Fouling of Polyalkylmethylsiloxane Composite Membranes during Pervaporation Separation of ABE-Fermentation Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
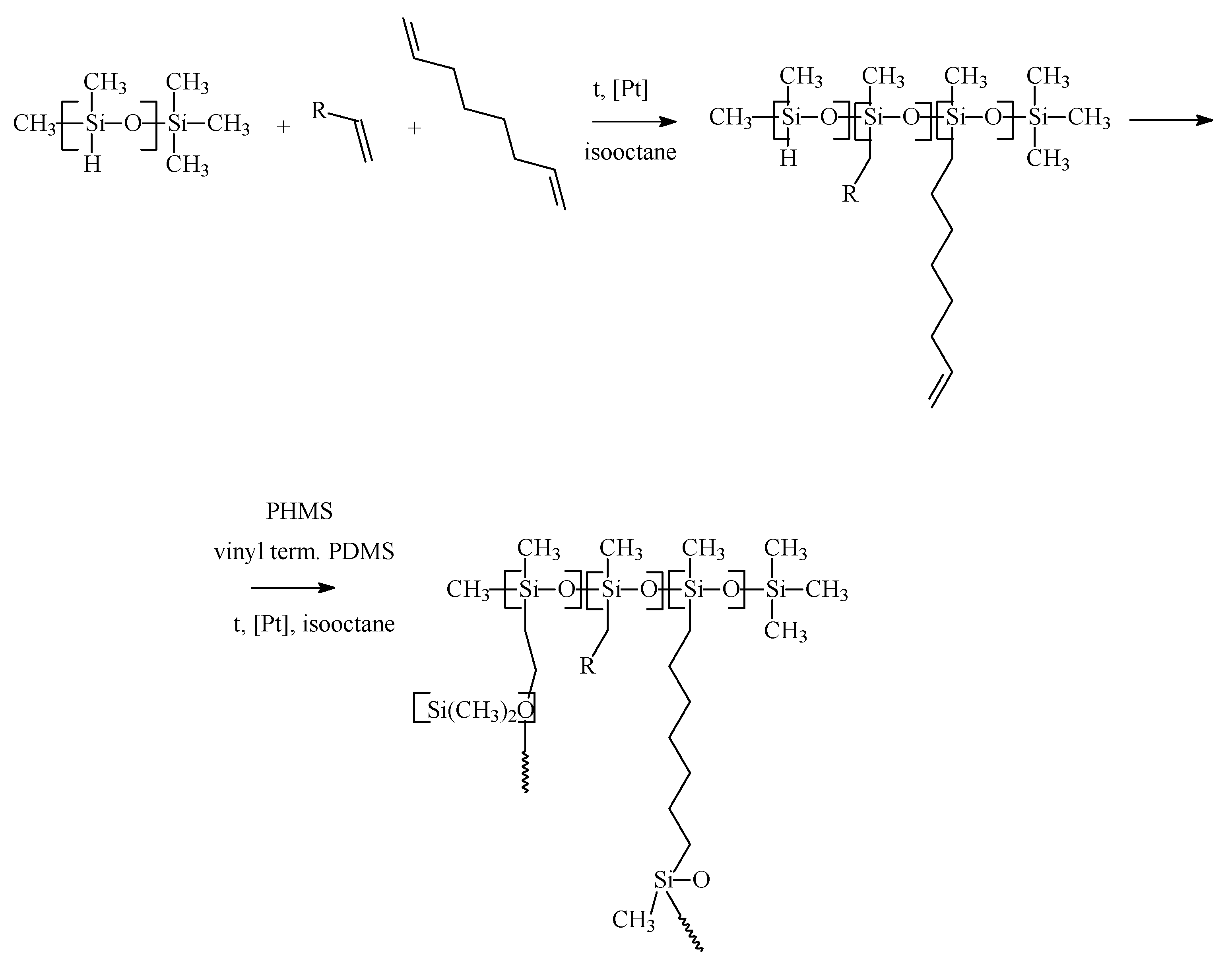
2.2. Membrane Materials Synthesis
2.3. Development Flat Sheet Composite Membranes
2.4. Prolonged Exposure of Composite Membranes in Fermentation Broth
2.5. Scanning Electron Microscopy and Energy Dispersive X-ray Spectral Analysis (EDX)
2.6. Contact Angle Measurement
2.7. Infrared Spectroscopy
2.8. Vacuum Pervaporation
3. Results
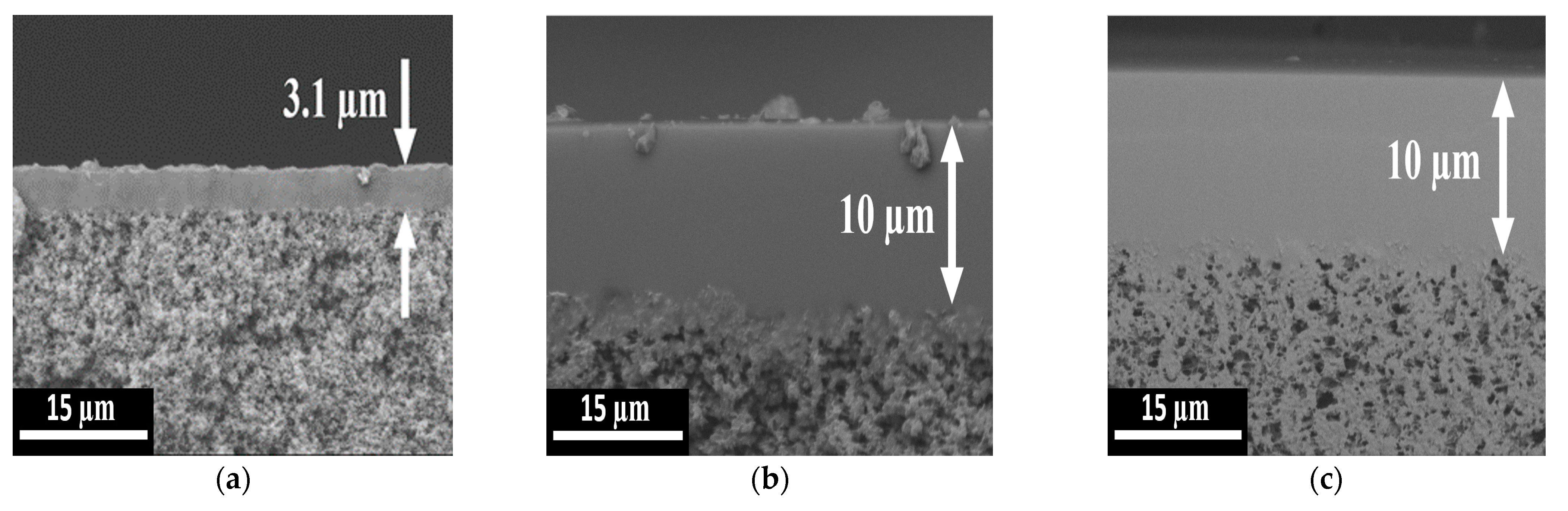
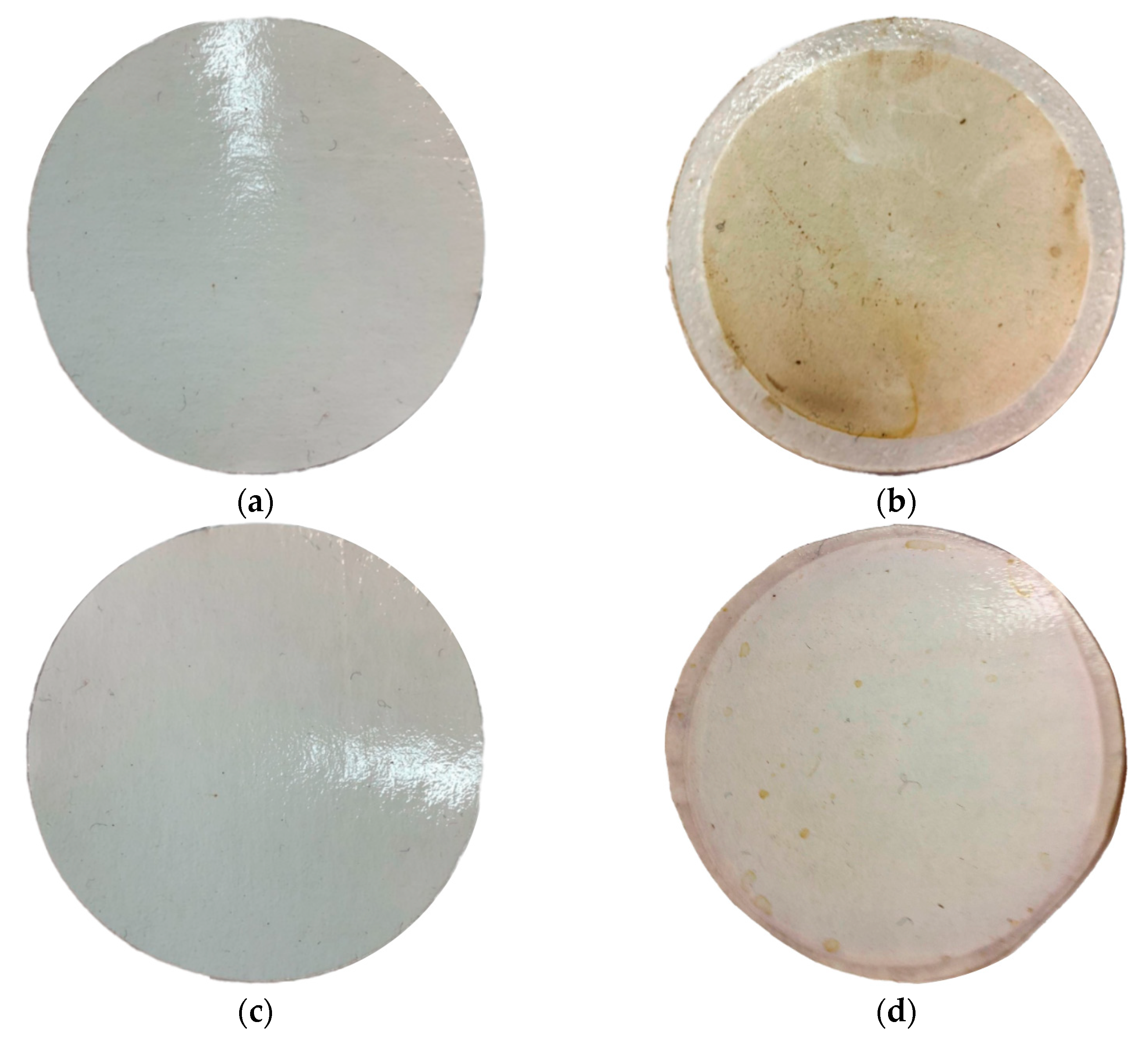
3.1. Composite Membrane Characterisation: Before and after Exposure to ABE-Fermentation Broth
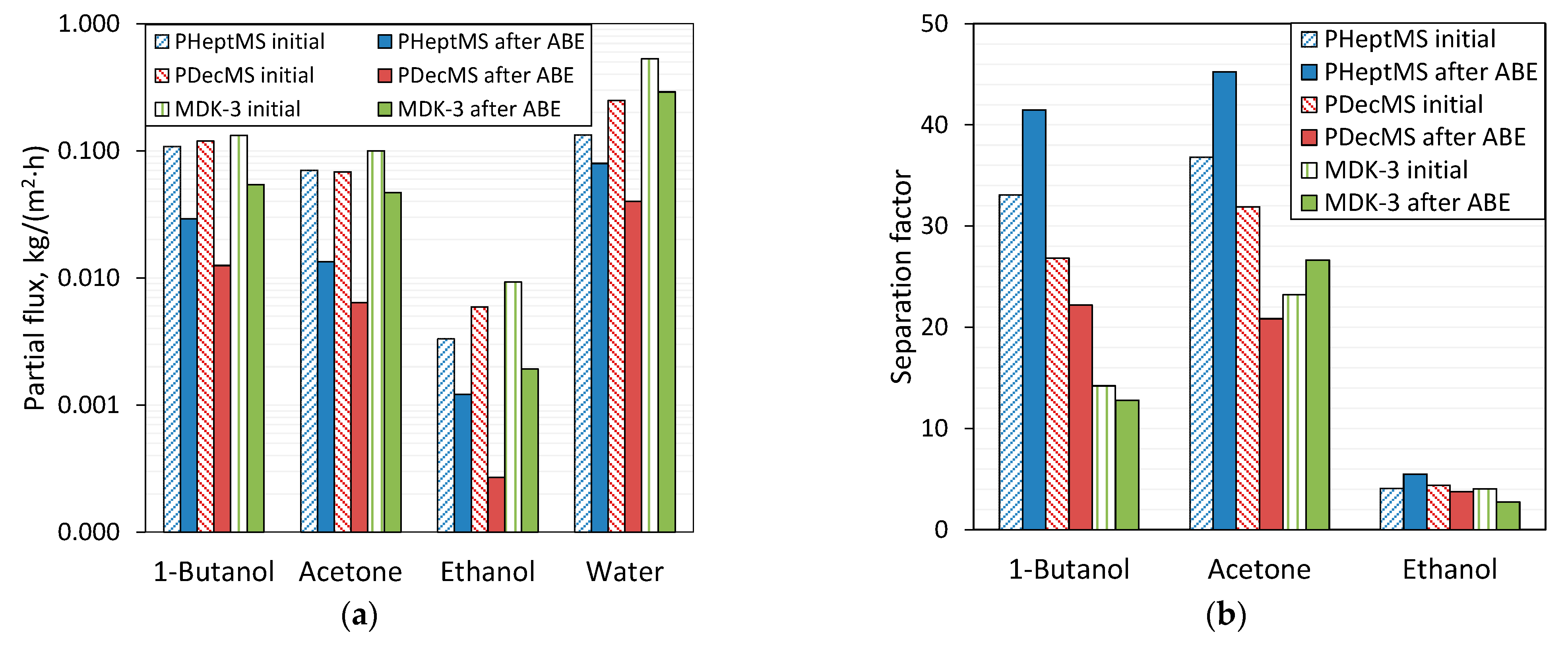
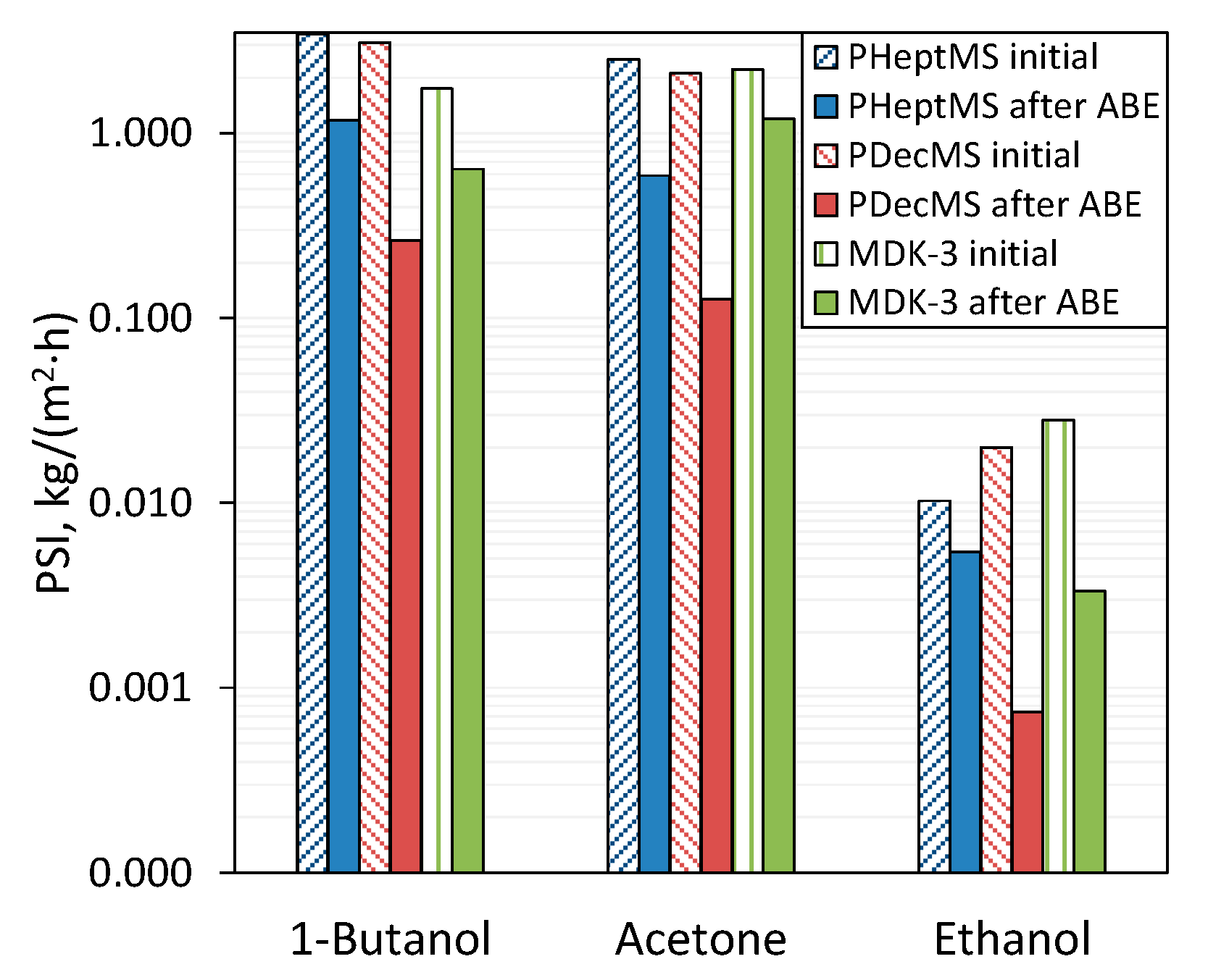
3.2. Comparison of the Pervaporation Properties of Composite Membranes before and after Exposure to ABE-Fermentation Broth
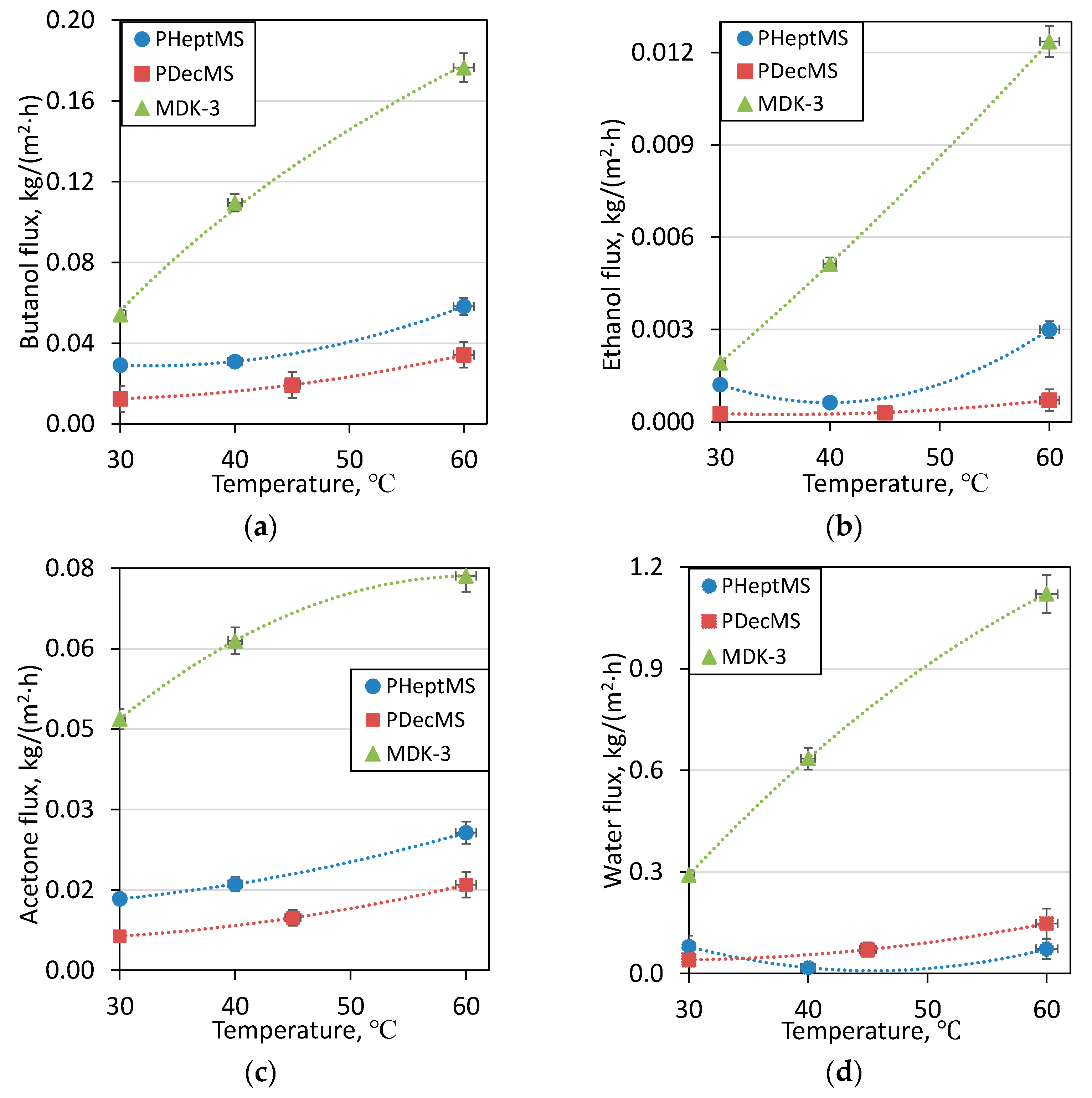
3.3. Study of the Effect of Temperature on the Primary Separation of ABE-Fermentation Broth
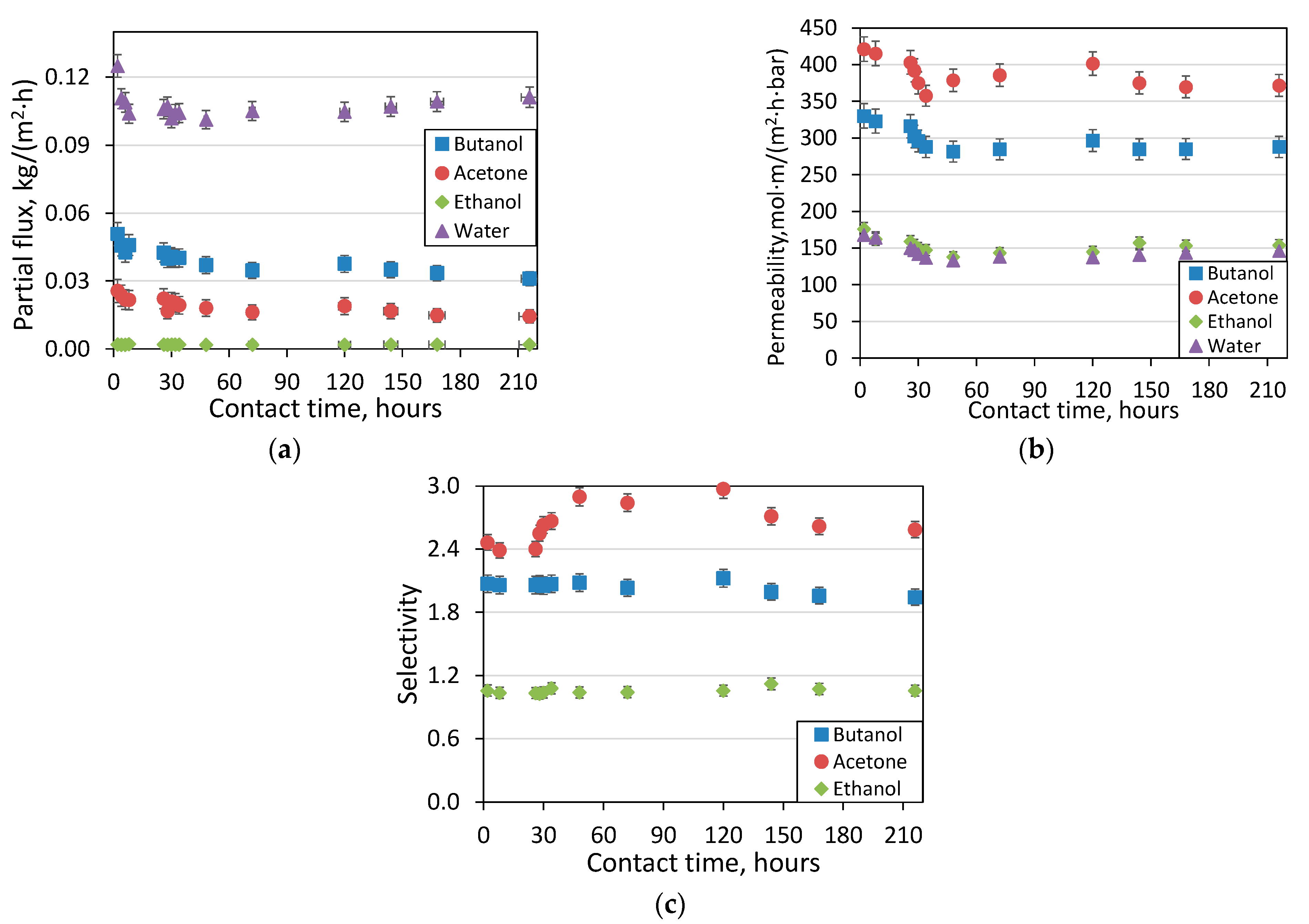
3.4. Dynamics of Contamination of the PHeptMS Membrane during Separation of ABE-Fermentation Broth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dürre, P. Biobutanol: An attractive biofuel. Biotechnol. J. Healthc. Nutr. Technol. 2007, 2, 1525–1534. [Google Scholar] [CrossRef]
- Maity, S.K. Opportunities, recent trends and challenges of integrated biorefinery: Part I. Renew. Sustain. Energy Rev. 2015, 43, 1427–1445. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative monomers based on lignocellulose and their use for polymer production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef]
- Akiyama, M.; Tsuge, T.; Doi, Y. Environmental life cycle comparison of polyhydroxyalkanoates produced from renewable carbon resources by bacterial fermentation. Polym. Degrad. Stab. 2003, 80, 183–194. [Google Scholar] [CrossRef]
- Wilke, D. Chemicals from biotechnology: Molecular plant genetics will challenge the chemical and the fermentation industry. Appl. Microbiol. Biotechnol. 1999, 52, 135–145. [Google Scholar] [CrossRef]
- Mahalingam, L.; Abdulla, R.; Sani, S.A.; Sabullah, M.K.; Faik, A.A.M.; Misson, M. Lignocellulosic Biomass–A Sustainable Feedstock for Acetone-Butanol-Ethanol Fermentation. Period. Polytech. Chem. Eng. 2022, 66, 279–296. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A.; Ankaram, S.; Duan, Y.; Awasthi, M.K. Biofuel production from biomass: Toward sustainable development. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–92. [Google Scholar]
- Lynd, L.R.; Cushman, J.H.; Nichols, R.J.; Wyman, C.E. Fuel ethanol from cellulosic biomass. Science 1991, 251, 1318–1323. [Google Scholar] [CrossRef]
- da Silva Trindade, W.R.; dos Santos, R.G. Review on the characteristics of butanol, its production and use as fuel in internal combustion engines. Renew. Sustain. Energy Rev. 2017, 69, 642–651. [Google Scholar] [CrossRef]
- Nanda, S.; Golemi-Kotra, D.; McDermott, J.C.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Fermentative production of butanol: Perspectives on synthetic biology. N. Biotechnol. 2017, 37, 210–221. [Google Scholar] [CrossRef]
- Nakagawa, H.; Harada, T.; Ichinose, T.; Takeno, K.; Matsumoto, S.; Kobayashi, M.; Sakai, M. Biomethanol production and CO2 emission reduction from forage grasses, trees, and crop residues. JARQ 2007, 41, 173–180. [Google Scholar] [CrossRef]
- Kang, A.; Lee, T.S. Converting sugars to biofuels: Ethanol and beyond. Bioengineering 2015, 2, 184–203. [Google Scholar] [CrossRef]
- García, V.; Päkkilä, J.; Ojamo, H.; Muurinen, E.; Keiski, R.L. Challenges in biobutanol production: How to improve the efficiency? Renew. Sustain. Energy Rev. 2011, 15, 964–980. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J. Microbiol. Biotechnol. 2003, 19, 595–603. [Google Scholar] [CrossRef]
- Qureshi, N.; Blaschek, H.P. Recent advances in ABE fermentation: Hyper-butanol producing Clostridium beijerinckii BA101. J. Ind. Microbiol. Biotechnol. 2001, 27, 287–291. [Google Scholar] [CrossRef]
- Schiel-Bengelsdorf, B.; Montoya, J.; Linder, S.; Dürre, P. Butanol fermentation. Environ. Technol. 2013, 34, 1691–1710. [Google Scholar] [CrossRef]
- Geddes, C.C.; Nieves, I.U.; Ingram, L.O. Advances in ethanol production. Curr. Opin. Biotechnol. 2011, 22, 312–319. [Google Scholar] [CrossRef]
- Kujawska, A.; Kujawski, J.; Bryjak, M.; Kujawski, W. ABE fermentation products recovery methods—A review. Renew. Sustain. Energy Rev. 2015, 48, 648–661. [Google Scholar] [CrossRef]
- Liu, S.; Qureshi, N. How microbes tolerate ethanol and butanol. New Biotechnol. 2009, 26, 117–121. [Google Scholar] [CrossRef]
- Luyben, W.L. Pressure-swing distillation for minimum-and maximum-boiling homogeneous azeotropes. Ind. Eng. Chem. Res. 2012, 51, 10881–10886. [Google Scholar] [CrossRef]
- Levario, T.J.; Dai, M.; Yuan, W.; Vogt, B.D.; Nielsen, D.R. Rapid adsorption of alcohol biofuels by high surface area mesoporous carbons. Microporous Mesoporous Mater. 2012, 148, 107–114. [Google Scholar] [CrossRef]
- Evans, P.J.; Wang, H.Y. Enhancement of butanol formation by Clostridium acetobutylicum in the presence of decanol-oleyl alcohol mixed extractants. Appl. Environ. Microbiol. 1988, 54, 1662–1667. [Google Scholar] [CrossRef]
- Oudshoorn, A.; Van Der Wielen, L.A.; Straathof, A.J. Assessment of options for selective 1-butanol recovery from aqueous solution. Ind. Eng. Chem. Res. 2009, 48, 7325–7336. [Google Scholar] [CrossRef]
- Vane, L.M. A review of pervaporation for product recovery from biomass fermentation processes. J. Chem. Technol. Biotechnol. 2005, 80, 603–629. [Google Scholar] [CrossRef]
- Rozicka, A.; Niemistö, J.; Keiski, R.L.; Kujawski, W. Apparent and intrinsic properties of commercial PDMS based membranes in pervaporative removal of acetone, butanol and ethanol from binary aqueous mixtures. J. Membr. Sci. 2014, 453, 108–118. [Google Scholar] [CrossRef]
- Niemistö, J.; Kujawski, W.; Keiski, R.L. Pervaporation performance of composite poly (dimethyl siloxane) membrane for butanol recovery from model solutions. J. Membr. Sci. 2013, 434, 55–64. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Wang, H.; Liu, L. A facile method of using sulfobetaine-containing copolymers for biofouling resistance. J. Appl. Polym. Sci. 2014, 131, 40789-1–40789-8. [Google Scholar] [CrossRef]
- García, V.; Pongrácz, E.; Muurinen, E.; Keiski, R.L. Recovery of n-butanol from salt containing solutions by pervaporation. Desalination 2009, 241, 201–211. [Google Scholar] [CrossRef]
- Tan, H.; Wu, Y.; Li, T. Pervaporation of n-butanol aqueous solution through ZSM-5-PEBA composite membranes. J. Appl. Polym. Sci. 2013, 129, 105–112. [Google Scholar] [CrossRef]
- Tong, C.; Bai, Y.; Wu, J.; Zhang, L.; Yang, L.; Qian, J. Pervaporation recovery of acetone-butanol from aqueous solution and fermentation broth using HTPB-based polyurethaneurea membranes. Sep. Sci. Technol. 2010, 45, 751–761. [Google Scholar] [CrossRef]
- Borisov, I.L.; Golubev, G.S.; Vasilevsky, V.P.; Volkov, A.V.; Volkov, V.V. Novel hybrid process for bio-butanol recovery: Thermopervaporation with porous condenser assisted by phase separation. J. Membr. Sci. 2017, 523, 291–300. [Google Scholar] [CrossRef]
- Atlaskin, A.A.; Petukhov, A.N.; Yanbikov, N.R.; Salnikova, M.E.; Sergeeva, M.S.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Evaluation of the absorbing pervaporation technique for ammonia recovery after the Haber process. Chem. Proc. Eng. 2018, 39, 323–333. [Google Scholar]
- Otvagina, K.V.; Penkova, A.V.; Dmitrenko, M.E.; Kuzminova, A.I.; Sazanova, T.S.; Vorotyntsev, A.V.; Vorotyntsev, I.V. Novel composite membranes based on chitosan copolymers with polyacrylonitrile and polystyrene: Physicochemical properties and application for pervaporation dehydration of tetrahydrofuran. Membranes 2019, 9, 38. [Google Scholar] [CrossRef]
- Liu, G.; Wei, W.; Jin, W. Pervaporation membranes for biobutanol production. ACS Sustain. Chem. Eng. 2014, 2, 546–560. [Google Scholar] [CrossRef]
- Nakayama, S.; Morita, T.; Negishi, H.; Ikegami, T.; Sakaki, K.; Kitamoto, D. Candida krusei produces ethanol without production of succinic acid; a potential advantage for ethanol recovery by pervaporation membrane separation. FEMS Yeast Res. 2008, 8, 706–714. [Google Scholar] [CrossRef]
- Guan, P.; Ren, C.; Shan, H.; Cai, D.; Zhao, P.; Ma, D.; Si, Z. Boosting the pervaporation performance of PDMS membrane for 1-butanol by MAF-6. Colloid Polym. Sci. 2021, 299, 1459–1468. [Google Scholar] [CrossRef]
- Li, S.Y.; Srivastava, R.; Parnas, R.S. Separation of 1-butanol by pervaporation using a novel tri-layer PDMS composite membrane. J. Membr. Sci. 2010, 363, 287–294. [Google Scholar] [CrossRef]
- Azimi, H.; Tezel, H.; Thibault, J. Optimization of the in situ recovery of butanol from ABE fermentation broth via membrane pervaporation. Chem. Eng. Res. Des. 2019, 150, 49–64. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, G.; Yuan, J.; Chen, T.; Xin, F.; Jiang, M.; Jin, W. In-situ recovery of bio-butanol from glycerol fermentation using PDMS/ceramic composite membrane. Sep. Purif. Technol. 2019, 229, 115811. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Volkov, A.V. Highly selective polysiloxane membranes for the separation of gases and liquids (review). Pet. Chem. 2021, 61, 571–590. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.S.; Lee, J.H. High performance and thermally stable PDMS pervaporation membranes prepared using a phenyl-containing tri-functional crosslinker for n-butanol recovery. Sep. Purif. Technol. 2020, 235, 116142. [Google Scholar] [CrossRef]
- Bennett, M.; Brisdon, B.J.; England, R.; Field, R.W. Performance of PDMS and organofunctionalised PDMS membranes for the pervaporative recovery of organics from aqueous streams. J. Membr. Sci. 1997, 137, 63–88. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Podtynnikov, I.A.; Golubev, G.S.; Volkov, V.V.; Borisov, I.L. Polyheptylmethylsiloxane-new material for pervaporation extraction of oxygenates from water. Membr. Membr. Technol. 2018, 8, 334–342. [Google Scholar]
- Grushevenko, E.A.; Podtynnikov, I.A.; Borisov, I.L. Highly selective pervaporation membrane for the isolation of 1-butanol from wastewater. J. Appl. Chem. 2019, 92, 1488–1496. [Google Scholar]
- Qureshi, N.; Blaschek, H.P. Production of acetone butanol ethanol (ABE) by a hyper-producing mutant strain of Clostridium beijerinckii BA101 and recovery by pervaporation. Biotechnol. Prog. 1999, 15, 594–602. [Google Scholar] [CrossRef]
- Qureshi, N.; Blaschek, H.P. Fouling studies of a pervaporation membrane with commercial fermentation media and fermentation broth of hyper-butanol-producing Clostridium beijerinckii BA101. Sep. Sci. Technol. 1999, 34, 2803–2815. [Google Scholar] [CrossRef]
- Dubreuil, M.F.; Vandezande, P.; Van Hecke, W.H.; Porto-Carrero, W.J.; Dotremont, C.T. Study on ageing/fouling phenomena and the effect of upstream nanofiltration on in-situ product recovery of n-butanol through poly [1-(trimethylsilyl)-1-propyne] pervaporation membranes. J. Membr. Sci. 2013, 447, 134–143. [Google Scholar] [CrossRef]
- Liu, G.; Wei, W.; Wu, H.; Dong, X.; Jiang, M.; Jin, W. Pervaporation performance of PDMS/ceramic composite membrane in acetone butanol ethanol (ABE) fermentation–PV coupled process. J. Membr. Sci. 2011, 373, 121–129. [Google Scholar] [CrossRef]
- Volkov, V.; Borisov, I.; Golubev, G.; Vasilevsky, V.; Matveev, D.; Bondarenko, G.; Volkov, A. Sorption-assisted thermopervaporation method for organics recovery from ABE fermentation broth. J. Chem. Technol. Biotechnol. 2020, 95, 40–51. [Google Scholar] [CrossRef]
- Qureshi, N.; Meagher, M.M.; Huang, J.; Hutkins, R.W. Acetone butanol ethanol (ABE) recovery by pervaporation using silicalite–silicone composite membrane from fed-batch reactor of Clostridium acetobutylicum. J. Membr. Sci. 2001, 187, 93–102. [Google Scholar] [CrossRef]
- Knozowska, K.; Kujawska, A.; Kujawa, J.; Kujawski, W.; Bryjak, M.; Chrzanowska, E.; Kujawski, J.K. Performance of commercial composite hydrophobic membranes applied for pervaporative reclamation of acetone, butanol, and ethanol from aqueous solutions: Binary mixtures. Sep. Purif. Technol. 2017, 188, 512–522. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Pan, Y.; Liu, G.; Wu, H.; Jiang, M.; Jin, W. Fluorinated PDMS membrane with anti-biofouling property for in-situ biobutanol recovery from fermentation-pervaporation coupled process. J. Membr. Sci. 2020, 609, 118225. [Google Scholar] [CrossRef]
- Lee, S.A.; Oh, S.H.; Lee, W. The effect of direct fluorination of polydimethylsiloxane films on their surface properties. J. Colloid Interface Sci. 2009, 332, 461–466. [Google Scholar] [CrossRef]
- Zheng, P.Y.; Li, X.Q.; Wu, J.K.; Wang, N.X.; Li, J.; An, Q.F. Enhanced butanol selectivity of pervaporation membrane with fluorinated monolayer on polydimethylsiloxane surface. J. Membr. Sci. 2018, 548, 215–222. [Google Scholar] [CrossRef]
- Borisov, I.L.; Golubev, G.S.; Patrusheva, E.V.; Sinekoy, S.P. Intensification of acetone-butanol-ethanol fermentation via products recovery: Thermopervaporation assisted by phase separation. Chem. Eng. Trans. 2018, 64, 43–48. [Google Scholar]
- Brunetti, A.; Zito, P.F.; Borisov, I.; Grushevenko, E.; Volkov, V.; Volkov, A.; Barbieri, G. CO2 separation from humidified ternary gas mixtures using a polydecylmethylsiloxane composite membrane. Fuel Process. Technol. 2020, 210, 106550. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters—A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Van Krevelen, D.W. Properties of Polymers: Their Correlation with Chemical Structure: Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Oxford, UK, 2009; p. 1030. [Google Scholar]
- Liu, F.; Liu, L.; Feng, X. Separation of acetone–butanol–ethanol (ABE) from dilute aqueous solutions by pervaporation. Sep. Purif. Technol. 2005, 42, 273–282. [Google Scholar] [CrossRef]
- Li, J.; Polka, H.M.; Gmehling, J. A gE model for single and mixed solvent electrolyte systems: 1. Model and results for strong electrolytes. Fluid Phase Equilibria 1994, 94, 89–114. [Google Scholar] [CrossRef]
- Lipnizki, F.; Hausmanns, S.; Field, R.W. Influence of impermeable components on the permeation of aqueous 1-propanol mixtures in hydrophobic pervaporation. J. Membr. Sci. 2004, 228, 129–138. [Google Scholar] [CrossRef]
- Ikegami, T.; Negishi, H.; Nakayama, S.; Kobayashi, G.; Sakaki, K. Pervaporative concentration of biobutanol from ABE fermentation broths by Clostridium saccharoperbutylacetonicum using silicone rubber-coated silicalite-1 membranes. Sep. Purif. Technol. 2014, 132, 206–212. [Google Scholar] [CrossRef]
- Huang, J.; Meagher, M.M. Pervaporative recovery of n-butanol from aqueous solutions and ABE fermentation broth using thin-film silicalite-filled silicone composite membranes. J. Membr. Sci. 2001, 192, 231–242. [Google Scholar] [CrossRef]
- Van Hecke, W.; Hofmann, T.; De Wever, H. Pervaporative recovery of ABE during continuous cultivation: Enhancement of performance. Bioresour. Technol. 2013, 129, 421–429. [Google Scholar] [CrossRef]
- Wu, H.; Chen, X.P.; Liu, G.P.; Jiang, M.; Guo, T.; Jin, W.Q.; Zhu, D.W. Acetone–butanol–ethanol (ABE) fermentation using Clostridium acetobutylicum XY16 and in situ recovery by PDMS/ceramic composite membrane. Bioprocess Biosyst. Eng. 2012, 35, 1057–1065. [Google Scholar] [CrossRef]
- Rom, A.; Esteve, D.; Friedl, A. Organophilic pervaporation of butanol from an aqueous solution with POMS. Chem. Eng. Trans. 2013, 35, 1315–1320. [Google Scholar]
- Li, Y.; Shi, J.; Liu, X.; Yu, Q.; Qiu, W.; Liu, L. Pervaporation Performance of Polydimethylsiloxane-Polyvinylidene Fluoride Composite Membrane for Butanol Recovery from Model Solutions and Acetone-Butanol-Ethanol Fermentation Broth. J. Biobased Mater. Bioenergy 2020, 14, 294–302. [Google Scholar] [CrossRef]
- Van Hecke, W.; De Wever, H. High-flux POMS organophilic pervaporation for ABE recovery applied in fed-batch and continuous set-ups. J. Membr. Sci. 2017, 540, 321–332. [Google Scholar] [CrossRef]







| Membrane | Condition | Element Weight Concentration, % | ||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Si | N | S | P | F | ||
| PHeptMS | initial | 50.7 | 14.3 | 35.0 | - | - | - | - |
| after ABE | 24.6 | 20.0 | 24.7 | 30.0 | 0.7 | - | - | |
| PDecMS | initial | 54.2 | 12.1 | 33.7 | - | - | - | - |
| after ABE | 56.3 | 18.1 | 24.9 | - | - | 0.7 | - | |
| MDK-3 | initial | 52.4 | 18.6 | 27.9 | - | - | - | 0.5 |
| after ABE | 52.3 | 20.6 | 25.5 | - | 0.1 | - | 0.9 | |
| Component/Polymer | δd | δp | δh | Δs-PHeptMS | Δs-PDecMS |
|---|---|---|---|---|---|
| Acetone | 15.5 | 10.4 | 7.0 | 8.25 | 8.95 |
| Butanol | 16.0 | 5.7 | 15.8 | 11.44 | 12.15 |
| Ethanol | 15.8 | 8.8 | 19.4 | 15.88 | 16.64 |
| Water | 15.5 | 16.0 | 42.3 | 39.83 | 40.57 |
| PHeptMS | 16.77 | 2.54 | 4.83 | - | - |
| PDecMS | 16.77 | 1.97 | 4.26 | - | - |
| Membrane | Condition | Θ by Water | Θ by Acetone | Θ by Ethanol | Θ by Butanol |
|---|---|---|---|---|---|
| MDK-3 | initial | 88 ± 2 | 41 ± 2 | 23 ± 2 | 26 ± 2 |
| after ABE | 74 ± 1.8 | 32 ± 1 | 21 ± 2 | 20 ± 1.3 | |
| PHeptMS | initial | 103 ± 2 | 31 ± 2 | 37 ± 2 | 23 ± 2 |
| after ABE | 91 ± 2 | 25 ± 1.6 | 34 ± 1.4 | 21 ± 1.1 | |
| PDecMS | initial | 106 ± 2 | 36 ± 2 | 41 ± 2 | 28 ± 2 |
| after ABE | 88 ± 1.3 | 24 ± 2.1 | 35 ± 1.2 | 23 ± 1.7 |
| Membrane | β Butanol/Water | β Acetone/Water | β Ethanol/Water | Temperature, °C |
|---|---|---|---|---|
| PHeptMS | 41.5 | 45.3 | 5.5 | 30 |
| 59.7 | 40.8 | 5.6 | 40 | |
| 74.4 | 60.9 | 10.5 | 60 | |
| PDecMS | 22.2 | 20.9 | 3.7 | 30 |
| 19.3 | 17.7 | 2.6 | 45 | |
| 16.9 | 14.6 | 4.2 | 60 | |
| MDK-3 | 12.8 | 26.6 | 2.7 | 30 |
| 14.7 | 20.6 | 3.5 | 40 | |
| 18.4 | 21.4 | 5.1 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokhmanka, T.N.; Grushevenko, E.A.; Arapova, O.V.; Bondarenko, G.N.; Golubev, G.S.; Borisov, I.L.; Volkov, A.V. Fouling of Polyalkylmethylsiloxane Composite Membranes during Pervaporation Separation of ABE-Fermentation Mixtures. Appl. Sci. 2023, 13, 3827. https://doi.org/10.3390/app13063827
Rokhmanka TN, Grushevenko EA, Arapova OV, Bondarenko GN, Golubev GS, Borisov IL, Volkov AV. Fouling of Polyalkylmethylsiloxane Composite Membranes during Pervaporation Separation of ABE-Fermentation Mixtures. Applied Sciences. 2023; 13(6):3827. https://doi.org/10.3390/app13063827
Chicago/Turabian StyleRokhmanka, Tatyana N., Evgenia A. Grushevenko, Olga V. Arapova, Galina N. Bondarenko, George S. Golubev, Ilya L. Borisov, and Alexey V. Volkov. 2023. "Fouling of Polyalkylmethylsiloxane Composite Membranes during Pervaporation Separation of ABE-Fermentation Mixtures" Applied Sciences 13, no. 6: 3827. https://doi.org/10.3390/app13063827
APA StyleRokhmanka, T. N., Grushevenko, E. A., Arapova, O. V., Bondarenko, G. N., Golubev, G. S., Borisov, I. L., & Volkov, A. V. (2023). Fouling of Polyalkylmethylsiloxane Composite Membranes during Pervaporation Separation of ABE-Fermentation Mixtures. Applied Sciences, 13(6), 3827. https://doi.org/10.3390/app13063827







