kazim Sahin. 2022. "The Use of Phytochemicals to Improve the Efficacy of Immune Checkpoint Inhibitors: Opportunities and Challenges" Applied Sciences 12, no. 20: 10548. https://doi.org/10.3390/app122010548
APA StyleGuven, D. C., Sahin, T. K., Rizzo, A., Ricci, A. D., Aksoy, S., & Sahin, K. (2022). The Use of Phytochemicals to Improve the Efficacy of Immune Checkpoint Inhibitors: Opportunities and Challenges. Applied Sciences, 12(20), 10548. https://doi.org/10.3390/app122010548








