Abstract
Supplementation with probiotics in sports is on the rise with the aim of improving health and athletic performance. Since intense exercise-induced muscle damage leads to an inflammatory process by increasing circulating inflammatory cytokines, probiotic supplementation may modulate and correct the inflammation. We systematically reviewed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines in the Scopus, Web of Science, and Medline databases for the 10 years until January 2023. This review aimed to evaluate probiotic supplementation as a strategy for modulating inflammation in healthy physically active adults or athletes. Studies were indexed to assess the effect of probiotic supplementation on cytokine behavior in the inflammatory response in physically active individuals. Of the 136 studies identified in the search, 13 met the inclusion criteria, and their quality was assessed using the McMaster Critical Review Form. The results of these trials indicated a significant improvement in inflammatory cytokines in probiotic-supplemented participants, with a significant increase in anti-inflammatory cytokines (IL-10) and a significant decrease in proinflammatory cytokines (IL-6, TNF-α, and IL-8). This would create uncertainty about probiotics’ effect on interleukins’ behavior after exercise, and further clinical trials are needed to establish a solid basis.
1. Introduction
The human microbiota is a set of microorganisms (bacteria, fungi, archaea, viruses, and parasites) that live in our body and can be differentiated into commensals, mutualists, and pathogens [1]. There are several microbial ecosystems in the human body, the most complex, diverse, and numerous of which are found in the gastrointestinal tract (GIT). The microbiota is specific to each individual and conditioned by their genotype, early exposure to microorganisms in their environment, diet, lifestyle changes, use of antibiotics, and quantity/quality of physical activity [2,3]. In total, the GIT in adults may harbor between 500 and 1000 species of microorganisms, the majority of which are bacteria belonging to the phyla Bacteroidetes (25%) and Firmicutes (60%), and to a lesser extent Proteobacteria, Verrucomicrobia, Fusobacteria, Cyanobacteria, Actinobacteria and Spirochaetes, archaea, fungi, protozoa, and viruses, among many others [4]. It has been described that the Firmicutes/Bacteroidetes (F/B) ratio is key to the homeostatic modulation of GIT. In this sense, increased F/B (obesity) or decreased F/B (inflammatory bowel disease) induce states of dysbiosis [5]. Additionally, a low amount of Proteobacteria together with a high presence of Bacteroidetes, Prevotella, and Ruminoccus is favorable for health [4].
Exercise induces physiological responses in the body that attempt to modulate the homeostatic adaptive processes, leading to recovery and tissue remodeling [6]. However, they do not always succeed, leading to metabolic, hormonal, neuro-physical, and immunological changes due to local muscle and systemic inflammatory hyper-responses to strenuous exercise, which is associated with reduced sports performance, increased fatigue, and the establishment of overtraining, putting the health of the athlete at risk [7,8]. In exercise-induced muscle damage (EIMD), an inflammatory process takes place as a consequence of the initial phase of mechanical muscle damage [9], with the release of interleukins (ILs) occurring, mainly IL-1β, IL-6, tumor necrosis factor-alpha (TNF-α), and macrophage inflammatory protein-1 (MIP-1), which enhance the inflammatory process [10,11]. To this inflammatory response must be added the inflammation caused by oxidative/metabolic stress that occurs during prolonged endurance exercise [9]. Exercise has an intensity- and/or time-dependent effect on the gut microbiota that is independent of the diet consumed [3]. Indeed, the behavior of the microbiota, which acts like an endocrine gland, inducing multifactorial changes in organ function, metabolism, immunity, and host behavior [12]. Regular physical activity and low/moderate-intensity exercise are beneficial adaptations and improve the long-term resistance of the intestinal barrier by reducing the response of heat shock proteins to heat stress by preventing the breakdown of tight junction proteins between the epithelial cells [12], such as those produced by therapeutic exercise in patients with inflammatory bowel disease [13]. However, high-intensity and long-duration exercise causes gastrointestinal disorders known as “exercise-induced gastrointestinal syndrome”, by damaging epithelial cells in the gut [14]. It is likely that the competition between muscle and intestinal blood flow, which is resolved in favor of the former during vigorous exercise, causes cell death of the lining epithelium, altering permeability, which facilitates endotoxemia, and triggers immune/inflammatory responses leading to EIMD inflammation [15,16].
The dysregulation of the inflammatory response needs to be modulated to optimize athletic performance and maintain the health of athletes. To this end, sports supplements have been used as a supplement to the athlete’s diet [17]. Thus, interest in probiotics as a sports nutritional supplement has grown exponentially in recent years. Probiotics are a class of dietary supplements consisting of “live microorganisms that, when administered in adequate amounts, confer a health benefit to the host” [18]. Probiotics may indirectly benefit athletic performance and health status by maintaining gastrointestinal function and health, reducing susceptibility to disease, and modulating host immune expression [16]. The ability of probiotics to improve an individual’s immunity is exerted by controlling the communication between immune and inflammatory cells, through the modulation of ILs, as probiotics bind to the membrane receptors of enterocytes, which critically influences the regulation (activation/inhibition) of ILs [16,19]. There are several pharmaceutical forms of probiotic microorganisms available. Commonly used probiotic strains belong to the genera Lactobacillus, Bifidobacterium, E. coli Nissle 1917 and Saccharomyces boulardii [20]. However, it may not necessarily provide the same health-promoting properties [16]. Furthermore, if a benefit is attributed to one strain, it cannot be extrapolated to other strains of the same species. Some strains of the Lactobacillus genus (paracasei CNCM I-4034; rhamnosus CNCM I-4036) and Bifidobacterium breve (CNCM I-4035) have anti-inflammatory effects [21].
In summary, there are many reasons to believe that probiotic supplementation can be used as a strategy to interfere with EIMD and/or microbiome inflammatory pathways, but its impact on EIMD-induced ILs remains to be established. Moht et al. [22] described the inflammatory effect in a population of healthy non-athlete adults and in a limited number of cytokines. Quero Calero et al. [23] also reported the effects of probiotics, prebiotics and/or symbiotics in athletes and active individuals, including their effects on the immune system, oxidative stress, gastrointestinal and respiratory symptoms, but not on EIMD-derived inflammatory cytokines. In 2022, Guo et al. [24] described probiotic supplementation on immune and inflammatory markers in athletes; however, they did not include some inflammatory (IL-15, IL-1alpha, IL-1RAa) or anti-inflammatory (INF-γ) cytokines. These investigators [24] did not describe or discuss in sufficient depth the posology or composition of probiotic supplementation. These authors included athletes in parallel controlled trials exclusively. Therefore, this review critically assesses the effects of probiotic supplementation in physically active adults to analyze the impact of probiotics on the behavior of interleukins in the inflammatory response and anti-inflammatory response in physically active adults and athletes, with a special interest in the composition and posology of supplemented probiotics with different clinical trial designs.
2. Methods
We formulated the research question using the PICOS model according to the standard methods proposed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [25]: (i) P (population): healthy, physically active or athletes adults without chronic diseases; (ii) I (intervention): supplementation with probiotics only (understood as live microorganisms); (iii) C (comparison): placebo/control group or pre/post comparison data group; (iv) O (outcomes): markers of inflammation: anti-inflammatory interleukins (IL-1Ra, IL-4, INF-γ, and IL-10) and pro-inflammatory interleukins (IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-15), and TNF-α; (v) S (study design): randomized, double-blind, placebo-controlled, crossover study.
2.1. Search Strategy
The SCOPUS, Web of Science (WOS), and Medline (PubMed) databases were searched for trials from the last 10 years of the database, up until January 2023. The search strategy included terms related to probiotics and the different outcome biomarkers, as well as a combination of these using the Medical Subject Headings (MeSH) index and Boolean operators: (probiotics OR probiotic-bacterium) AND (exercise OR athletes OR sport or physical activity) AND (inflammatory OR immune system OR cytokines OR biomarkers). All trials were included in an Excel spreadsheet to identify possible duplicates.
2.2. Selection Criteria
The following inclusion criteria were applied for the final selection of trials: (a) healthy adults without chronic diseases, physically active (excluding animal/in vitro studies); (b) studies evaluating probiotic supplementation alone (excluding any combination with any other supplements or prebiotics); (c) clinical trials, randomized or not, placebo/control group or of pre/post comparison (excluding systematic reviews, meta-analyses, popular articles, letters to the editor or opinion articles and any other non- original studies); (d) measured variables (primary or secondary) that were biomarkers of inflammation: anti-inflammatory interleukins (IL-1Ra, IL-4, INF-γ, and IL-10) and pro-inflammatory interleukins (IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-15), and TNF-α; and (e) trials with clear information on dosage and duration of probiotic supplementation (understood as live microorganisms). All records that did not meet these criteria were excluded.
2.3. Quality Assessment
The methodological quality of studies was assessed using the McMaster University Occupational Therapy Evidence-Based Practice Research Group [26] as a critical appraisal tool. These guidelines are suitable for the assessment of randomized and non-randomized studies because they are a comprehensive and reliable tool for assessing the methodological quality of quantitative evidence.
3. Results
3.1. Study Selection
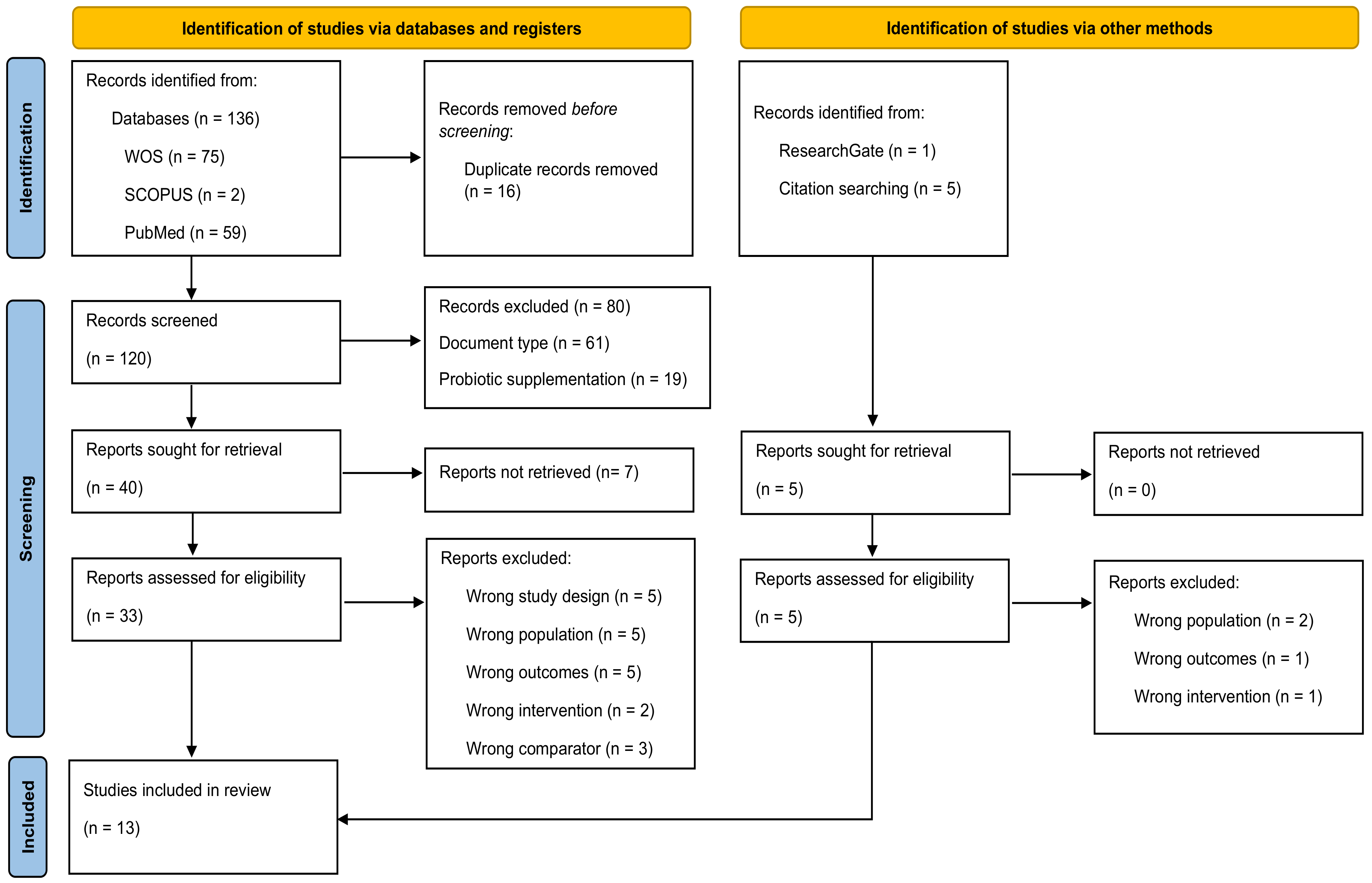
The literature search yielded 142 studies, of which 136 studies were retrieved from the electronic databases WOS, SCOPUS, and PubMed, and six studies were retrieved from other sources, such as ResearchGate and reference lists of relevant studies. After the exclusion of 16 duplicates, we examined a total of 120 identified articles from databases were retrieved. After title and abstract assessment, 40 articles were considered as potential studies. After full-text review and assessment of potential registries, 13 studies [27,28,29,30,31,32,33,34,35,36,37,38,39] were included in the systematic review (Figure 1).
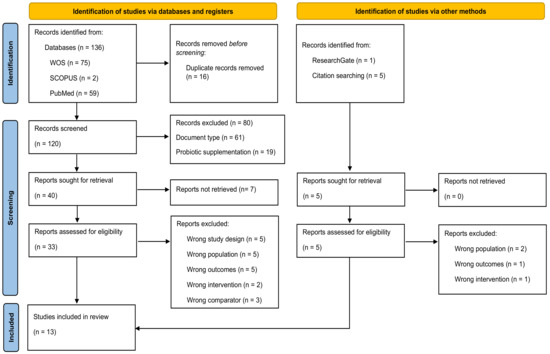
Figure 1.
Flow diagram depicting the identification and selection processes of relevant studies according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [25].
3.2. Quality Assessment
Four studies [27,28,30,39] had results ≥ 15 points, corresponding to “excellent” quality, and nine [29,31,32,33,34,35,36,37,38] studies had results between 13 and 14 points, corresponding to “very good” quality (Table 1).

Table 1.
Results of the methodological quality assessment of included studies—McMaster Critical Review Form for Quantitative Studies [26].
3.3. Characteristics of the Participants and the Intervention
The total number of physically active adults included in this systematic review was 300 (248 men, 4 women, and 48 unspecified). A total of 269 participants were endurance athletes [22,23,24,25,26,27,29,31,32,33,34], 27 were elite athletes [39], and 31 participants were strength athletes [33,35]. Six studies used a supplement manufactured for the study [22,25,26,29,30,34], six studies used a proprietary commercial supplement [29,32,33,36,37,38], and one study did not report [28]. Regarding the strains used, eight studies [28,29,30,35,36,37,38,39] used mixed strains, and five studies used single strains [27,31,32,33,34]. The doses of probiotics used ranged from 0.25 × 108 Colony Forming Units (CFU) to 250,000 × 108 CFU. The timing of probiotic supplementation was: two trials [33,37] after breakfast, one trial [38] post-exercise, one trial [28] after dinner, and three trials [32,34,36] twice a day and six trials [27,29,30,31,35,39] once a day without specifying the exact time of the supplementation. The duration of the intervention ranged from 1 week [32] to 14 weeks [36]. The pharmaceutical form used for probiotics supplementation was: capsules [27,29,30,34,35,37,38,39], powder [28,33,36], and drinks [31,32] (Table 2).

Table 2.
Characteristics of participants and supplementation protocols of the selected studies.
3.4. Outcome Measures
Table 3 summarizes the content of the studies included in this systematic review. It includes information on the author(s), year of publication, country, the sample studied, level of sports, gender, number, age, height, weight, body mass index, and maximum volume of oxygen uptake (VO2max) of the participants. The study design includes the control group if the study included one; the supplementation protocol, which specifies the type of probiotics used, the dose, and time of administration; the interleukins analyzed; the main effects; and finally, the results.

Table 3.
Studies included in the systematic review of the effect of Probiotics supplementation on interleukin response in healthy adults.
3.5. Anti-Inflammatory Cytokines
Significant increases (p < 0.05) in IL-10 (anti-inflammatory) have been observed in the control group [30,31] and at baseline [32,34,37], contrasting with significant decreases (p < 0.05) in IL-10 observed in trained runners [29]. Only one study reported a significant increase (p < 0.05) in IL-4 [34] in the intervention group compared to the baseline. Shing et al. [29] described a favorable trend of increased IL-1Ra in the intervention group after 4 weeks of supplementation with a probiotics mixture of 4.5 × 1010 CFU (Table 3). Huang et al. [34] reported a significant increase (p < 0.05) in INF-γ in the supplemented group compared to the control group. Two studies [32,33] showed no change when comparing both conditions (intervention and control).
3.6. Pro-Inflammatory Cytokines
The major pro-inflammatory cytokines TNF-α [28,29,30,31,32,33,34,36,39], IL-6 [27,28,29,30,31,32,33,34,35,36,37,38,39], IL-1α [38], IL-1β [28,30,31,32,33] and IL-8 [28,32,33,34,37,38] were evaluated as markers of exercise inflammation in the studies included in this systematic review (Table 3).
Pugh et al. [38] showed a substantial, non-significant decrease (p > 0.05) for IL-1α in seven supplemented elite cyclists compared to the control group; however, a trend towards an increase was observed in the group supplemented with a probiotics mixture (2.5 × 1013 CFU) compared to baseline [38]. In four studies [30,31,32,33], no changes in IL-1β were observed in supplemented groups compared to control groups (Table 3).
For IL-6, three trials [29,34,38] reported significant reductions (p < 0.05) in the probiotic group compared with the non-supplemented group. In 7 of the studies [30,31,32,33,36,37,39] included in this systematic review, no changes were observed in the intervention group compared to the control group, although 4 studies [31,32,36,37] reported significant increases (p < 0.05) in IL-6 throughout the study in adults supplemented with probiotics (Table 3).
Only the study by Huang et al. [34] in triathletes described a significant decrease (p < 0.05) in IL-8 in the supplemented group with L-Plantarum PS128 (3 × 1010 CFU) compared to the control group, although significant increases (p < 0.05) in IL-8 have been observed in physically active adults following probiotic supplementation [32,37] (Table 3).
Significant decreases (p < 0.05) in TNF-α were reported in two studies [29,34] included in this review, in triathletes [34] and runners [29] supplemented with 3 × 1010 CFU and 4.5 × 1010 CFU, respectively, compared to the control group. Probiotic supplementation showed no effect on TNF-α compared with the control group in four studies [30,31,33,39] that were included in this systematic review. In addition, significant increases (p < 0.05) were found in the supplemented group compared with baseline in endurance athletes (32) and marathoners [31]. Five studies [28,29,33,34,36] included in this systematic review showed a non-significant increase (p > 0.05) in TNF-α in the supplemented group at the end of the study compared to the baseline (Table 3).
4. Discussion
This systematic review aimed to analyze the effect of probiotic supplementation on cytokine levels in physically active healthy adults or athletes. Thirteen trials were selected because they met the inclusion and/or exclusion criteria. In general, it is difficult to assess the effect of probiotics on the modulation of exercise-induced inflammation, as it would be influenced by the type/duration of exercise, the amount/type of each probiotic used, and the duration of supplementation. Therefore, there is no clear evidence of consistent beneficial effects of probiotic supplementation on the ability of probiotics to modulate immune and/or inflammatory dysfunction after exercise. The duration of probiotic supplementation varied widely between trials. It is likely that effects on inflammatory markers require several months of supplementation rather than a few weeks. The heterogeneity in the duration of supplementation may also explain the uncertainty in the results. For example, no changes were observed when comparing the two study conditions (intervention and control) [30,31,32,33,36,37,39], and significant increases in inflammatory cytokines occurred in the intervention condition throughout the study [31,32,36,37,38]. However, there was a significant increase in the anti-inflammatory cytokines, IL-10, [30,31], accompanied by a significant decrease in the pro-inflammatory cytokines, IL-6 [29,34,38], TNF-α [29,34], and IL-8 [34] in the intervention group compared to the control group. In addition, no probiotic-related adverse effects were reported. Therefore, the results of this systematic review have been divided into sections for a more precise analysis.
4.1. Probiotics Supplementation
The trials selected for this review used different strains of probiotics in the supplementation periods: Lactobacillus salivarius [27], Lactococcus lactis [30,36], Bacillus coagulans [33], Bifidobacterium animalis [28,30,37,38,39], Lactobacillus acidophilus [28,29,30,36,37,38], Lactobacillus casei [29,31,32], Lactobacillus helveticus Lafti [39], Enterococcus faecium [36,39], Bifidobacterium longum [39], Bacillus subtilis [39], Lactobacillus lactis [30], Bifidobacterium bifidum [29,30,36,37,38], Bifidobacterium breve [29,35], Bifidobacterium lactis [29,36], Lactobacillus brevis [36], L-plantarum [29,34], L-rhamnosus [29], Streptococcus thermophilus [29,35] and L. fermentum [29]. The efficacy of probiotics depends not only on the type of strain, but also on the dose administered [40]. However, there is no specific/standard dose induces beneficial effects or that generates changes in the physiological homeostasis of the supplemented individuals [41]. The International Olympic Committee (ICO), in 2018, noted moderate support for the use of probiotics in athletes with 1.0 × 1010 CFU per day [42] and oral doses of probiotics have ranged from 108 to 1010 CFU per day [43], although the doses of probiotics used in this review ranged from 0.25 × 108 to 25 × 1012 CFU [27,28,29,30,31,32,33,34,35,36,37,38,39].
It is important to consider the possible risks or side effects that probiotic supplementation may cause. For example, probiotics may be responsible for mild gastrointestinal symptoms such as abdominal pain, nausea, loose stool, and bloating, which disappear within a few days of taking them [44]. In general, probiotic supplementation is safe, but caution should be exercised in people with serious health conditions, such as severe acute pancreatitis, inflammatory bowel disease, liver disease, and human immunodeficiency virus No adverse effects were reported in the 13 trials analyzed in this systematic review [27,28,29,30,31,32,33,34,35,36,37,38,39].
4.2. Anti-Inflammatory Cytokines
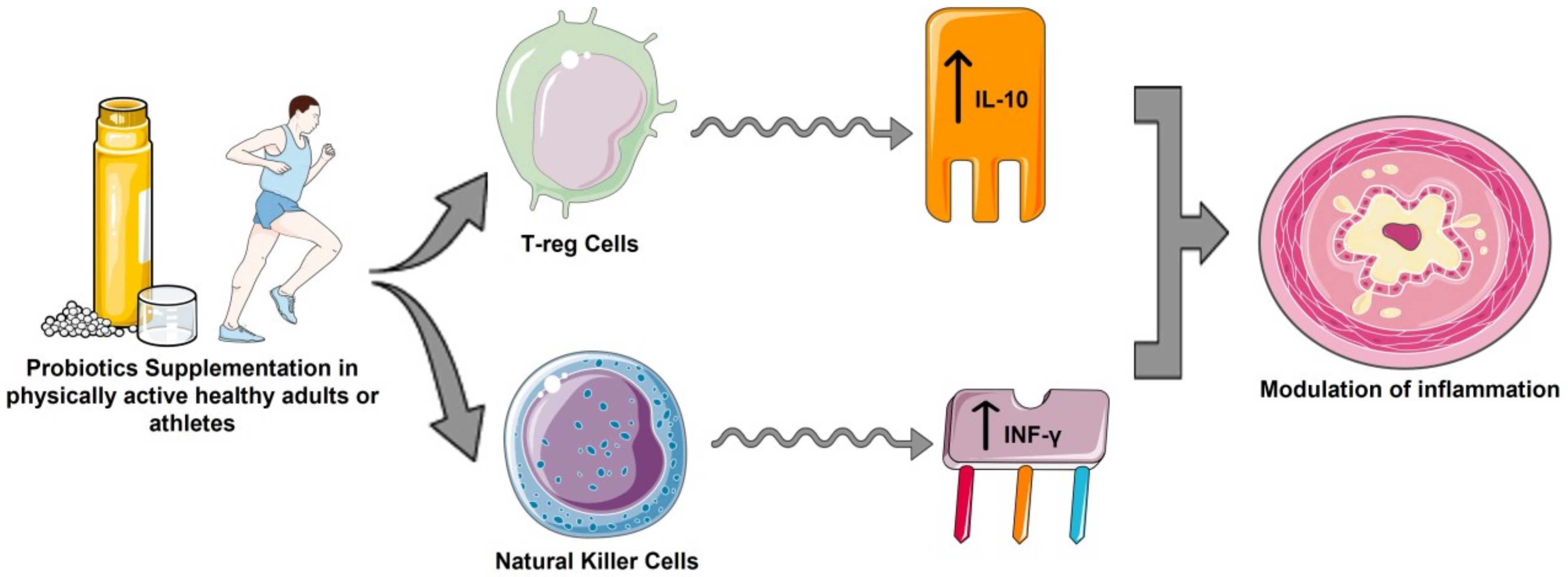
IL-10 triggers an increased anti-inflammatory response, and is one of the cytokines with the most potent anti-inflammatory action. The increase in IL-10 and IL-1Ra levels after exercise occurred after an increase in plasma IL-6 [45,46], which could justify the increases in IL-10 in the supplemented group in 10 studies [28,29,30,31,32,33,34,37,38], and significant in four studies [31,32,34,37], compared to baseline. A significant increase in IL-10 in the control group was also observed in marathon runners [30,31], and a significant increase was observed in elite cyclists [38]. This may suggest that probiotics play an additional role to exercise in the stimulation and release of IL-10 in the face of exacerbated inflammatory responses generated by high-intensity exercise situations [45]. The immunoregulatory effects of probiotics may be due to the action of Treg cells and their ability to increase the synthesis of IL-10 to attenuate excessive inflammatory responses (Figure 2), as occurs in inflammatory bowel disease and some autoimmune diseases [47]. These findings are in contrast to those described in a recent meta-analysis of 5 randomized clinical trials in which healthy athletes supplemented with probiotics showed a significant reductions in IL-10 concentrations compared with the control group [24]. These differences may be due to the magnitude of the global anti-inflammatory effect of probiotics, since by reducing proinflammatory cytokines levels, they no longer stimulate the production of anti-inflammatory cytokines such as IL-10, IL-4, IL-1Ra and INF-γ [18,24].
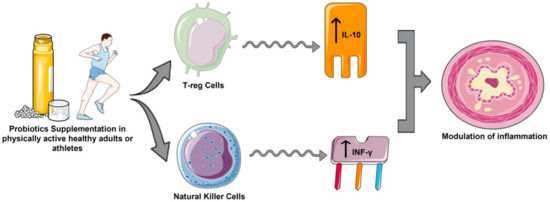
Figure 2.
Anti-inflammatory effects of probiotics in physically active healthy adults or athletes. (Created by Authors: Fernández-Lázaro et al. for this study).
In a previous study [24], the group of athletes supplemented with probiotics showed a significant advantage in IFN-γ levels compared to the control group. These results are similar to those described by Huang et al. [34], although Batatinha et al. [28] described a slight downward trend for IFN-γ in marathoners. Probiotics could condition NK cells by interacting with intestinal epithelial cells, inducing an increase in IFN-γ secretion (Figure 2) [47]. In the 13 studies [27,28,29,30,31,32,33,34,35,36,37,38,39] included in this review, no significant increase in IL-4 levels was observed compared with the placebo group. This may be a consequence of the adaptation processes of IL-4 to continuous/regular exercise [48].
4.3. Pro-Inflammatory Cytokines
Acute muscle inflammation caused by intense contractions during exercise could lead to leukocyte infiltration and increased levels of inflammatory cytokines such as TNF-α, IL-8, and IL-6. IL-6 is a key member of the cytokine network, and plays a critical role in acute inflammation [10,11]. Thus, pro-inflammatory cytokines were elevated after an exercise in the supplemented group, including TNF-α (31,32), IL-6 (31,32,36,37), IL-8 (32,37), IL-1α [38], and IL-1β [31].
In the results evaluated (Table 3), probiotics significantly reduced the production of the pro-inflammatory cytokines TNF-α [29,34], IL-6 [29,34,38], and IL-8 [34] compared to the control group. The inflammatory power of probiotics may be due to their effect on inflammatory signaling cascades by deregulating intracellular pathways of immune cells mitogen-activated protein kinases (MAPKs) that act on transcription factors: Janus kinase (JAK/STAT), nuclear factor κB pathway (NF-κB), Jun-1, and Fos [47]. The potential inhibition of NF-κB by the action of probiotics would trigger the suppression of the activation and phosphorylation of JAK/STAT proteins and inhibit MAPK signaling through its interaction with three key members of this pathway, including JNK, p38, and ERK [17]. Another possible effect of probiotics is to act on histamine, specifically on the H2 receptors of antigen-presenting cells, leading to a reduction in TNF-α, and MIP-1 [47]. The pathway mediated by probiotics metabolites such as short-chain fatty acids (SCFA), such as propionate, acetate, and butyrate [18], would have anti-inflammatory activity by binding to specific receptors on intestinal epithelial cells and suppressing the production of pro-inflammatory cytokines by the immune cells [47]. For example, it has recently been described that oats could promote the intestinal microbiota through an increased production of SFCA [49]. In this sense, Guo et al. [24] recently showed that probiotic supplementation significantly reduced the level of TNF-α and showed a significant decrease in IL-1β, IL-6, and IL-8. However, only 4/13 studies covered herein were reviewed by Gao et al. [24].
5. Future Scenarios
While it is true that potential benefits to inflammatory response have been described compared to non-supplemented athletes, specifically based on the significant increases in IL-10 [31,32,34,37], and significant decreases in TNF-α [29,34], IL-6 [29,34,38], and IL-8 [34], probiotics were unable to control the inflammatory response after exercise [31,32,36,37,38]. This would create uncertainty regarding the effect of probiotics on the behavior of cytokines after exercise. Furthermore, it is difficult to specifically recommend single-strain or mixed-strain formulations of probiotics, as the benefits experienced are strain-specific and will depend on the dose and time of supplementation, as well as the duration, intensity, and type of exercise. However, these findings could be encouraging as they would represent an important improvement in supplementation strategies aimed at improving the inflammatory response and therefore exercise performance, although further studies are needed to confirm this. It should be noted that supplementation should always be prescribed on an individual basis and supervised by a specialist.
6. Strengths and Limitations
The authors of this review acknowledge several limitations. Firstly, a limited number of manuscripts met the inclusion criteria, with a total of 13 records, although our systematic approach followed the PRISMA method [25] and the search was conducted using three electronic databases as SCOPUS, WOS, and Medline (PubMed). The McMaster methodological quality assessment tool [26] was used to ensure that all selected records met minimum quality criteria, the high rate of papers excluded for insufficient quality, and a several and a range of outcomes commonly used in sports supplementation research were included. Secondly, the high heterogeneity of the studies in terms of outcomes, supplement dosage, and intervention duration warrants caution in interpreting the results.
7. Conclusions
Overall, the probiotics selected in the studies in this systematic review may have some anti-inflammatory effects by modulating pro-inflammatory cytokines and stimulating anti-inflammatory cytokines. However, further studies are needed to identify probiotic strains that may play a critical role in inflammatory homeostasis, as well as studies to establish approaches to modulate the concentration or composition of probiotics.
Author Contributions
D.F.-L.: conceived and designed the research, analyzed and interpreted the data, drafted the paper, and approved the final version submitted for publication; E.G. and N.S.-S.: analyzed and interpreted the data, drafted the paper, and approved the final version submitted for publication K.R.F., R.R., J.M.-A. and R.M.A. analyzed and interpreted the data and critically reviewed the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors want to thank the Neurobiology Research Group, Department of Cellular Biology, Genetics, Histology and Pharmacology, Faculty of Medicine, of the University of Valladolid for their collaboration on infrastructure computer support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CFU | Colony Forming Units |
| EIMD | Exercise-Induced Muscle Damage |
| GIT | Gastrointestinal Tract |
| ICO | International Olympic Committee |
| ILs | Interleukins |
| JAK | Janus Kinase |
| MAPKs | Mitogen-Activated Protein Kinases |
| MeSH | Medical Subject Headings |
| MIP-1 | Macrophage Inflammatory Protein-1 |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyzes |
| SCFA | Short-Chain Fatty Acids |
| TNF-α | Tumor Necrosis Factor-alpha |
| VO2max | Maximum Volume of Oxygen |
| WOS | Web of Science |
References
- Eloe-Fadrosh, E.A.; Rasko, D.A. The Human Microbiome: From Symbiosis to Pathogenesis. Annu. Rev. Med. 2013, 64, 145–163. [Google Scholar] [CrossRef]
- Moles, L.; Otaegui, D. The Impact of Diet on Microbiota Evolution and Human Health. Is Diet an Adequate Tool for Microbiota Modulation? Nutrients 2020, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Del Campo-Moreno, R.; Alarcón-Cavero, T.; D’Auria, G.; Delgado-Palacio, S.; Ferrer-Martínez, M. Microbiota in human health: Characterization and transfer techniques. Enferm. Infecc. Microbiol. Clin. 2018, 36, 241–245. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; González-Bernal, J.J.; Sánchez-Serrano, N.; Navascués, L.J.; Del Río, A.A.; Mielgo-Ayuso, J. Physical Exercise as a Multimodal Tool for COVID-19: Could It Be Used as a Preventive Strategy? Int. J. Environ. Res. Public Health 2020, 17, 8496. [Google Scholar] [CrossRef] [PubMed]
- Kreher, J.B.; Schwartz, J.B. Overtraining Syndrome: A Practical Guide. Sports Health 2012, 4, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.L. Cytokine hypothesis of overtraining: A physiological adaptation to excessive stress? Med. Sci. Sports Exerc. 2000, 32, 317–331. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Navascués, L.J.; Martínez, A.C.; Seco-Calvo, J. The Role of Selenium Mineral Trace Element in Exercise: Antioxidant Defense System, Muscle Performance, Hormone Response, and Athletic Performance. A Systematic Review. Nutrients 2020, 12, 1790. [Google Scholar] [CrossRef]
- Stožer, A.; Vodopivc, P.; Bombek, L.K. Pathophysiology of Exercise-Induced Muscle Damage and Its Structural, Functional, Metabolic, and Clinical Consequences. Physiol. Res. 2020, 69, 565–598. [Google Scholar] [CrossRef]
- Malm, C. Exercise-induced muscle damage and inflammation: Fact or fiction? Acta Physiol. Scand. 2001, 171, 233–239. [Google Scholar] [CrossRef]
- Zuhl, M.; Schneider, S.; Lanphere, K.; Conn, C.; Dokladny, K.; Moseley, P. Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 2014, 48, 980–986. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Hernández-Burgos, N.; Cobreros Mielgo, R.; García-Lázaro, S. Evaluation of physical activity as a therapeutic adjuvant for patients with inflammatory bowel disease: A review. Investig. Clin. 2022, 63, 304–322. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Mika, A.S.; McCubbin, A.J. The impact of exercise modality on exercise-induced gastrointestinal syndrome and associated gastrointestinal symptoms. J. Sci. Med. Sport. 2022, 25, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Lee, E.C.; Armstrong, E.M. Interactions of Gut Microbiota, Endotoxemia, Immune Function, and Diet in Exertional Heatstroke. J. Sports Med. 2018, 2018, 5724575. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Calvo, J.S.; Martínez, A.C.; García, A.C.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef]
- Miles, M.P. Probiotics in sports nutrition. Probiotics Adv. Food Health Appl. 2022, 1, 277–295. [Google Scholar]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef]
- Theodorakopoulou, M.; Perros, E.; Giamarellos-Bourboulis, E.J.; Dimopoulos, G. Controversies in the management of the critically ill: The role of probiotics. Int. J. Antimicrob. Agents 2013, 42, S41–S44. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Robles-Sánchez, C.; Abadía-Molina, F.; Morón-Calvente, V.; Sáez-Lara, M.J.; Ruiz-Bravo, A.; Jiménez-Valera, M.; Gil, Á.; Gómez-Llorente, C.; Fontana, L. Adamdec1, Ednrb and Ptgs1/Cox1, inflammation genes upregulated in the intestinal mucosa of obese rats, are downregulated by three probiotic strains. Sci. Rep. 2017, 7, 1939. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Basile, A.J.; Crawford, M.S.; Sweazea, K.L.; Carpenter, K.C. Probiotic Supplementation Has a Limited Effect on Circulating Immune and Inflammatory Markers in Healthy Adults: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2020, 120, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Calero, C.D.Q.; Rincón, E.O.; Marqueta, P.M. Probiotics, prebiotics and synbiotics: Useful for athletes and active individuals? A systematic review. Benef. Microbes 2020, 27, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.T.; Peng, Y.C.; Yen, H.Y.; Wu, J.C.; Hou, W.H. Effects of Probiotic Supplementation on Immune and Inflammatory Markers in Athletes: A Meta-Analysis of Randomized Clinical Trials. Medicina 2022, 58, 1188. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Law, M.; Stewart, C.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998; pp. 1–9. [Google Scholar]
- Axelrod, C.L.; Brennan, C.J.; Cresci, G.; Paul, D.; Hull, M.; Fealy, C.E.; Kirwan, J.P. UCC118 supplementation reduces exercise-induced gastrointestinal permeability and remodels the gut microbiome in healthy humans. Physiol. Rep. 2019, 7, e14276. [Google Scholar] [CrossRef]
- Batatinha, H.; Tavares-Silva, E.; Leite, G.S.F.; Resende, A.S.; Albuquerque, J.A.T.; Arslanian, C.; Fock, R.A.; Lancha AHJr Lira, F.S.; Krüger, K.; Thomatieli-Santos, R.; et al. Probiotic supplementation in marathonists and its impact on lymphocyte population and function after a marathon: A randomized placebo-controlled double-blind study. Sci. Rep. 2020, 10, 18777. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef]
- Tavares-Silva, E.; Caris, A.V.; Santos, S.A.; Ravacci, G.R.; Thomatieli-Santos, R.V. Effect of Multi-Strain Probiotic Supplementation on URTI Symptoms and Cytokine Production by Monocytes after a Marathon Race: A Randomized, Double-Blind, Placebo Study. Nutrients 2021, 13, 1478. [Google Scholar] [CrossRef]
- Aisberg, M.; Paixão, V.; Almeida, E.B.; Santos, J.M.B.; Foster, R.; Rossi, M.; Pithon-Curi, T.C.; Gorjão, R.; Momesso, C.M.; Andrade, M.S.; et al. Daily Intake of Fermented Milk Containing Lactobacillus casei Shirota (Lcs) Modulates Systemic and Upper Airways Immune/Inflammatory Responses in Marathon Runners. Nutrients 2019, 11, 1678. [Google Scholar] [CrossRef]
- Gill, S.K.; Allerton, D.M.; Ansley-Robson, P.; Hemmings, K.; Cox, M.; Costa, R.J.S. Does Short-Term High Dose Probiotic Supplementation Containing Lactobacillus casei Attenuate Exertional-Heat Stress Induced Endotoxaemia and Cytokinaemia? Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Hoffman, M.W.; Zelicha, H.; Gepner, Y.; Willoughby, D.S.; Feinstein, U.; Ostfeld, I. The Effect of 2 Weeks of Inactivated Probiotic Bacillus coagulans on Endocrine, Inflammatory, and Performance Responses during Self-Defense Training in Soldiers. J. Strength Cond. Res. 2019, 33, 2330–2337. [Google Scholar] [CrossRef]
- Huang, W.C.; Wei, C.C.; Huang, C.C.; Chen, W.L.; Huang, H.Y. The Beneficial Effects of Lactobacillus plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Stone, J.D.; Turner, S.M.; Anzalone, A.J.; Eimerbrink, M.J.; Pane, M.; Amoruso, A.; Rowlands, D.S.; Oliver, J.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 Supplementation Attenuates Performance and Range-of-Motion Decrements Following Muscle Damaging Exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef]
- Lamprecht, M.; Bogner, S.; Schipper, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.N.; Sparks, A.S.; Doran, D.A.; Fleming, S.C.; Langan-Evans, C.; Kirk, B.; Fearn, R.; Morton, J.P.; Close, G.L. Four weeks of probiotic supplementation reduces GI symptoms during a marathon race. Eur. J. Appl. Physiol. 2019, 119, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.N.; Wagenmakers, A.J.M.; Doran, D.A.; Fleming, S.C.; Fielding, B.A.; Morton, J.P.; Close, G.L. Probiotic supplementation increases carbohydrate metabolism in trained male cyclists: A randomized, double-blind, placebo-controlled crossover trial. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E504–E513. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.; Tamir, S.; Golan, R.; Weinstein, A.; Weinstein, Y. The effect of probiotic supplementation on performance, inflammatory markers and gastro-intestinal symptoms in elite road cyclists. J. Int. Soc. Sports Nutr. 2021, 18, 36. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sport Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Pugh, J.; O’Sullivan, O.; Black, K.; Townsend, J.R.; Pyne, D.B.; Wardenaar, F.C.; West, N.P.; Whisner, C.M.; McFarland, L.V. Best Practices for Probiotic Research in Athletic and Physically Active Populations: Guidance for Future Randomized Controlled Trials. Front. Nutr. 2022, 9, 809983. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory Effects of Probiotics During Pathogenic Infections with Emphasis on Immune Regulation. Front. Immunol. 2021, 12, 571. [Google Scholar] [CrossRef]
- Shehzad, A.; Rabail, R.; Munir, S.; Jan, H.; Fernández-Lázaro, D.; Aadil, R.M. Impact of Oats on Appetite Hormones and Body Weight Management: A Review. Curr. Nutr. Rep. 2023. online ahead of print. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).