The Differences in Transient Characteristics of Postural Control between Young and Older Adults across Four Different Postural Tasks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Procedures
2.3. Data Acquisition and Analysis
2.4. Statistical Analysis
3. Results
3.1. Correlations
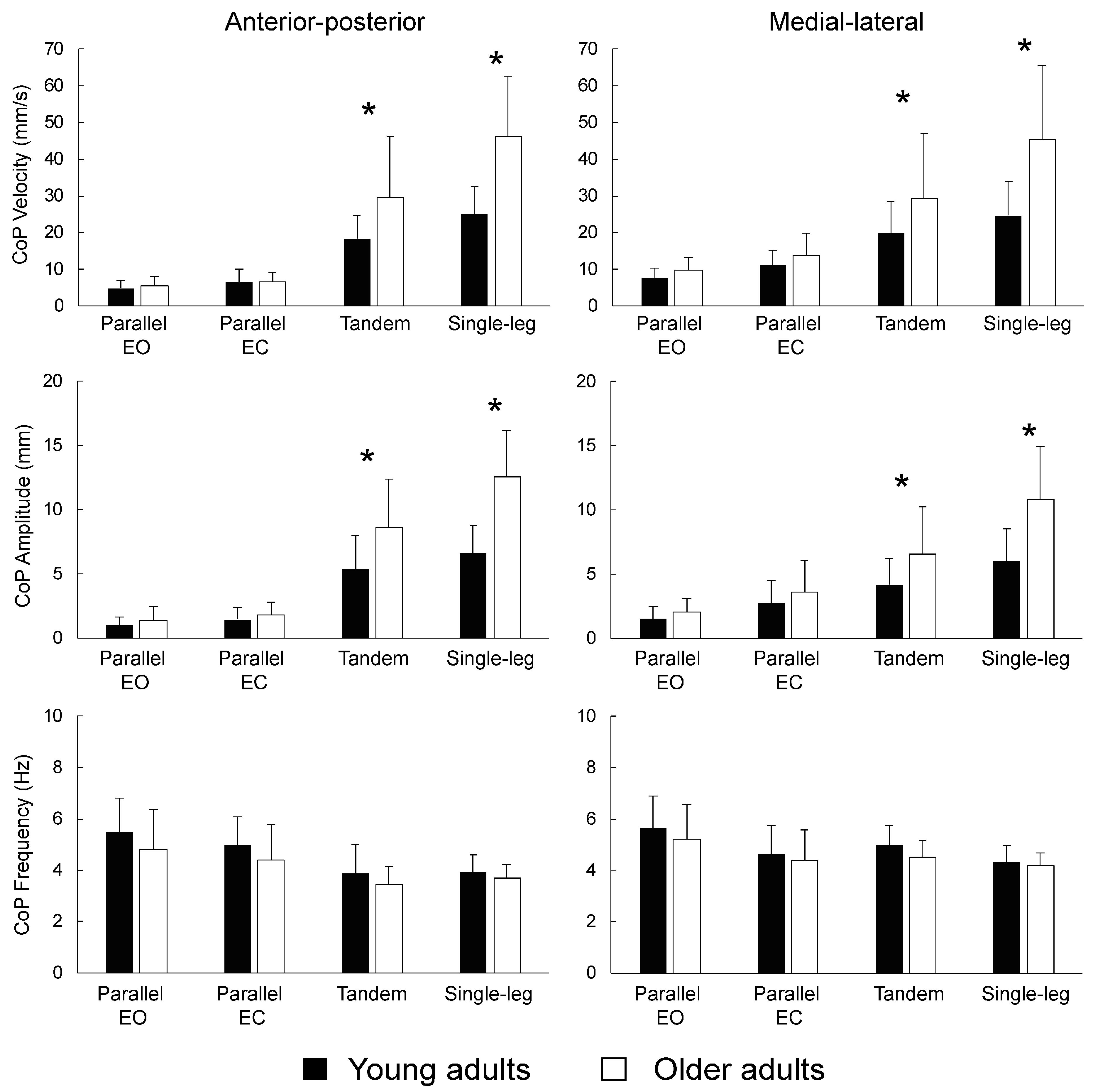
3.2. Difference between Young and Older Adults in Traditional Whole-Trial Estimates
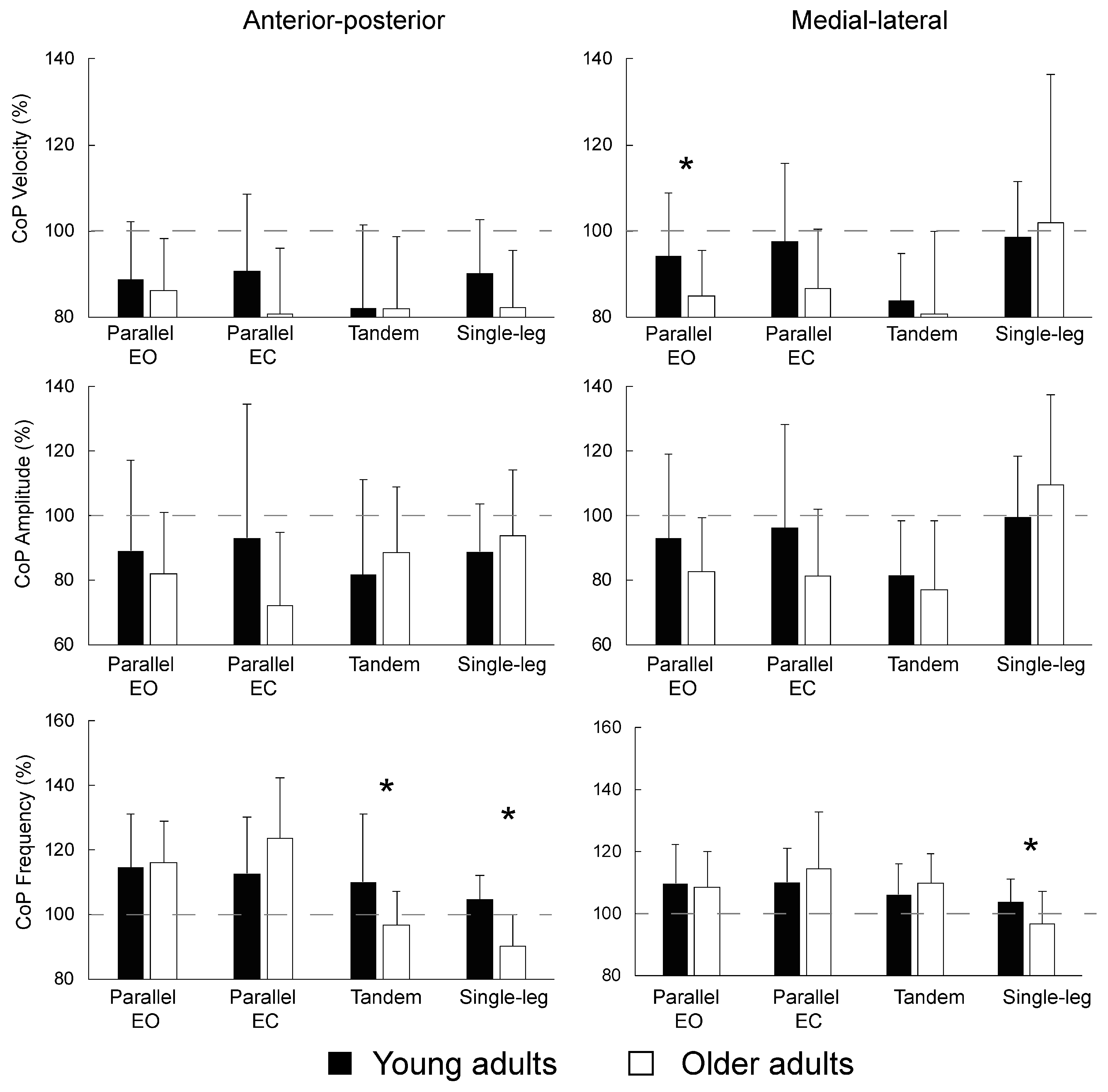
3.3. Difference between Young and Older Adults in Transient Characteristics of Postural Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakagawa, H.B.; Ferraresi, J.R.; Prata, M.G.; Scheicher, M. Postural Balance and Functional Independence of Elderly People According to Gender and Age: Cross-Sectional Study. Sao Paulo Med. J. 2017, 135, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Osoba, M.; Rao, A.; Agrawal, S.K.; Lalwani, A. Balance and Gait in the Elderly: A Contemporary Review. Laryngoscope Investig. Otolaryngol. 2019, 4, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Kozinc, Ž.; Löfler, S.; Hofer, C.; Carraro, U.; Šarabon, N. Diagnostic Balance Tests for Assessing Risk of Falls and Distinguishing Older Adult Fallers and Non-Fallers: A Systematic Review with Meta-Analysis. Diagnostics 2020, 10, 667. [Google Scholar] [CrossRef]
- Miyashita, T.L.; Cote, C.; Terrone, D.; Diakogeorgiou, E. Detecting Changes in Postural Sway. J. Biomech. 2020, 107, 109868. [Google Scholar] [CrossRef] [PubMed]
- McGough, E.L.; Hsu, L.Y.; Thompson, H.J.; Teri, L. Concurrent Validity of Postural Sway Measures in Older Adults With Cognitive Impairment. Phys. Occup. Ther. Geriatr. 2018, 36, 399–410. [Google Scholar] [CrossRef]
- Carpenter, M.G.; Frank, J.S.; Winter, D.A.; Peysar, G.W. Sampling Duration Effects on Centre of Pressure Summary Measures. Gait Posture 2001, 13, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.A.; Chaudhari, A.M.W.; Worthen-Chaudhari, L.C.; Bigelow, K.E.; Monfort, S.M. A New Perspective on Transient Characteristics of Quiet Stance Postural Control. PLoS ONE 2020, 15, e0237246. [Google Scholar] [CrossRef] [PubMed]
- Peterka, R.J. Sensorimotor Integration in Human Postural Control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef] [Green Version]
- Maurer, C.; Mergner, T.; Peterka, R.J. Multisensory Control of Human Upright Stance. Exp. Brain Res. 2006, 171, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Assländer, L.; Peterka, R.J. Sensory Reweighting Dynamics in Human Postural Control. J. Neurophysiol. 2014, 111, 1852–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozinc, Ž.; Šarabon, N. Transient Body Sway Characteristics during Single-Leg Quiet Stance in Ballet Dancers and Young Adults. J. Biomech. 2021, 115, 110195. [Google Scholar] [CrossRef]
- Carroll, J.P.; Freedman, W. Nonstationary Properties of Postural Sway. J. Biomech. 1993, 26, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Cooper, S.A.; Doan, J.B.; Clark Dickin, D.; Whishaw, I.Q.; Pellis, S.M.; Suchowersky, O. Parkinsonian Deficits in Sensory Integration for Postural Control: Temporal Response to Changes in Visual Input. Park. Relat. Disord. 2006, 12, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Kozinc, Ž.; Trajković, N.; Šarabon, N. Transient Characteristics of Body Sway during Single-Leg Stance in Athletes with a History of Ankle Sprain. Gait Posture 2021, 86, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kozinc, Ž.; Šarabon, N. The Effects of Leg Preference on Transient Characteristics of Body Sway During Single-Leg Stance: A Cross-Sectional Study. Front. Hum. Neurosci. 2021, 14, 617222. [Google Scholar] [CrossRef]
- Parreira, R.B.; Boer, M.C.; Rabello, L.; Costa, V.D.S.P.; De Oliveira, E.; Da Silva, R.A. Age-Related Differences in Center of Pressure Measures during One-Leg Stance Are Time Dependent. J. Appl. Biomech. 2013, 29, 312–316. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, E.; Seiger, Å.; Hirschfeld, H. One-Leg Stance in Healthy Young and Elderly Adults: A Measure of Postural Steadiness? Clin. Biomech. 2004, 19, 688–694. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring Balance in the Elderly: Validation of an Instrument. Can. J. Public Health 1992, 83, S7–S11. [Google Scholar]
- Schober, P.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Van der Kooij, H.; Campbell, A.D.; Carpenter, M.G. Sampling Duration Effects on Centre of Pressure Descriptive Measures. Gait Posture 2011, 34, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Le Clair, K.; Riach, C. Postural Stability Measures: What to Measure and for How Long. Clin. Biomech. 1996, 11, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Colledge, N.R.; Cantley, P.; Peaston, I.; Brash, H.; Lewis, S.; Wilson, J.A. Ageing and Balance: The Measurement of Spontaneous Sway by Posturography. Gerontology 1994, 40, 273–278. [Google Scholar] [CrossRef]
- Hsiao, D.; Belur, P.; Myers, P.S.; Earhart, G.M.; Rawson, K.S. The Impact of Age, Surface Characteristics, and Dual-Tasking on Postural Sway. Arch. Gerontol. Geriatr. 2020, 87, 103973. [Google Scholar] [CrossRef]
- Horak, F.B. Postural Orientation and Equilibrium: What Do We Need to Know about Neural Control of Balance to Prevent Falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef] [Green Version]
- Lui, K.Y.; Hewston, P.; Deshpande, N. Visual–Vestibular Interaction for Postural Control during Sit-to-Stand: Effects of Aging. Motor Control 2019, 23, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Barela, A.M.F.; Caporicci, S.; de Freitas, P.B.; Jeka, J.J.; Barela, J.A. Light Touch Compensates Peripheral Somatosensory Degradation in Postural Control of Older Adults. Hum. Mov. Sci. 2018, 60, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Reed, C.A.; DuBois, C.K.; Hutchison, K.A.; Huppert, T.J.; Monfort, S.M. Influence of Serial Subtraction Tasks on Transient Characteristics of Postural Control. Hum. Mov. Sci. 2022, 83, 102950. [Google Scholar] [CrossRef] [PubMed]
- Boisgontier, M.P.; Beets, I.A.M.; Duysens, J.; Nieuwboer, A.; Krampe, R.T.; Swinnen, S.P. Age-Related Differences in Attentional Cost Associated with Postural Dual Tasks: Increased Recruitment of Generic Cognitive Resources in Older Adults. Neurosci. Biobehav. Rev. 2013, 37, 1824–1837. [Google Scholar] [CrossRef] [PubMed]
- Schoene, D.; Valenzuela, T.; Lord, S.R.; De Bruin, E.D. The Effect of Interactive Cognitive-Motor Training in Reducing Fall Risk in Older People: A Systematic Review. BMC Geriatr. 2014, 14, 107. [Google Scholar] [CrossRef] [Green Version]
- Bernard-Demanze, L.; Lacour, M. The Fall in Older Adults: Physical and Cognitive Problems. Curr. Aging Sci. 2017, 10, 185–200. [Google Scholar]
- Feller, K.J.; Peterka, R.J.; Horak, F.B. Sensory Re-Weighting for Postural Control in Parkinson’s Disease. Front. Hum. Neurosci. 2019, 13, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Group | N | Age (Years) | Body Mass (kg) | Body Height (m) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Younger males | 10 | 23.4 | 3.2 | 76.4 | 8.2 | 1.82 | 0.04 |
| Younger females | 10 | 24.1 | 2.5 | 58.6 | 5.5 | 1.71 | 0.06 |
| Older males | 5 | 70.2 | 3.3 | 85.2 | 8.2 | 1.77 | 0.09 |
| Older females | 10 | 74.5 | 6.6 | 64.5 | 9.1 | 1.59 | 0.06 |
| Task | Descriptions of the Tasks and the Transition to the Task Positions |
|---|---|
| Parallel EO | Participants assumed the parallel stance position on the force plate and closed their eyes. The eyes were opened (as instructed by the examiner) after 15 s, simultaneously with the start of the data recording. The arms were hanging loosely at the sides. |
| Parallel EC | Participants assumed the parallel stance position on the force plate. After that, they closed their eyes (as instructed by the examiner) simultaneously with the start of the data recording. The arms were hanging loosely at the sides. |
| Tandem | Participants stood with their feet parallel, at the posterior edge of the force plate. When ready, they transitioned the dominant leg forward and assumed the tandem stance. The data recording started manually 1 s after they assumed the tandem position. The hands were placed on the hips. |
| Single-leg | Participants stood with their feet parallel, at the middle of the force plate. When ready, they transitioned the weight to their dominant leg, assuming a single-leg stance. The data recording started ~1 s after they assumed this position. The hip of the free leg was at 0°, and the thigh was parallel to the standing leg, while the knee was flexed at ~90°. The hands were placed on the hips. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozinc, Ž.; Marjanov, N.; Šarabon, N. The Differences in Transient Characteristics of Postural Control between Young and Older Adults across Four Different Postural Tasks. Appl. Sci. 2023, 13, 3485. https://doi.org/10.3390/app13063485
Kozinc Ž, Marjanov N, Šarabon N. The Differences in Transient Characteristics of Postural Control between Young and Older Adults across Four Different Postural Tasks. Applied Sciences. 2023; 13(6):3485. https://doi.org/10.3390/app13063485
Chicago/Turabian StyleKozinc, Žiga, Nika Marjanov, and Nejc Šarabon. 2023. "The Differences in Transient Characteristics of Postural Control between Young and Older Adults across Four Different Postural Tasks" Applied Sciences 13, no. 6: 3485. https://doi.org/10.3390/app13063485





