Novel Proton-Conducting Layered Perovskites Based on BaLa2In2O7 Produced by Cationic Co-Doping
Abstract
:1. Introduction
2. Experimental Procedure
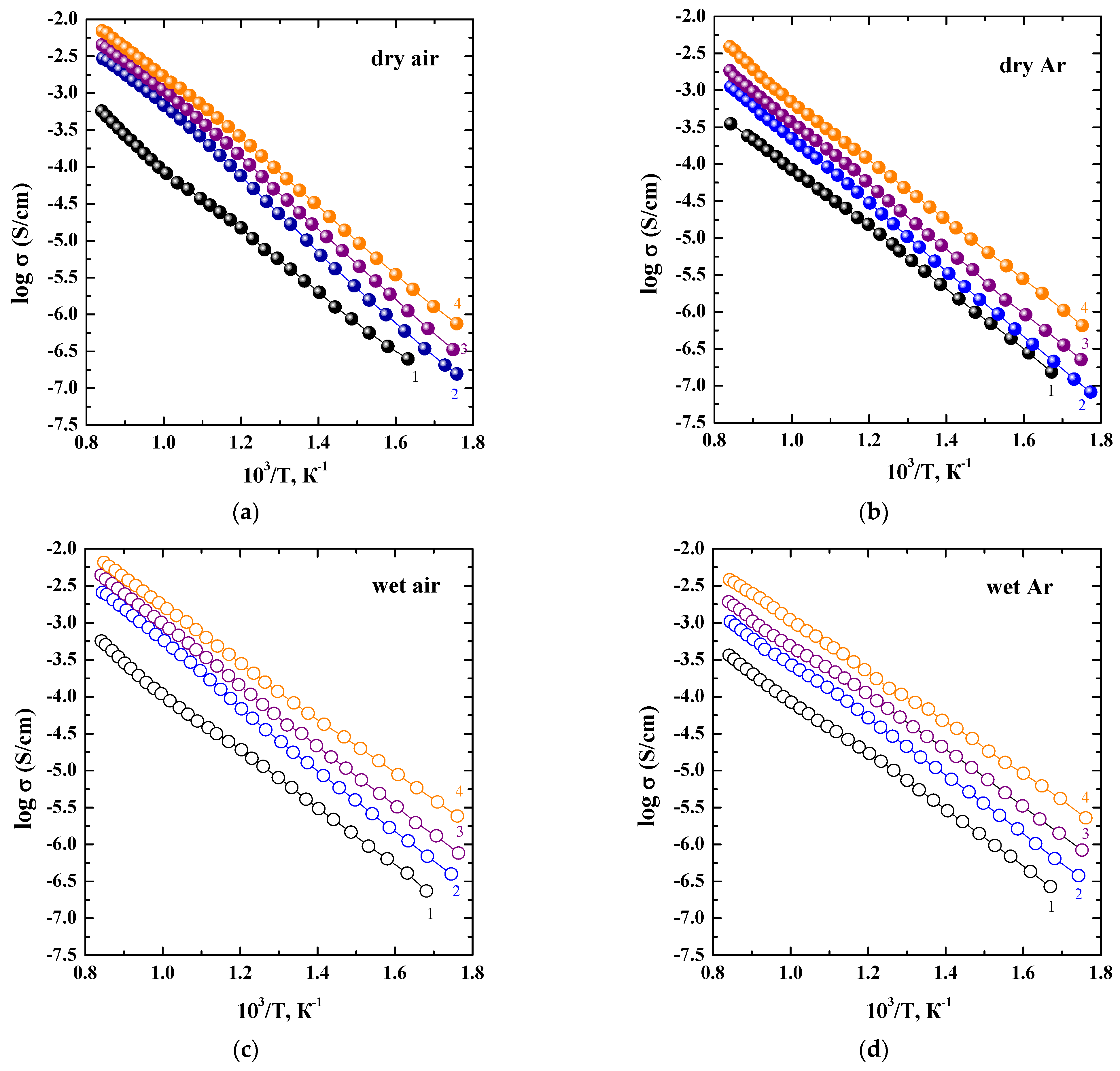
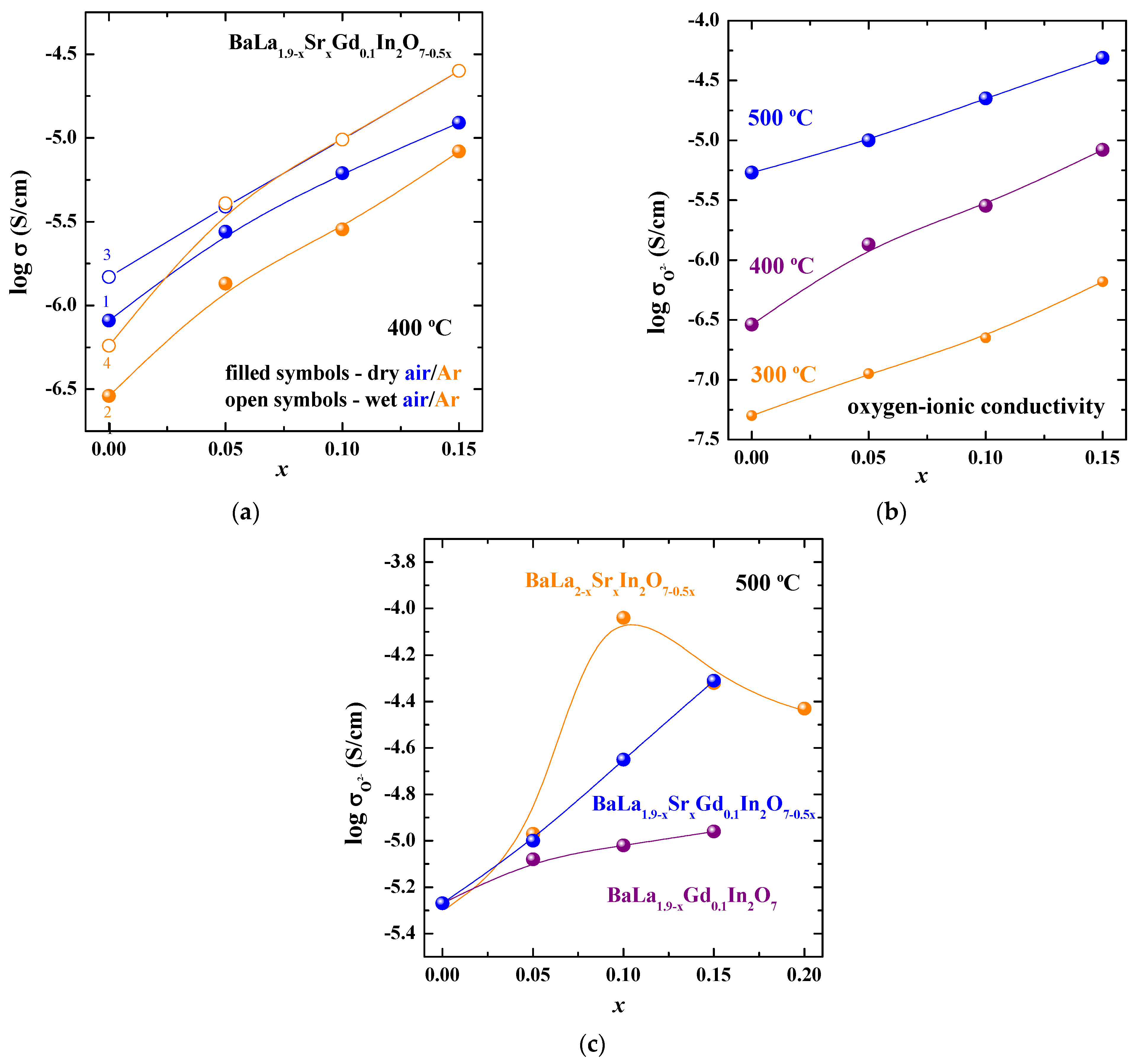
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Hu, Y.H. Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Nayak, A.P.; Sasmal, A. Recent advance on fundamental properties and synthesis of barium zirconate for proton conducting ceramic fuel cell. J. Clean. Prod. 2023, 386, 135827. [Google Scholar] [CrossRef]
- Guo, R.; He, T. High-entropy perovskite electrolyte for protonic ceramic fuel cells operating below 600 °C. ACS Mater. Lett. 2022, 4, 1646–1652. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Zhao, S.; Xia, L.; Zhu, M.; Han, M.; Ni, M. Modelling of an integrated protonic ceramic electrolyzer cell (PCEC) for methanol synthesis. J. Power Sources 2023, 559, 232667. [Google Scholar] [CrossRef]
- Liu, F.; Ding, D.; Duan, C. Protonic ceramic electrochemical cells for synthesizing sustainable chemicals and fuels. Adv. Sci. 2023, 2023, 2206478. [Google Scholar] [CrossRef]
- Kim, D.; Bae, K.T.; Kim, K.J.; Im, H.-N.; Jang, S.; Oh, S.; Lee, S.W.; Shin, T.H.; Lee, K.T. High-performance protonic ceramic electrochemical cells. ACS Energy Lett. 2022, 7, 2393–2400. [Google Scholar] [CrossRef]
- Tian, H.; Luo, Z.; Song, Y.; Zhou, Y.; Gong, M.; Li, W.; Shao, Z.; Liu, M.; Liu, X. Protonic ceramic materials for clean and sustainable energy: Advantages and challenges. Int. Mater. Rev. 2022, 2022, 1–29. [Google Scholar] [CrossRef]
- Ji, H.I.; Lee, J.H.; Son, J.W.; Yoon, K.J.; Yang, S.; Kim, B.K. Protonic ceramic electrolysis cells for fuel production: A brief review. J. Korean Ceram. Soc. 2020, 57, 480–494. [Google Scholar] [CrossRef]
- Corigliano, O.; Pagnotta, L.; Fragiacomo, P. On the technology of solid oxide fuel cell (SOFC) energy systems for stationary power generation: A review. Sustainability 2022, 14, 15276. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.; Yan, J.; Liu, Y.; Jiang, H.; Zhang, H.; Tang, J.; Liu, Q. Research progresses on the application of perovskite in adsorption and photocatalytic removal of water pollutants. J. Hazard. Mater. 2023, 442, 130024. [Google Scholar] [CrossRef]
- Tarasova, N. Layered perovskites BaLnnInnO3n+1 (n = 1, 2) for electrochemical applications: A mini review. Membranes 2023, 13, 34. [Google Scholar] [CrossRef]
- Zvonareva, I.A.; Medvedev, D.A. Proton-conducting barium stannate for high-temperature purposes: A brief review. J. Eur. Ceram. Soc. 2023, 43, 198–207. [Google Scholar] [CrossRef]
- Aminudin, M.A.; Kamarudin, S.K.; Lim, B.H.; Majilan, E.H.; Masdar, M.S.; Shaari, N. An overview: Current progress on hydrogen fuel cell vehicles. Int. J. Hydrog. Energy 2023, 48, 4371–4388. [Google Scholar] [CrossRef]
- Liu, F.; Fang, L.; Diercks, D.; Kazempoor, P.; Duan, C. Rationally designed negative electrode for selective CO2-to-CO conversion in protonic ceramic electrochemical cells. Nano Energy 2022, 102, 107722. [Google Scholar] [CrossRef]
- Liu, F.; Duan, C. Direct-hydrocarbon proton-conducting solid oxide fuel cells. Sustainability 2021, 13, 4736. [Google Scholar] [CrossRef]
- Qiao, Z.; Li, S.; Li, Y.; Xu, N.; Xiang, K. Structure, mechanical properties, and thermal conductivity of BaZrO3 doped at the A-B site. Ceram. Int. 2022, 48, 12529–12536. [Google Scholar] [CrossRef]
- Guo, R.; Li, D.; Guan, R.; Kong, D.; Cui, Z.; Zhou, Z.; He, T. Sn–Dy–Cu triply doped BaZr0.1Ce0.7Y0.2O3−δ: A chemically stable and highly proton-conductive electrolyte for low-temperature solid oxide fuel cells. ACS Sustain. Chem. Eng. 2022, 10, 5352–5362. [Google Scholar] [CrossRef]
- Gu, Y.; Luo, G.; Chen, Z.; Huo, Y.; Wu, F. Enhanced chemical stability and electrochemical performance of BaCe0.8Y0.1Ni0.04Sm0.06O3-δ perovskite electrolytes as proton conductors. Ceram. Int. 2022, 48, 10650–10658. [Google Scholar] [CrossRef]
- Medvedev, D.A. Current drawbacks of proton-conducting ceramic materials: How to overcome them for real electrochemical purposes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100549. [Google Scholar] [CrossRef]
- Kasyanova, A.N.; Zvonareva, I.A.; Tarasova, N.A.; Bi, L.; Medvedev, D.A.; Shao, Z. Electrolyte materials for protonic ceramic electrochemical cells: Main limitations and potential solutions. Mater. Rep. Energy 2022, 2, 100158. [Google Scholar] [CrossRef]
- Lybye, D.; Poulsen, F.W.; Mogensen, M. Conductivity of A- and B-site doped LaAlO3, LaGaO3, LaScO3 and LaInO3 perovskites. Solid State Ion. 2000, 128, 91–103. [Google Scholar] [CrossRef]
- Gorelov, V.P.; Stroeva, A.Y. Solid proton conducting electrolytes based on LaScO3. Russ. J. Electrochem. 2012, 48, 949–960. [Google Scholar] [CrossRef]
- Kuzmin, A.V.; Lesnichyova, A.S.; Tropin, E.S.; Stroeva, A.Y.; Vorotnikov, V.A.; Solodyankina, D.M.; Belyakov, S.A.; Plekhanov, M.S.; Farlenkov, A.S.; Osinkin, D.A.; et al. LaScO3-based electrolyte for protonic ceramic fuel cells: Influence of sintering additives on the transport properties and electrochemical performance. J. Power Sources 2020, 466, 228255. [Google Scholar] [CrossRef]
- Kuzmin, A.V.; Stroeva, A.Y.; Plekhanov, M.S.; Gorelov, V.P.; Farlenkov, A.S. Chemical solution deposition and characterization of the La1-xSrxScO3-α thin films on La1-xSrxMnO3-α substrate. Int. J. Hydrog. Energy 2018, 43, 19206–19212. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, T.; Naito, H.; Yugami, H. Electrical conductivity of Al-doped La1−xSrxScO3 perovskite-type oxides as electrolyte materials for low-temperature SOFC. Solid State Ion. 2003, 159, 217–222. [Google Scholar] [CrossRef]
- Lybye, D.; Bonanos, N. Proton and oxide ion conductivity of doped LaScO3. Solid State Ion. 1999, 125, 339–344. [Google Scholar] [CrossRef]
- Stroeva, A.Y.; Gorelov, V.P.; Balakireva, V.B. Conductivity of La1−xSrxSc1−yMgyO3−α (x = y = 0.01–0.20) in reducing atmosphere. Russ. J. Electrochem. 2010, 46, 784–788. [Google Scholar] [CrossRef]
- Ito, N.; Matsumoto, H.; Kawasaki, Y.; Okada, S.; Ishihara, T. The effect of Zn addition to La1−xSrxScO3-δ systems as a B-site dopant. Chem. Lett. 2009, 38, 582–583. [Google Scholar] [CrossRef]
- Yugami, H.; Kato, H.; Iguchi, F. Protonic SOFCs using perovskite-type conductors. Adv. Sci. Technol. 2014, 95, 66–71. [Google Scholar] [CrossRef]
- Kato, H.; Iguchi, F.; Yugami, H. Compatibility and performance of La0.675Sr0.325Sc0.99Al0.01O3 perovskite-type oxide as an electrolyte material for SOFCs. Electrochemistry 2014, 82, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Ruddlesden, S.N.; Popper, P. The compound Sr3Ti2O7 and its structure. Acta Cryst. 1958, 11, 54–55. [Google Scholar] [CrossRef] [Green Version]
- Fujii, K.; Esaki, Y.; Omoto, K.; Yashima, M.; Hoshikawa, A.; Ishigaki, T.; Hester, J.R. New perovskite-related structure family of oxide-ion conducting materials NdBaInO4. Chem. Mater. 2014, 26, 2488–2491. [Google Scholar] [CrossRef]
- Fujii, K.; Shiraiwa, M.; Esaki, Y.; Yashima, M.; Kim, S.J.; Lee, S. Improved oxide-ion conductivity of NdBaInO4 by Sr doping. J. Mater. Chem. A 2015, 3, 11985. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, T.; Yan, Y.; Sakai, T.; Ida, S. Oxide ion conductivity in doped NdBaInO4. Solid State Ion. 2016, 288, 262. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Lu, F.; Xu, J.; Kuang, X. Acceptor doping and oxygen vacancy migration in layered perovskite NdBaInO4-based mixed conductors. J. Phys. Chem. C 2016, 120, 6416–6426. [Google Scholar] [CrossRef]
- Fijii, K.; Yashima, M. Discovery and development of BaNdInO4—A brief review. J. Ceram. Soc. Jpn. 2018, 126, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Troncoso, L.; Alonso, J.A.; Aguadero, A. Low activation energies for interstitial oxygen conduction in the layered perovskites La1+xSr1-xInO4+d. J. Mater. Chem. A 2015, 3, 17797–17803. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Fernández-Díaz, M.T.; Aguadero, A. Introduction of interstitial oxygen atoms in the layered perovskite LaSrIn1-xBxO4+δ system (B=Zr, Ti). Solid State Ion. 2015, 282, 82–87. [Google Scholar] [CrossRef]
- Troncoso, L.; Mariño, C.; Arce, M.D.; Alonso, J.A. Dual oxygen defects in layered La1.2Sr0.8-xBaxInO4+d (x = 0.2, 0.3) oxide-ion conductors: A neutron diffraction study. Materials 2019, 12, 1624. [Google Scholar] [CrossRef] [Green Version]
- Tarasova, N.; Animitsa, I.; Galisheva, A.; Korona, D. Incorporation and conduction of protons in Ca, Sr, Ba-doped BaLaInO4 with Ruddlesden-Popper structure. Materials 2019, 12, 1668. [Google Scholar] [CrossRef] [Green Version]
- Troncoso, L.; Arce, M.D.; Fernández-Díaz, M.T.; Mogni, L.V.; Alonso, J.A. Water insertion and combined interstitial-vacancy oxygen conduction in the layered perovskites La1.2Sr0.8-xBaxInO4+δ. New J. Chem. 2019, 43, 6087–6094. [Google Scholar] [CrossRef]
- Zhou, Y.; Shiraiwa, M.; Nagao, M.; Fujii, K.; Tanaka, I.; Yashima, M.; Baque, L.; Basbus, J.F.; Mogni, L.V.; Skinner, S.J. Protonic conduction in the BaNdInO4 structure achieved by acceptor doping. Chem. Mater. 2021, 33, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, M.; Kido, T.; Fujii, K.; Yashima, M. High-temperature proton conductors based on the (110) layered perovskite BaNdScO4. J. Mat. Chem. A 2021, 9, 8607. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Materials AIILnInO4 with Ruddlesden-Popper structure for electrochemical applications: Relationship between ion (oxygen-ion, proton) conductivity, water uptake and structural changes. Materials 2022, 15, 114. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A.; Medvedev, D. Layered and hexagonal perovskites as novel classes of proton-conducting solid electrolytes: A focus review. Electrochem. Mater. Technol. 2022, 1, 20221004. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I.; Belova, K.; Abakumova, E.; Cheremisina, P.; Medvedev, D. Oxygen ion and proton transport in alkali-earth doped layered perovskites based on BaLa2In2O7. Inorganics 2022, 10, 161. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I.; Verinkina, E. Synthesis, hydration processes and ionic conductivity of novel gadolinium-doped ceramic materials based on layered perovskite BaLa2In2O7 for electrochemical purposes. Processes 2022, 10, 2536. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I.; Abakumova, E. Cation and oxyanion doping of layered perovskite BaNd2In2O7: Oxygen-ion and proton transport. Int. J. Hydrog. Energy 2022, in press. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I.; Abakumova, E.; Gnatyuk, V.; Zvonareva, I. Novel protonic conductor SrLa2Sc2O7 with layered structure for electrochemical devices. Materials 2022, 15, 8867. [Google Scholar] [CrossRef]
- Caldes, M.; Michel, C.; Rouillon, T.; Hervieu, M.; Raveau, B. Novel indates Ln2BaIn2O7, n = 2 members of the Ruddlesden–Popper family (Ln = La, Nd). J. Mater. Chem. 2002, 12, 473–476. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
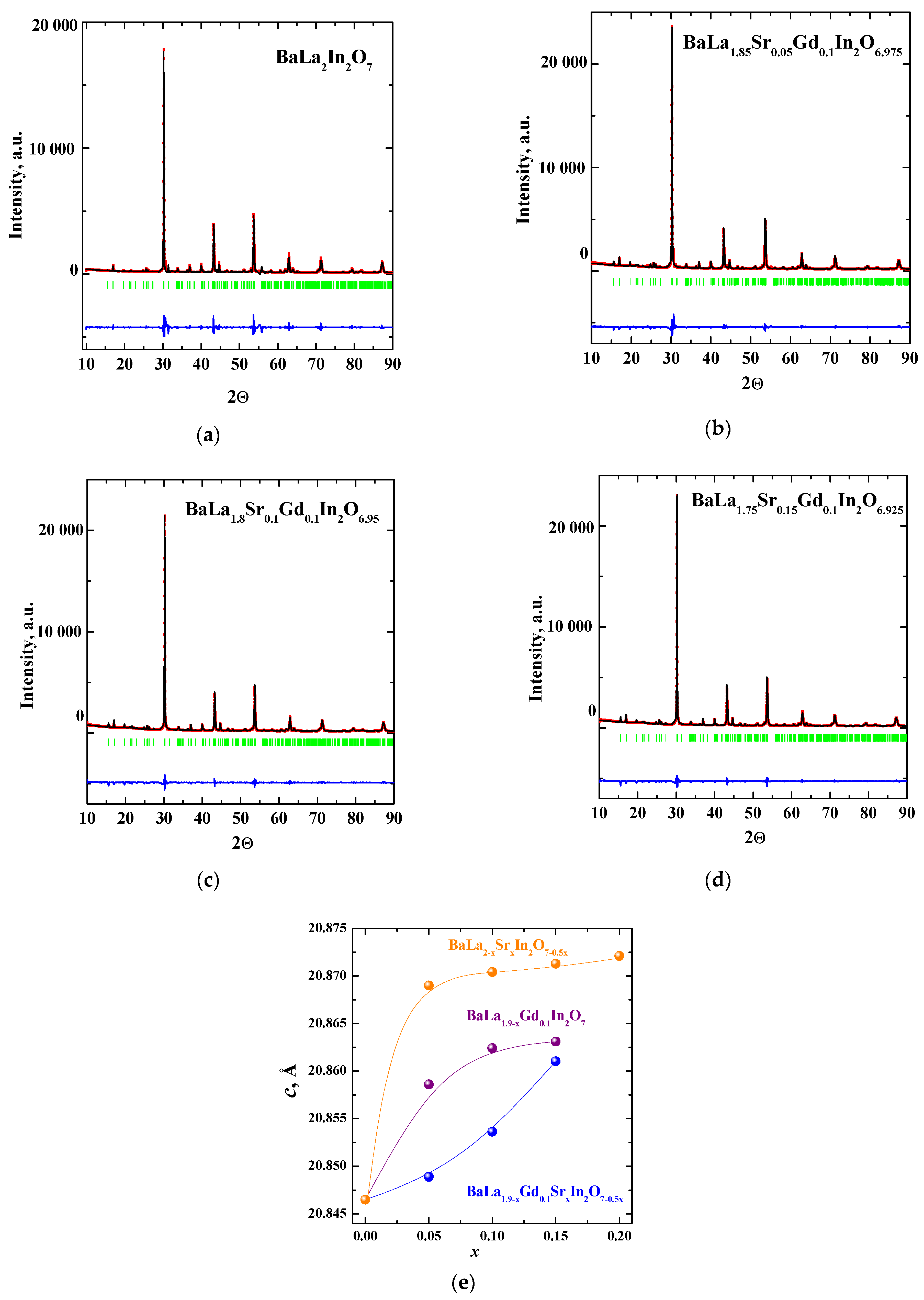
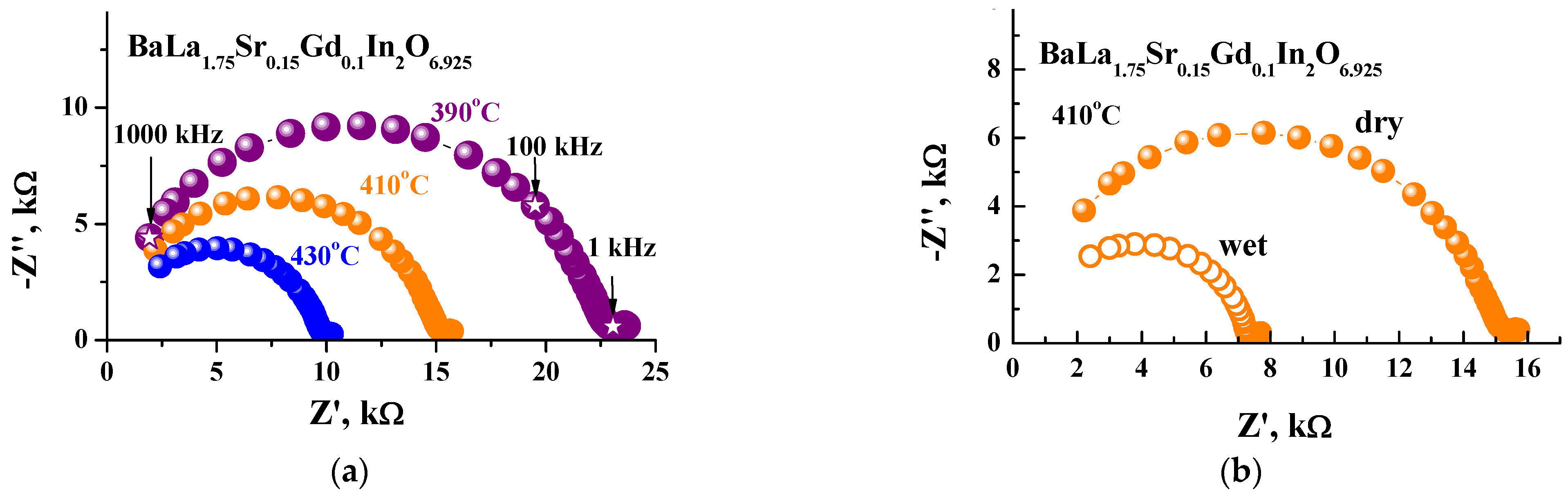

| Sample | a, b (Å) | c (Å) | Vcell (Å3) | Water Uptake (mol) |
|---|---|---|---|---|
| 0 | 5.914(9) | 20.846(5) | 729.3365 | 0.17 |
| 0.05 | 5.918(5) | 20.848(9) | 730.3086 | 0.16 |
| 0.10 | 5.919(2) | 20.853(6) | 730.6461 | 0.17 |
| 0.15 | 5.920(3) | 20.861(0) | 731.1770 | 0.18 |
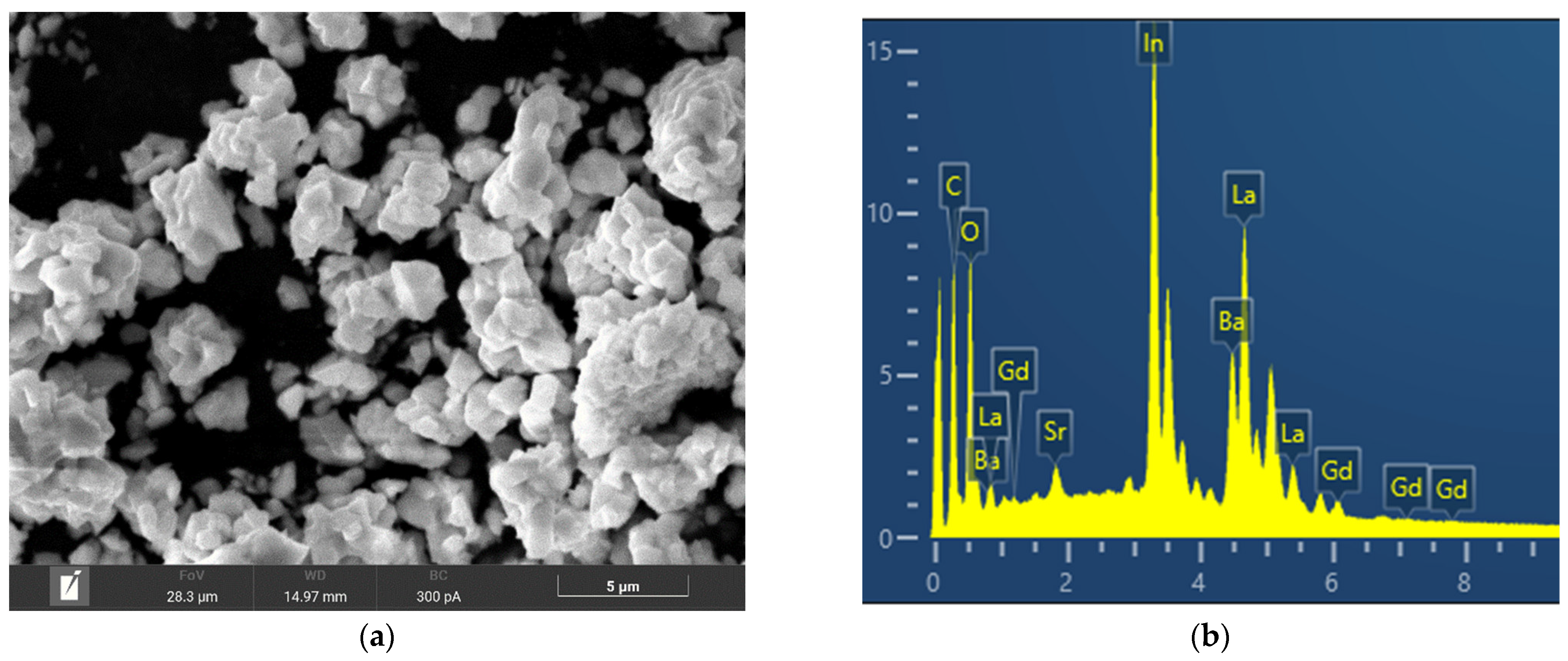
| Composition | Content of Element, Atomic % | ||||
|---|---|---|---|---|---|
| Ba | La | In | Sr | Gd | |
| 1 | 20.2 (20.0) | 40.1 (40.0) | 39.7 (40.0) | - | - |
| 2 | 20.1 (20.0) | 37.3 (37.0) | 39.8 (40.0) | 0.9 (1.0) | 1.9 (2.0) |
| 3 | 20.3 (20.0) | 36.0 (36.0) | 39.8 (40.0) | 1.9 (2.0) | 2.0 (2.0) |
| 4 | 20.2 (20.0) | 35.3 (35.0) | 39.7 (40.0) | 2.8 (3.0) | 2.0 (2.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasova, N.; Bedarkova, A.; Animitsa, I.; Abakumova, E.; Trofimov, A.; Verinkina, E. Novel Proton-Conducting Layered Perovskites Based on BaLa2In2O7 Produced by Cationic Co-Doping. Appl. Sci. 2023, 13, 3449. https://doi.org/10.3390/app13063449
Tarasova N, Bedarkova A, Animitsa I, Abakumova E, Trofimov A, Verinkina E. Novel Proton-Conducting Layered Perovskites Based on BaLa2In2O7 Produced by Cationic Co-Doping. Applied Sciences. 2023; 13(6):3449. https://doi.org/10.3390/app13063449
Chicago/Turabian StyleTarasova, Nataliia, Anzhelika Bedarkova, Irina Animitsa, Ekaterina Abakumova, Alexey Trofimov, and Evgeniya Verinkina. 2023. "Novel Proton-Conducting Layered Perovskites Based on BaLa2In2O7 Produced by Cationic Co-Doping" Applied Sciences 13, no. 6: 3449. https://doi.org/10.3390/app13063449





