Translational Echocardiography: The Dog as a Clinical Research Model of Cardiac Dysfunction
Abstract
:1. Introduction
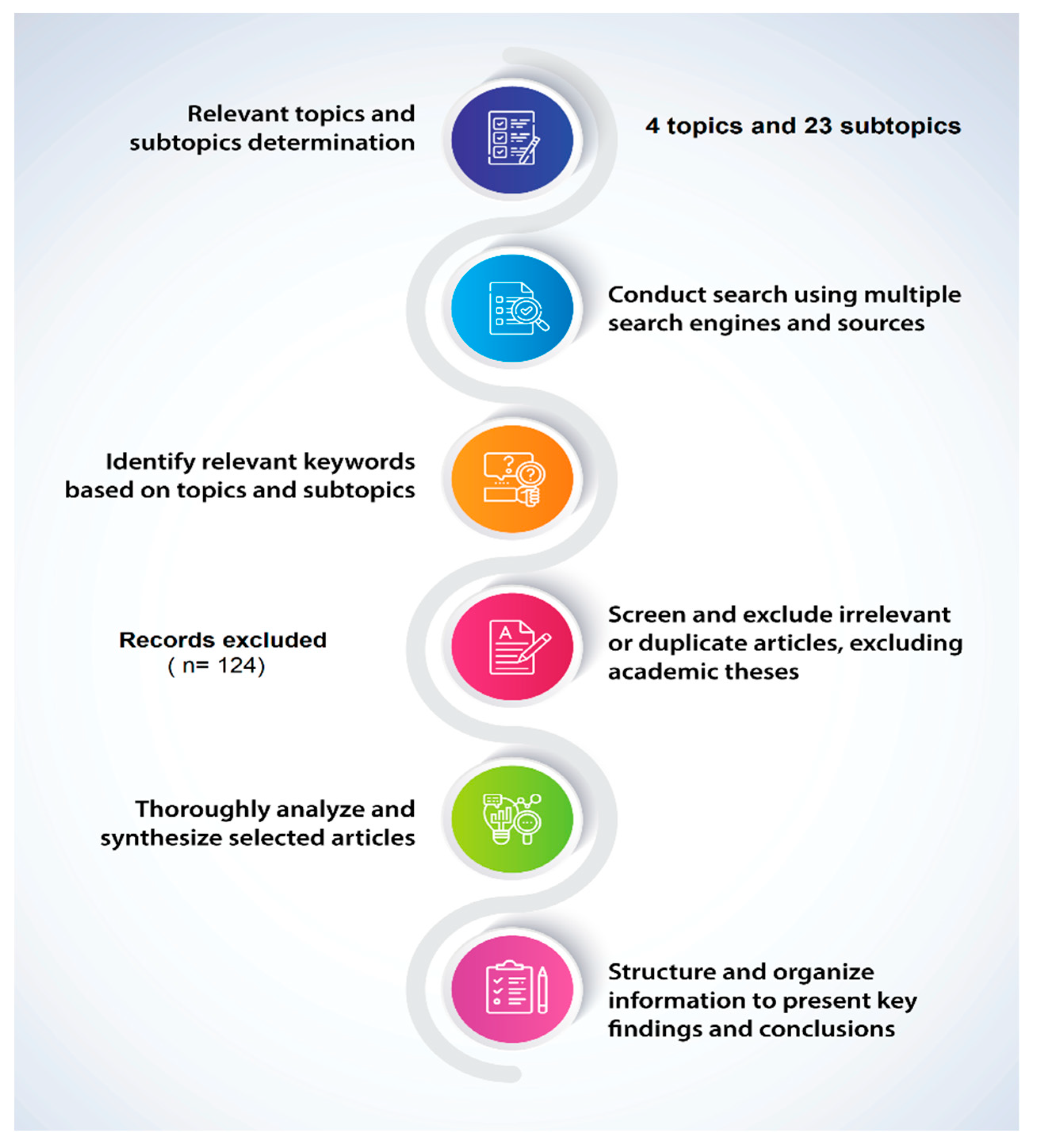
2. Review Methodology
- Step 1.
- Conducting the search. The search was conducted using a comprehensive information search program that utilizes multiple search engines and information sources such as PubMed Central (PMC), Core, DIGI-TAL.CSIC, DOAB, DOAJ, EBSCO host, Elsevier B.V, Redib, Scopus, and Web of Science, available through the Academic Information System of the Autonomous University of Baja California.
- Step 2.
- Identification of keywords. The authors identified relevant keywords based on the four topics and 23 subtopics proposed by the authors included in this review.
- Step 3.
- Selection and analysis of academic information and articles. In this process, the authors screened and excluded irrelevant or duplicate articles. In this analysis, academic theses were excluded.
- Step 4.
- Documentation and synthesis of results. The selected articles were thoroughly analyzed and synthesized, with the information structured and organized to clearly present the key findings and conclusions.
3. Cardiac Anatomical Similarities between the Human and the Dog
3.1. Auricular Anatomical Coincidences
3.2. Ventricular Chambers in Both Species
3.3. Similarities in Cardiac Valves
3.4. The Coronary System
4. Myxomatous Mitral Valve Disease in Dogs as a Clinical Model in the Study of the Pathology in Humans
4.1. (MDMV) Comparative Pathology and Pathophysiology
4.2. Mitral Valve Disease Epidemiology
4.3. Canine Myxomatous Mitral Valve Disease. Comparative Transthoracic Echocardiography with Human Mitral Valve Prolapse
4.4. Comparative Assessment of Mitral Regurgitation Severity
4.5. Mitral Regurgitation Quantification in the Canine Model
4.6. Left Heart Remodeling Assessment and LA Evaluation
4.7. Comparative Left Ventricle Assessment
4.8. Key Main Useful Echocardiographic Considerations for the Approach of Mitral Endocardiosis in Canine Patients
4.8.1. Evaluation of the Regurgitant Area
4.8.2. Presence of Vena Contracta
4.8.3. Degree of Myxomatous Degeneration
4.8.4. Mitral Valve Prolapse
4.8.5. Vmax Wave E
4.8.6. E-Wave Deceleration Time
4.8.7. E/IVRT Ratio
4.8.8. E/é Ratio of the Mitral Annulus
5. Dilated Cardiomyopathy (DCM), a Human and Canine Common Disease
5.1. Echocardiographic Measurements on DCM
5.1.1. Measurement of Left Ventricular Volume by Simpson’s Method of Disks and Left Ventricular M-Mode
5.1.2. E-Point to Septal Separation or EPSS: An Echocardiographic Parameter for Accurate Assessment of Left Ventricular Performance
5.1.3. Sphericity Index (SI)
5.1.4. Tissue Doppler and Speckle Tracking
6. Canine Patients with Naturally Occurring Cardiac Diseases—Learning from a Potential Echocardiography Research Model
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Gaar-Humphreys, K.R.; Spanjersberg, T.C.F.; Santarelli, G.; Grinwis, G.C.M.; Szatmári, V.; Roelen, B.A.J.; Vink, A.; van Tintelen, J.P.; Asselbergs, F.W.; Fieten, H.; et al. Genetic Basis of Dilated Cardiomyopathy in Dogs and Its Potential as a Bidirectional Model. Animals 2022, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Fan, H.; Liu, Z.; He, J.Q. Large Mammalian Animal Models of Heart Disease. J. Cardiovasc. Dev. Dis. 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löwa, A.; Jevtić, M.; Gorreja, F.; Hedtrich, S. Alternatives to animal testing in basic and preclinical research of atopic dermatitis. Exp. Dermatol. 2018, 27, 476–483. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.; Fernández-Friera, L.; Villalba, M.; López-Melgar, B.; España, S.; Mateo, J.; Mota, R.A.; Jiménez-Borreguero, J.; Ruiz-Cabello, J. Cardiovascular imaging: What have we learned from animal models? Front. Pharmacol. 2015, 6, 227. [Google Scholar] [CrossRef] [Green Version]
- Demiris, G.; Oliver, D.P.; Washington, K.T. Defining and analyzing the problem. In Behavioral Intervention Research in Hospice and Palliative Care; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–39. [Google Scholar]
- Getty, R. General heart and blood vessels. In Sisson and Grossman’s: The Anatomy of the Domestic Animals, 5th ed.; Sisson, S., Grossman, J.D., Getty, R., Eds.; Saunders: Philadelphia, PA, USA, 1975; pp. 164–175. [Google Scholar]
- Michaelsson, M.; Ho, S.Y. Congenital Heart Malformations in Mammals: An Illustrated Text; Imperial College Press: River Edge, NJ, USA; London, UK, 2000. [Google Scholar]
- Crick, S.J.; Sheppard, M.N.; Ho, S.Y.; Gebstein, L.; Anderson, R.H. Anatomy of the pig heart: Comparisons with normal human cardiac structure. J. Anat. 1998, 193, 105–119. [Google Scholar] [CrossRef]
- Queiroz, L.L.; Moura, L.R.; Moura, V.M.B.D. Morphometric assessment of canine heart without macroscopically visible changes caused by cardiac disease. Ciênc. Anim. Bras. 2018, 19, e43748. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.J.; Laizzo, P.A. Comparative cardiac anatomy. In Handbook of Cardiac Anatomy, Physiology, and Devices; Iaizzo, P., Ed.; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Rodriguez, E.R.; Tan, C.D. Structure and Anatomy of the Human Pericardium. Prog. Cardiovasc. Dis. 2017, 59, 327–340. [Google Scholar] [CrossRef]
- Evans, H.E. The heart and arteries. In Miller’s Anatomy of the Dog, 3rd ed.; Miller, M.E., Evans, H.E., Eds.; Saunders: Philadelphia, PA, USA, 1993; pp. 586–602. [Google Scholar]
- Holt, J.P. The normal pericardium. Am. J. Cardiol. 1970, 26, 455–465. [Google Scholar] [CrossRef]
- Naimark, W.A.; Lee, J.M.; Limeback, H.; Cheung, D.T. Correlation of structure and viscoelastic properties in the pericardia of four mammalian species. Am. J. Physiol. 1992, 263, H1095–H1106. [Google Scholar] [CrossRef]
- Spodick, D.H. The Pericardium: A Comprehensive Textbook; Dekker: New York, NY, USA, 1997. [Google Scholar]
- Czum, J.M.; Silas, A.M.; Althoen, M.C. Evaluation of the Pericardium with CT and MR. ISRN Cardiol. 2014, 2014, 174908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakovljevic, D.G. Physical activity and cardiovascular aging: Physiological and molecular insights. Exp. Gerontol. 2018, 109, 67–74. [Google Scholar] [CrossRef]
- Singh, B. Dyce, Sack, and Wensing’s Textbook of Veterinary Anatomy, 5th ed.; Elsevier: St. Louis, MO, USA, 2017. [Google Scholar]
- Walmsley, R. Anatomy of human mitral valve in adult cadaver and comparative anatomy of the valve. Br. Heart J. 1978, 40, 351–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarniceriu, C.C.; Hurjui, L.L.; Tanase, D.M.; Nedelcu, A.H.; Gradinaru, I.; Ursaru, M.; Stefan Rudeanu, A.; Delianu, C.; Lozneanu, L. The Pulmonary Venous Return from Normal to Pathological—Clinical Correlations and Review of Literature. Medicina 2021, 57, 293. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, G.B.; Hussain, S.T.; Ramankutty, R.M.; Lytle, B.W.; Blackstone, E.H. Reconstruction of fibrous skeleton: Technique, pitfalls and results. Multimed. Man. Cardiothorac. Surg. 2014, 2014, mmu004. [Google Scholar] [CrossRef]
- Krawczyk-Ożóg, A.; Hołda, M.K.; Sorysz, D.; Koziej, M.; Siudak, Z.; Dudek, D.; Klimek-Piotrowska, W. Morphologic variability of the mitral valve leaflets. J. Thorac. Cardiovasc. Surg. 2017, 154, 1927–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.A.; Nguyen, E.T.; Dennie, C.; Wald, R.M.; Crean, A.M.; Yoo, S.J.; Jimenez-Juan, L. Computed tomography and magnetic resonance imaging of the coronary sinus: Anatomic variants and congenital anomalies. Insights Imaging 2014, 5, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Bigler, M.R.; Seiler, C. The Human Coronary Collateral Circulation, Its Extracardiac Anastomoses and Their Therapeutic Promotion. Int. J. Mol. Sci. 2019, 20, 3726. [Google Scholar] [CrossRef] [Green Version]
- Teunissen, P.F.; Horrevoets, A.J.; van Royen, N. The coronary collateral circulation: Genetic and environmental determinants in experimental models and humans. J. Mol. Cell. Cardiol. 2012, 52, 897–904. [Google Scholar] [CrossRef]
- Palmisano, A.; Nicoletti, V.; Colantoni, C.; Monti, C.B.; Pannone, L.; Vignale, D.; Darvizeh, F.; Agricola, E.; Schaffino, S.; De Cobelli, F.; et al. Dynamic changes of mitral valve annulus geometry at preprocedural CT: Relationship with functional classes of regurgitation. Eur. Radiol. Exp. 2021, 5, 34. [Google Scholar] [CrossRef]
- Coutinho, G.F.; Antunes, M.J. Current status of the treatment of degenerative mitral valve regurgitation. Rev. Port. Cardiol. 2021, 40, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.L.; Marques, L.C.; Aiello, V.D. Myxomatous degeneration of the mitral valve. Autops. Case Rep. 2018, 8, e2018058. [Google Scholar] [CrossRef] [PubMed]
- Oyama, M.A.; Elliott, C.; Loughran, K.A.; Kossar, A.P.; Castillero, E.; Levy, R.J.; Ferrari, G. Comparative pathology of human and canine myxomatous mitral valve degeneration: 5HT and TGF-β mechanisms. Cardiovasc. Pathol. 2020, 46, 107196. [Google Scholar] [CrossRef] [PubMed]
- Merryman, W.D.; Youn, I.; Lukoff, H.D.; Krueger, P.M.; Guilak, F.; Hopkins, R.A.; Sacks, M.S. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: Implications for collagen biosynthesis. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H224–H231. [Google Scholar] [CrossRef] [PubMed]
- Sacks, M.S.; He, Z.; Baijens, L.; Wanant, S.; Shah, P.; Sugimoto, H.; Yoganathan, A.P. Surface strains in the anterior leaflet of the functioning mitral valve. Ann. Biomed. Eng. 2002, 30, 1281–1290. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, K.P.; Ring, L.; Rana, B.S. Anatomy of the mitral valve: Understanding the mitral valve complex in mitral regurgitation. Eur. J. Echocardiogr. 2010, 11, i3–i9. [Google Scholar] [CrossRef] [Green Version]
- Fox, P.R. Pathology of myxomatous mitral valve disease in the dog. J. Vet. Cardiol. 2012, 14, 103–126. [Google Scholar] [CrossRef]
- Markby, G.; Summers, K.M.; MacRae, V.E.; Del-Pozo, J.; Corcoran, B.M. Myxomatous degeneration of the canine mitral valve: From gross changes to molecular events. J. Comp. Pathol. 2017, 156, 371–383. [Google Scholar] [CrossRef] [Green Version]
- Saremi, F.; Sánchez-Quintana, D.; Mori, S.; Muresian, H.; Spicer, D.E.; Hassani, C.; Anderson, R.H. Fibrous skeleton of the heart: Anatomic overview and evaluation of pathologic conditions with CT and MR imaging. RadioGraphics 2017, 37, 1330–1351. [Google Scholar] [CrossRef]
- Jimenez, J.H.; Soerensen, D.D.; Zhaoming, H.; He, S.; Yoganathan, A.P. Effects of a saddle shaped annulus on mitral valve function and chordal force distribution: An in vitro study. Ann. Biomed. Eng. 2003, 31, 1171–1181. [Google Scholar] [CrossRef]
- Padala, M.; Hutchison, R.A.; Croft, L.R.; Jimenez, J.H.; Gorman, R.C.; Gorman, J.H. Saddle shape of the mitral annulus reduces systolic strains on the P2 segment of the posterior mitral leaflet. Ann. Thorac. Surg. 2009, 88, 1499–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connell, P.S.; Han, R.I.; Grande-Allen, K.J. Differentiating the aging of the mitral valve from human and canine myxomatous degeneration. J. Vet. Cardiol. 2012, 14, 31–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aupperle, H.; Disatian, S. Pathology, protein expression and signaling in myxomatous mitral valve degeneration: Comparison of dogs and humans. J. Vet. Cardiol. 2012, 14, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Combs, M.D.; Yutzey, K.E. Heart valve development: Regulatory networks in development and disease. Circ. Res. 2009, 105, 408–421. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, S.; Ferrari, G.; Gorman, R.C.; Gorman, J.H.; Schoen, F.J.; Sacks, M.S. Heart valve biomechanics and underlying mechanobiology. Comp. Physiol. 2016, 6, 1743–1780. [Google Scholar]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; EnriquezSarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Marechaux, S.; Illman, J.E.; Huynh, J.; Michelena, H.I.; Nkomo, V.T.; Tribouilloy, C. Functional anatomy and pathophysiologic principles in mitral regurgitation: Non-invasive assessment. Prog. Cardiovasc. Dis. 2017, 60, 289–304. [Google Scholar] [CrossRef]
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef]
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef]
- Borgarelli, M.; Buchanan, J.W. Historical review, epidemiology and natural history of degenerative mitral valve disease. J. Vet. Cardiol. 2012, 14, 93–101. [Google Scholar] [CrossRef]
- Parker, H.G.; Kilroy-Glynn, P. Myxomatous mitral valve disease in dogs: Does size matter? J. Vet. Cardiol. 2012, 14, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, T.W.; Wiles, B.M.; Llewellyn-Zaidi, A.M.; Evans, K.M.; O’Neill, D.G. Longevity and mortality in Kennel Club registered dog breeds in the UK in 2014. Canine. Genet. Epidemiol. 2018, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagardi, M.; Bionda, A.; Locatelli, C.; Cortellari, M.; Frattini, S.; Negro, A.; Crepaldi, P.; Brambilla, P.G. Echocardiographic Evaluation of the Mitral Valve in Cavalier King Charles Spaniels. Animals 2020, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Hyun, C. Mitral valve prolapse in Cavalier King Charles spaniel: A review and case study. J. Vet. Sci. 2005, 6, 67–73. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Michelena, H.I. Mitral regurgitation in the 21st century. Prog. Cardiovasc. Dis. 2017, 60, 285–288. [Google Scholar] [CrossRef]
- Atkins, C.; Bonagura, J.; Ettinger, S.; Fox, P.; Gordon, S.; Haggstrom, J.; Hamlin, R.; Keene, B.; Luis-Fuentes, V.; Stepien, R. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J. Vet. Intern. Med. 2009, 23, 1142–1150. [Google Scholar] [CrossRef]
- Borgarelli, M.; Crosara, S.; Lamb, K.; Savarino, P.; La Rosa, G.; Tarducci, A.; Haggstrom, J. Survival characteristics and prognostic variables of dogs with preclinical chronic degenerative mitral valve disease attributable to myxomatous degeneration. J. Vet. Intern. Med. 2012, 26, 69–75. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Boon, J.A. Acquired heart disease: Mitral insufficiency. In Manual of Veterinary Echocardiography, 1st ed.; Boon, J.A., Ed.; Williams and Wilkins: Baltimore, MD, USA, 1998; pp. 261–286. [Google Scholar]
- Serres, F.; Chetboul, V.; Tissier, R.; Poujol, L.; Gouni, V.; Carlos Sampedrano CPouchelon, J.L. Comparison of 3 ultrasound methods for quantifying left ventricular systolic function: Correlation with disease severity and prognostic value in dogs with mitral valve disease. J. Vet. Intern. Med. 2008, 22, 566–577. [Google Scholar] [CrossRef]
- Bonagura, J.D.; Schober, K.E. Can ventricular function be assessed by echocardiography in chronic canine mitral valve disease? J. Small Anim. Pract. 2009, 50, 12–24. [Google Scholar] [CrossRef]
- Chetboul, V. Advanced techniques in echocardiography in small animals. Vet. Clin. Small Anim. Pract. 2010, 40, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-I.; Lu, T.-L.; Choi, R.; Hyun, C. Echocardiographic Features in Canine Myxomatous Mitral Valve Disease: An Animal Model for Human Mitral Valve Prolapse. In Proceedings of the Advanced Concepts in Endocarditis-2021, Virtual, 25 August 2021. [Google Scholar] [CrossRef] [Green Version]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Scientific document Committee of the European Association of cardiovascular imaging: Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouni, V.; Serres, F.J.; Pouchelon, J.L.; Tissier, R.; Lefebvre, H.P.; Nicolle, A.P.; Sampedrano, C.C.; Chetboul, V. Quantification of mitral valve regurgitation in dogs with degenerative mitral valve disease by use of the proximal isovelocity surface area method. J. Am. Vet. Med. Assoc. 2007, 231, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; Nihoyannopoulos, P.; Otto, C.M.; Quinones, M.A.; Rakowski, H.; et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 777–802. [Google Scholar] [CrossRef]
- Chetboul, V.; Tissier, R. Echocardiographic assessment of canine degenerative mitral valve disease. J. Vet. Cardiol. 2012, 14, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Chetboul, V.; Serres, F.; Tissier, R.; Lefebvre, H.P.; Sampedrano, C.C.; Gouni, V.; Poujol, L.; Hawa, G.; Pouchelon, J.L. Association of plasma N-terminal pro-B-type natriuretic peptide concentration with mitral regurgitation severity and outcome in dogs with asymptomatic degenerative mitral valve disease. J. Vet. Intern. Med. 2009, 23, 984–994. [Google Scholar] [CrossRef]
- Muzzi, R.A.; de Araújo, R.B.; Muzzi, L.A.; Pena, J.L.; Silva, E.F. Regurgitant jet area by Doppler color flow mapping: Quantitative assessment of mitral regurgitation severity in dogs. J. Vet. Cardiol. 2003, 5, 33–38. [Google Scholar] [CrossRef]
- Sargent, J.; Muzzi, R.; Mukherjee, R.; Somarathne, S.; Schranz, K.; Stephenson, H.; Connolly, D.; Brodbelt, D.; Fuentes, V.L. Echocardiographic predictors of survival in dogs with myxomatous mitral valve disease. J. Vet. Cardiol. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Weissman, N.J.; Zamorano, J.L. Quantitation of mitral regurgitation. Circulation 2012, 126, 2005–2017. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for non-invasive evaluation of native Valvular regurgitation: A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Vezzosi, T.; Grosso, G.; Tognetti, R.; Meucci, V.; Patata, V.; Marchesotti, F.; Domenech, O. The Mitral INsufficiency Echocardiographic score: A severity classification of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2021, 35, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.; Connolly, D.J.; Watts, V.; Mõtsküla, P.; Volk, H.A.; Lamb, C.R.; Luis Fuentes, V. Assessment of mitral regurgitation in dogs: Comparison of results of echocardiography with magnetic resonance imaging. J. Small Anim. Pract. 2015, 56, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biner, S.; Rafique, A.; Rafii, F.; Tolstrup, K.; Noorani, O.; Shiota, T.; Gurudevan, S.; Siegel, R.J. Reproducibility of proximal isovelocity surface area, vena contracta, and regurgitant jet area for assessment of mitral regurgitation severity. JACC Cardiovasc. Imaging 2010, 3, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Paiva, R.M.; Garcia-Guasch, L.; Manubens, J.; Montoya-Alonso, J.A. Proximal isovelocity surface area variability during systole in dogs with mitral valve prolapse. J. Vet. Cardiol. 2011, 13, 267–270. [Google Scholar] [CrossRef]
- Tidholm, A.; Bodegård-Westling, A.; Höglund, K.; Häggström, J.; Ljungvall, I. Real-time 3-dimensional echocardiographic assessment of effective regurgitant orifice area in dogs with myxomatous mitral valve disease. J. Vet. Intern. Med. 2017, 31, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Borgarelli, M.; Savarino, P.; Crosara, S.; Santilli, R.A.; Chiavegato, D.; Poggi, M.; Bellino, C.; La Rosa, G.; Zanatta, R.; Haggstrom, J.; et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J. Vet. Intern. Med. 2008, 22, 120–128. [Google Scholar] [CrossRef]
- Hansson, K.; Häggström, J.; Kvart, C.; Lord, P. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet. Radiol. Ultrasound 2002, 43, 568–575. [Google Scholar] [CrossRef]
- Cornell, C.C.; Kittleson, M.D.; Torre, P.D.; Häggström, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 2004, 18, 311–321. [Google Scholar] [CrossRef]
- Schober, K.E.; Hart, T.M.; Stern, J.A.; Li, X.; Samii, V.F.; Zekas, L.J.; Scansen, B.A.; Bonagura, J.D. Detection of congestive heart failure in dogs by Doppler echocardiography. J. Vet. Intern. Med. 2010, 24, 1358–1368. [Google Scholar] [CrossRef]
- Boswood, A.; Häggström, J.; Gordon, S.G.; Wess, G.; Stepien, R.L.; Oyama, M.A.; Keene, B.W.; Bonagura, J.; MacDonald, K.A.; Patteson, M.; et al. Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: The EPIC study—A randomized clinical trial. J. Vet. Intern. Med. 2016, 30, 1765–1779. [Google Scholar] [CrossRef]
- Reynolds, C.A.; Brown, D.C.; Rush, J.E.; Fox, P.R.; Nguyenba, T.P.; Lehmkuhl, L.B.; Gordon, S.G.; Kellihan, H.B.; Stepien, R.L.; Lefbom, B.K.; et al. Prediction of first onset of congestive heart failure in dogs with degenerative mitral valve disease: The PREDICT cohort study. J. Vet. Cardiol. 2012, 14, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.R.; Dell’Italia, L.J.; Tillson, M.; Killingsworth, C.; Denney, T.; Hathcock, J.; Botzman, L. Left ventricular remodeling in preclinical experimental mitral regurgitation of dogs. J. Vet. Cardiol. 2012, 14, 73–92. [Google Scholar] [CrossRef]
- Menciotti, G.; Borgarelli, M. Review of Diagnostic and Therapeutic Approach to Canine Myxomatous Mitral Valve Disease. Vet. Sci. 2017, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- McGinley, J.C.; Berretta, R.M.; Chaudhary, K.; Rossman, E.; Bratinov, G.D.; Gaughan, J.P.; Houser, S.; Margulies, K.B. Impaired contractile reserve in severe mitral valve regurgitation with a preserved ejection fraction. Eur. J. Heart Fail. 2007, 9, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Baron Toaldo, M.; Romito, G.; Guglielmini, C.; Diana, A.; Pelle, N.G.; Contiero, B.; Cipone, M. Prognostic value of echocardiographic indices of left atrial morphology and function in dogs with myxomatous mitral valve disease. J. Vet. Intern. Med. 2018, 32, 914–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef] [PubMed]
- Di Marcello, M.; Terzo, E.; Locatelli, C.; Palermo, V.; Sala, E.; Dall’Aglio, E.; Bussadori, C.M.; Spalla, I.; Brambilla, P.G. Assessment of mitral regurgitation severity by Doppler color flow mapping of the vena contracta in dogs. J. Vet. Intern. Med. 2014, 28, 1206–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, H.D.; Häggström, J. Mitral valve prolapse in the dog: A model of mitral valve prolapse in man. Cardiovasc. Res. 2000, 47, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Osuga, T.; Morishita, K.; Suzuki, S.; Morita, T.; Yokoyama, N.; Ohta, H.; Yamasaki, M.; Takiguchi, M. Prognostic value of left atrial function in dogs with chronic mitral valvular heart disease. J. Vet. Intern. Med. 2014, 28, 1746–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesselowski, S.; Borgarelli, M.; Menciotti, G.; Abbott, J. Echocardiographic anatomy of the mitral valve in healthy dogs and dogs with myxomatous mitral valve disease. J. Vet. Cardiol. 2015, 17, 97–106. [Google Scholar] [CrossRef]
- Hezzell, M.J.; Boswood, A.; Moonarmart, W.; Elliott, J. Selected echocardiographic variables change more rapidly in dogs that die from myxomatous mitral valve disease. J. Vet. Cardiol. 2012, 14, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, G.J.; Park, C. Rate of left ventricular pressure change by Doppler echocardiography in dogs with chronic mitral valve disease at different stages of congestive heart failure. Vet. Radiol. Ultrasound 2018, 59, 758–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chetboul, V.; Bussadori, C.; De Madron, É. Clinical Echocardiography of the Dog and Cat; Elsevier: Amsterdam, The Netherlands, 2016; pp. 127–138. [Google Scholar]
- Kim, H.T.; Han, S.M.; Song, W.J.; Kim, B.; Choi, M.; Yoon, J.; Youn, H.Y. Retrospective study of degenerative mitral valve disease in small-breed dogs: Survival and prognostic variables. J. Vet. Sci. 2017, 18, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, H.M. Usefulness of conventional and tissue Doppler echocardiography to predict congestive heart failure in dogs with myxomatous mitral valve disease. J. Vet. Intern. Med. 2015, 29, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.; Rivero, J.; McCullough, S.D.; West, E.; Opotowsky, A.R.; Waxman, A.B.; Systrom, D.M.; Shah, A.M. E/e’ Ratio in Patients with Unexplained Dyspnea: Lack of Accuracy in Estimating Left Ventricular Filling Pressure. Circ. Heart Fail. 2015, 8, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Simpson, S.; Kordtomeikel, K.; Wong, S.; Bennison, S.; El-Gendy, S.A.; Cobb, M.; Rutland, C.S. Diagnosis, Prognosis, Management, Treatment, Research and Advances in Canine Dilated Cardiomyopathy. In Canine Genetics, Health and Medicine; Rutland, C., Ed.; IntechOpen: London, UK, 2021; Available online: https://www.intechopen.com/chapters/76601 (accessed on 17 November 2022). [CrossRef]
- Egenvall, A.; Bonnett, B.N.; Häggström, J. Heart Disease as a Cause of Death in Insured Swedish Dogs Younger than 10 Years of Age. J. Vet. Intern. Med. 2006, 20, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.M.; Creevy, K.E.; Promislow, D.E. Mortality in north american dogs from 1984 to 2004: An investigation into age-, size-, and breed-related causes of death. J. Vet. Intern. Med. 2011, 25, 187–198. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [Green Version]
- Mausberg, T.B.; Wess, G.; Simak, J.; Keller, L.; Drögemüller, M.; Drögemüller, C.; Webster, M.T.; Stephenson, H.; Dukes-McEwan, J.; Leeb, T. A locus on chromosome 5 is associated with dilated cardiomyopathy in Doberman Pinschers. PLoS ONE 2011, 6, e20042. [Google Scholar] [CrossRef] [Green Version]
- Dixon, J.A.; Spinale, F.G. Large animal models of heart failure: A critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009, 2, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Wess, G.; Domenech, O.; Dukes-McEwan, J.; Häggström, J.; Gordon, S. European Society of Veterinary Cardiology screening guidelines for dilated cardiomyopathy in Doberman Pinschers. J. Vet. Cardiol. 2017, 19, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Wess, G.; Schulze, A.; Butz, V.; Simak, J.; Killich, M.; Keller, L.J.; Maeurer, J.; Hartmann, K. Prevalence of dilated cardiomyopathy in doberman pinschers in various age groups. J. Vet. Intern. Med. 2010, 24, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Bonagura, J.D.; Visser, L.C. Echocardiographic assessment of dilated cardiomyopathy in dogs. J. Vet. Cardiol. 2022, 40, 15–50. [Google Scholar] [CrossRef] [PubMed]
- Wess, G.; Killich, M.; Hartmann, K. Comparison of pulsed wave and color Doppler myocardial velocity imaging in healthy dogs. J. Vet. Intern. Med. 2010, 24, 360–366. [Google Scholar] [CrossRef]
- Chetboul, V.; Carlos, C.; Blot, S.; Thibaud, J.L.; Escriou, C.; Tissier, R.; Retortillo, J.L.; Pouchelon, J.L. Tissue Doppler assessment of diastolic and systolic alterations of radial and longitudinal left ventricular motions in Golden Retrievers during the preclinical phase of cardiomyopathy associated with muscular dystrophy. Am. J. Vet. Res. 2004, 65, 1335–1341. [Google Scholar] [CrossRef]
- O’Sullivan, M.L.; O’Grady, M.R.; Minors, S.L. Assessment of diastolic function by Doppler echocardiography in normal Doberman Pinschers and Doberman Pinschers with dilated cardiomyopathy. J. Vet. Intern. Med. 2007, 21, 81–91. [Google Scholar] [CrossRef]
- Chetboul, V.; Gouni, V.; Sampedrano, C.C.; Tissier, R.; Serres, F.; Pouchelon, J.L. Assessment of regional systolic and diastolic myocardial function using tissue Doppler and strain imaging in dogs with dilated cardiomyopathy. J. Vet. Intern. Med. 2007, 21, 719–730. [Google Scholar] [CrossRef]
- Klaeboe, L.G.; Edvardsen, T. Echocardiographic assessment of left ventricular systolic function. J. Echocardiogr. 2019, 17, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Dandel, M.; Hetzer, R. Echocardiographic strain and strain rate imaging–clinical applications. Int. J. Cardiol. 2009, 132, 11–24. [Google Scholar] [CrossRef]
- Loen, V.; Vos, M.A.; van der Heyden, M.A.G. The canine chronic atrioventricular block model in cardiovascular preclinical drug research. Br. J. Pharmacol. 2022, 179, 859–881. [Google Scholar] [CrossRef]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef] [PubMed]
- Romero Borges, R.; Valido Díaz, A.; Bernal Llerenas, T.; Fimia Duarte, R.; Iannacone, R. Biomodel of arterial hypertension in Wistar rats administered 10% saline solution. Biotempo 2018, 15, 75–82. [Google Scholar]
- Zaragoza, C.; Gomez-Guerrero, C.; Martin-Ventura, J.L.; Blanco-Colio, L.; Lavin, B.; Mallavia, B.; Tarin, C.; Mas, S.; Ortiz, A.; Egido, J. Animal models of cardiovascular diseases. J. Biomed. Biotechnol. 2011, 2011, 497841. [Google Scholar] [CrossRef] [PubMed]
- Esser, L.C.; Borkovec, M.; Bauer, A.; Häggström, J.; Wess, G. Left ventricular M-mode prediction intervals in 7651 dogs: Population-wide and selected breed -specific values. J. Vet. Intern. Med. 2020, 34, 2242–2252. [Google Scholar] [CrossRef]
- de Madron, É. Normal Views: 2D, TM, Spectral, and Color Doppler. In Clinical Echocardiography of the Dog and Cat; Chetboul, V., Bussadori, C., de Madron, É, Eds.; Elsevier Publishing: St. Louis, MO, USA, 2016. [Google Scholar]
- Coffman, M.; Guillot, E.; Blondel, T.; Garelli-Paar, C.; Feng, S.; Heartsill, S.; Atkins, C.E. Clinical efficacy of a benazepril and spironolactone combination in dogs with congestive heart failure due to myxomatous mitral valve disease: The BEnazepril Spironolactone STudy (BESST). J. Vet. Intern. Med. 2021, 35, 1673–1687. [Google Scholar] [CrossRef]
- Bellenger, N.G.; Burgess, M.I.; Ray, S.G.; Lahiri, A.; Coats, A.J.; Cleland, J.G.; Pennell, D.J. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur. Heart J. 2000, 21, 1387–1396. [Google Scholar] [CrossRef] [Green Version]
- Grothues, F.; Smith, G.C.; Moon, J.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
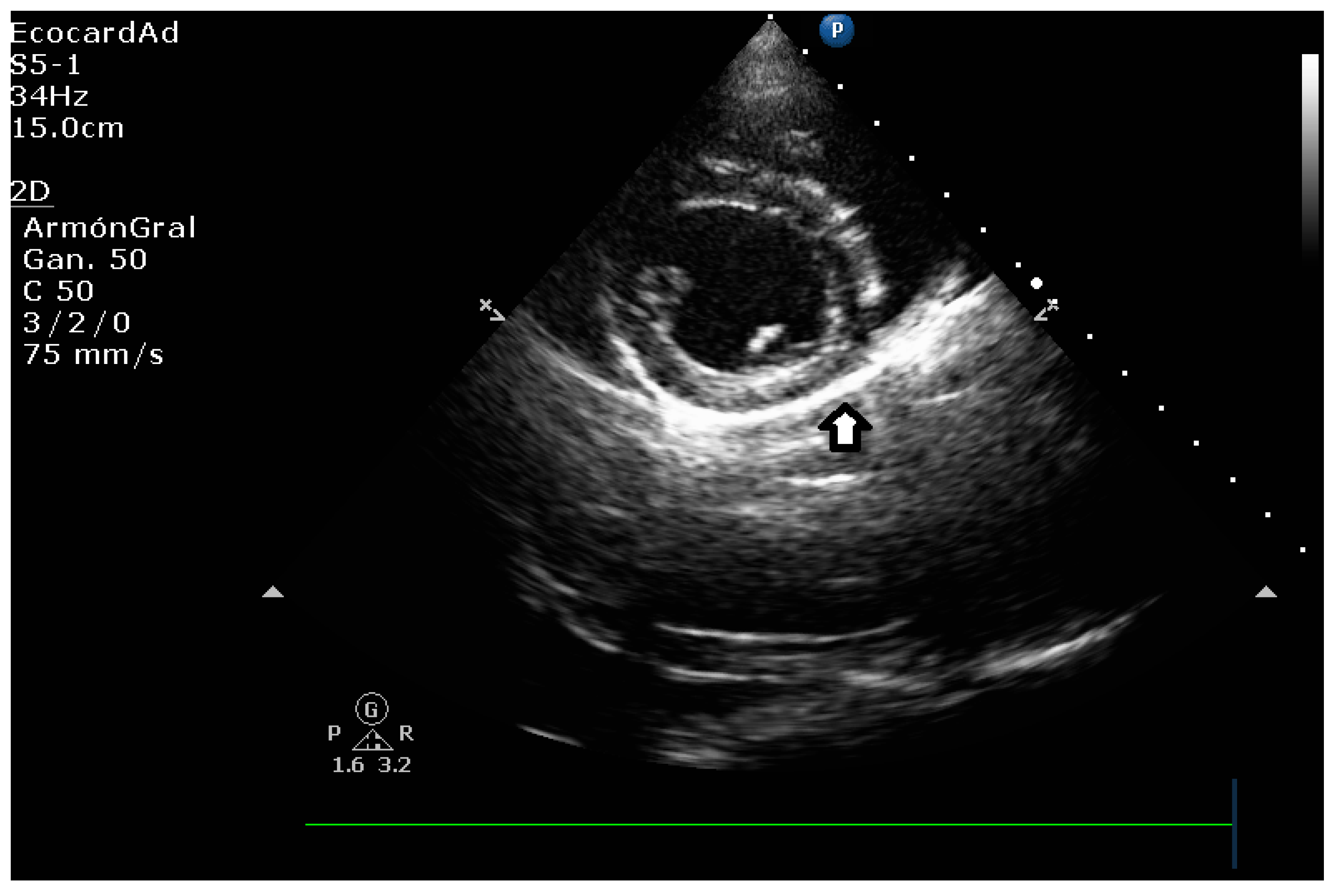
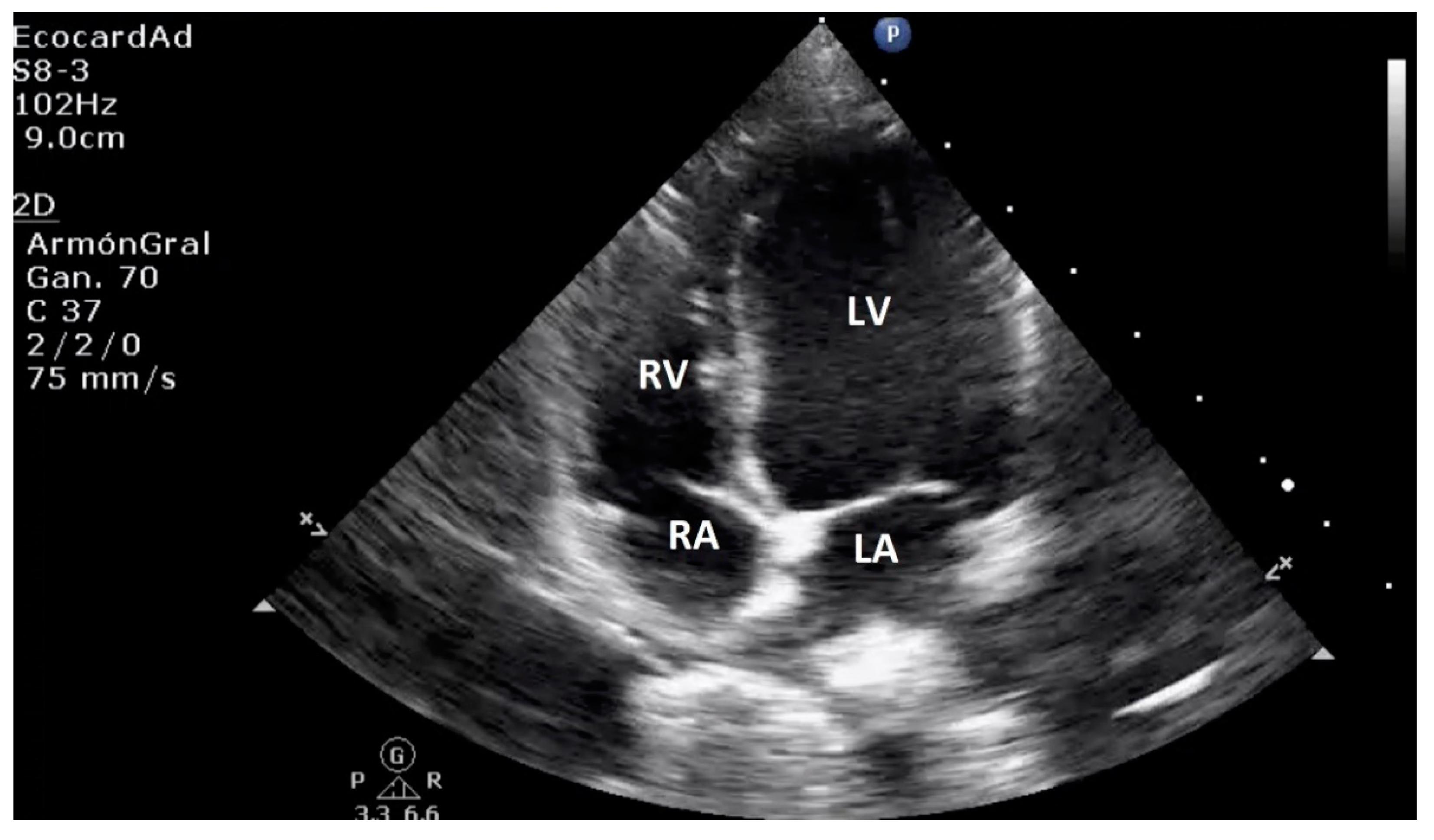
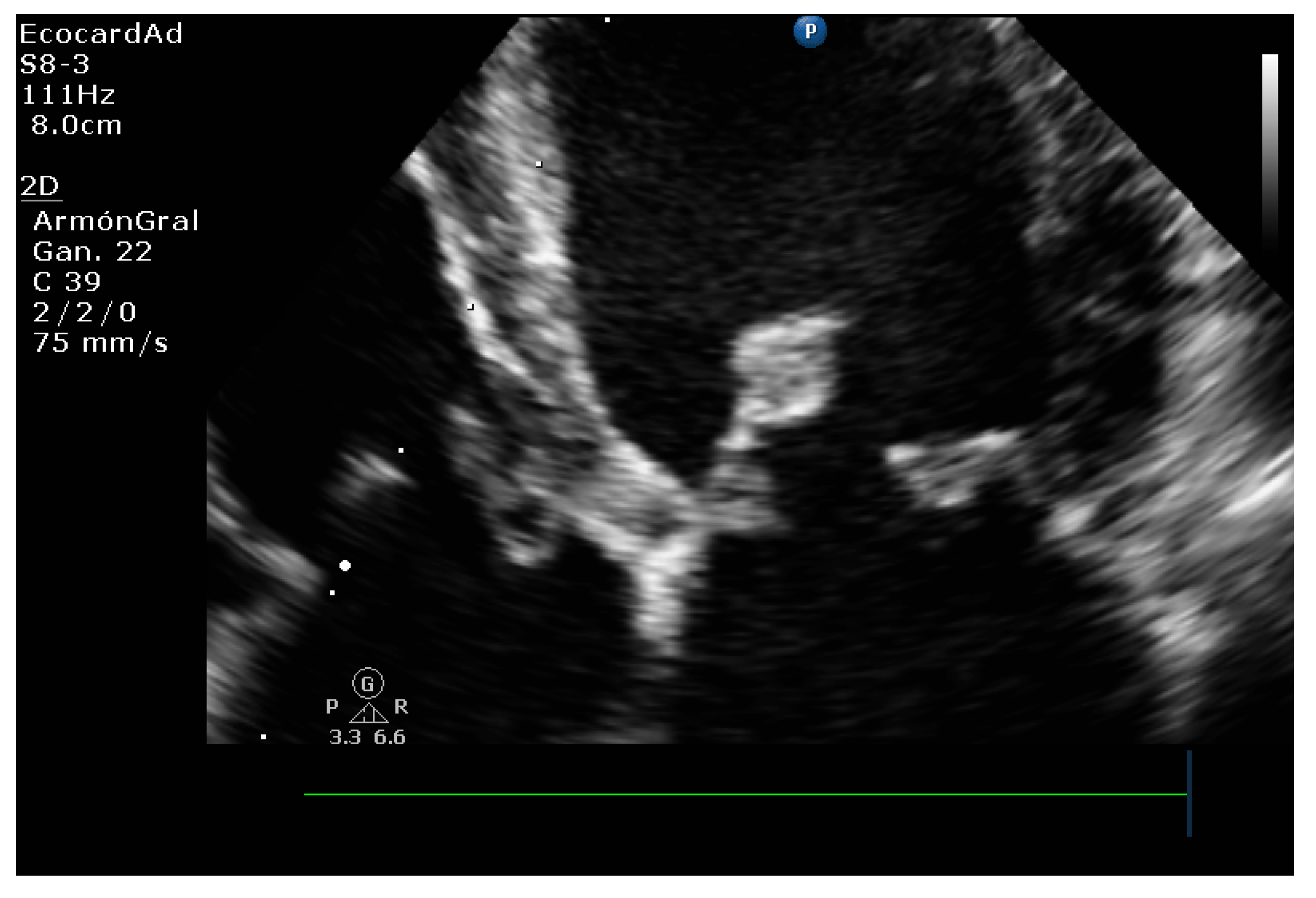

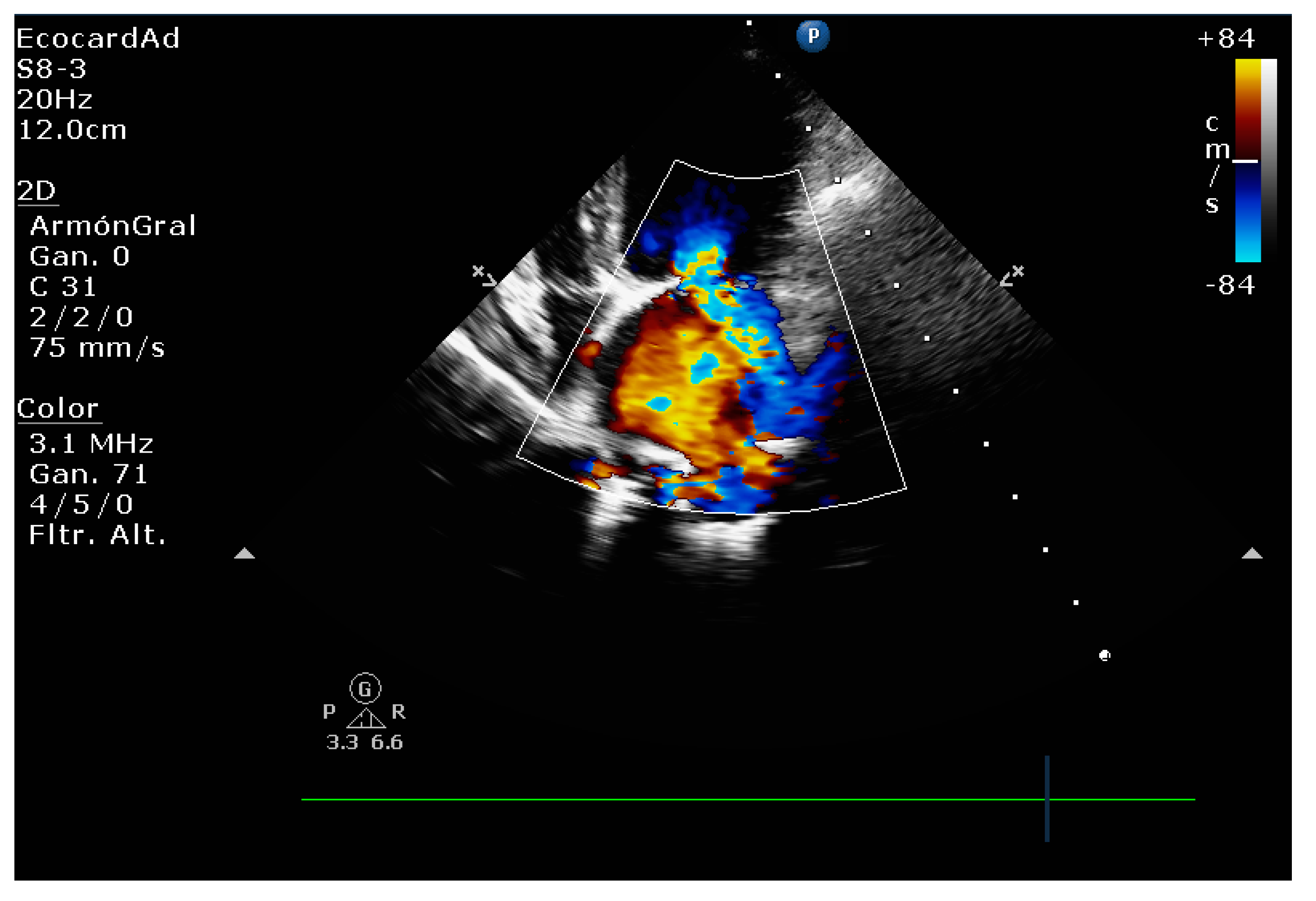





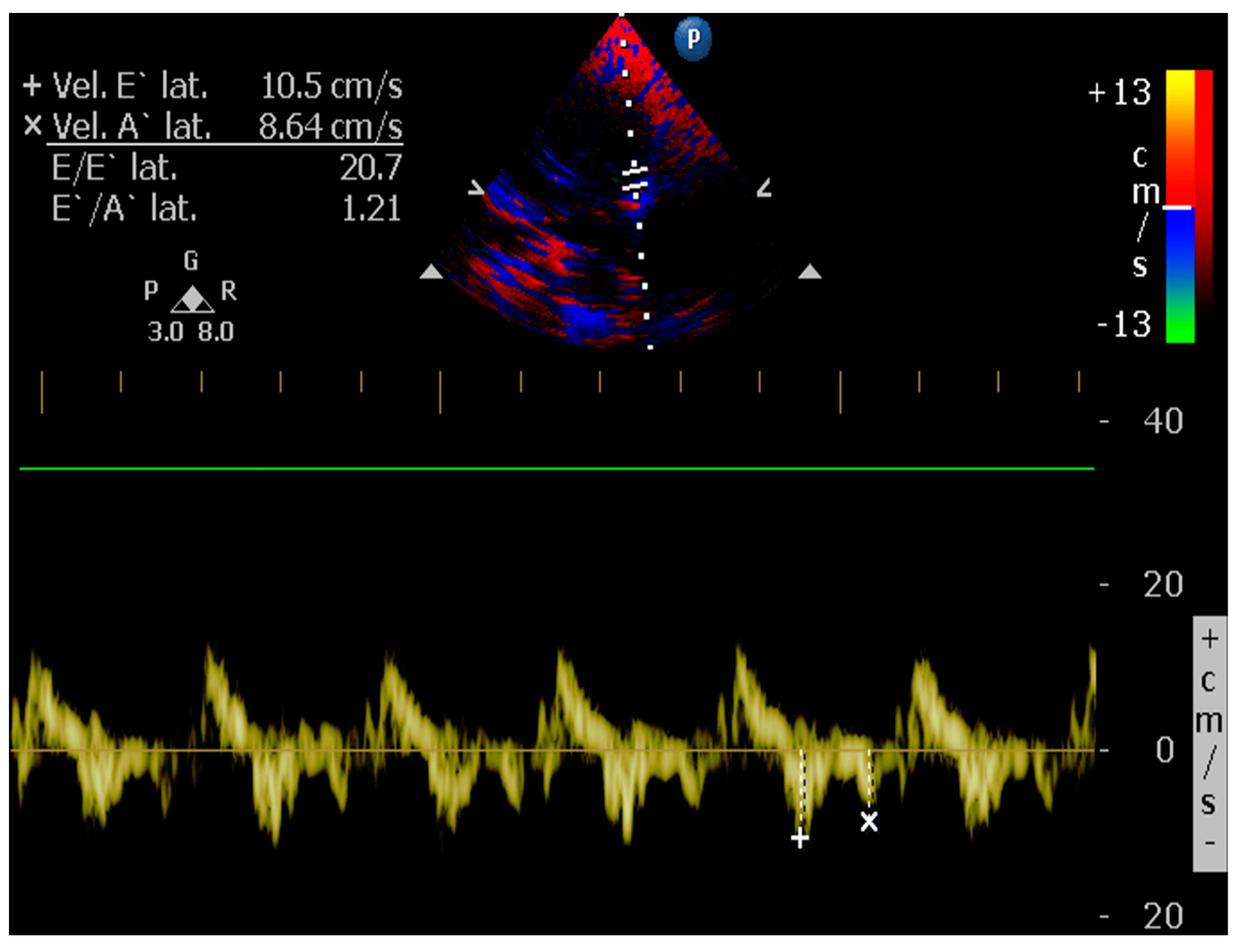
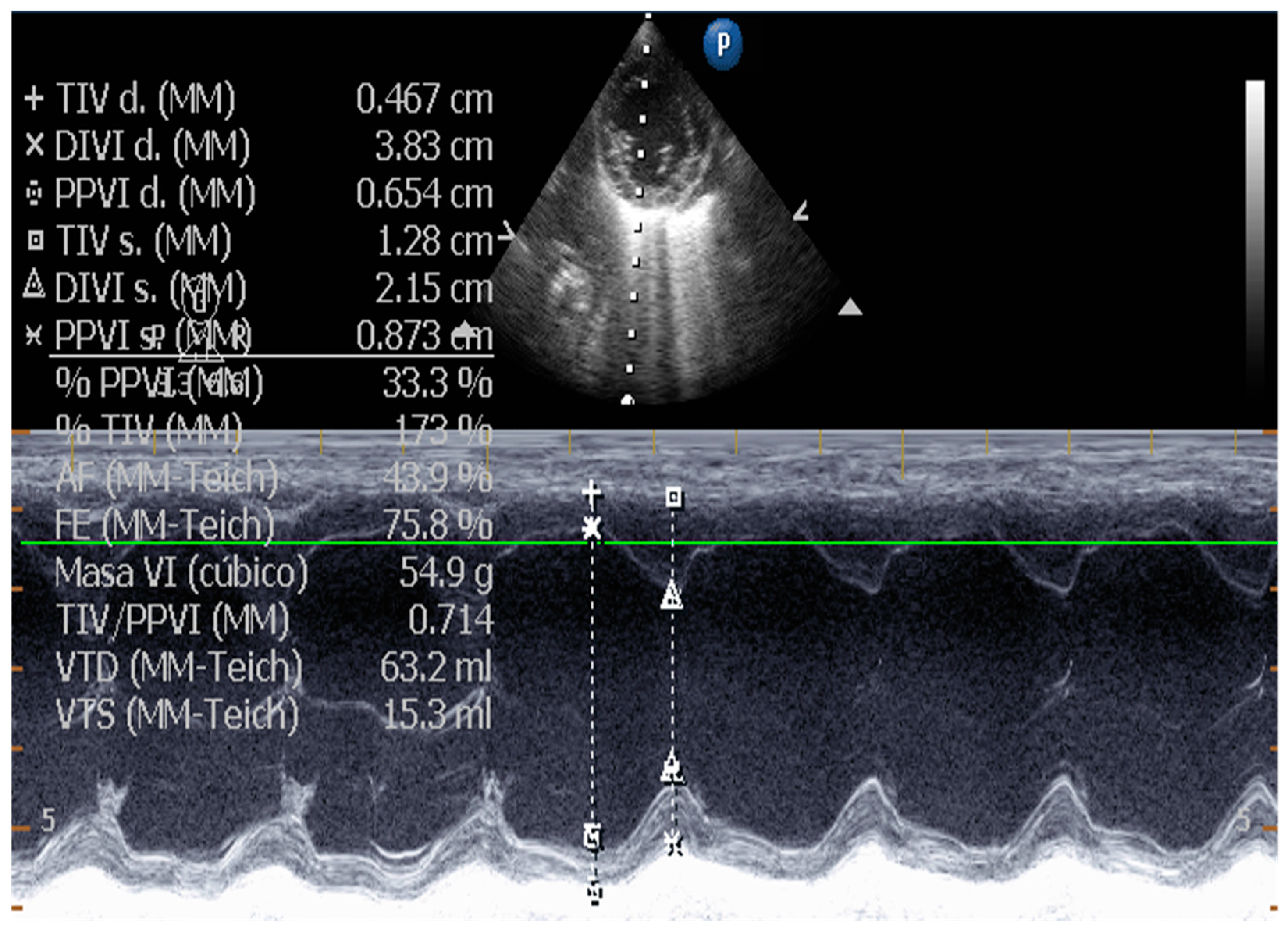
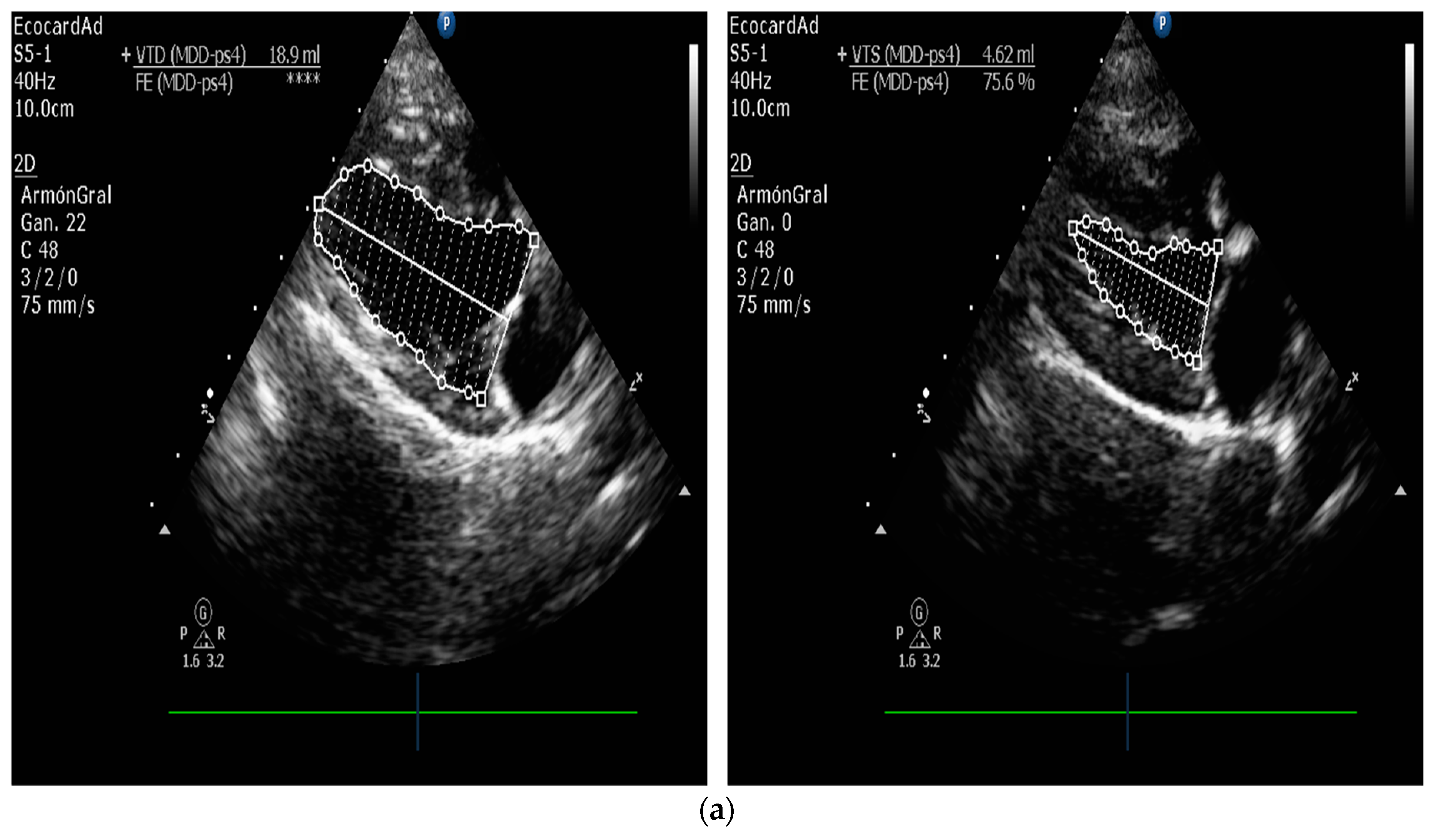


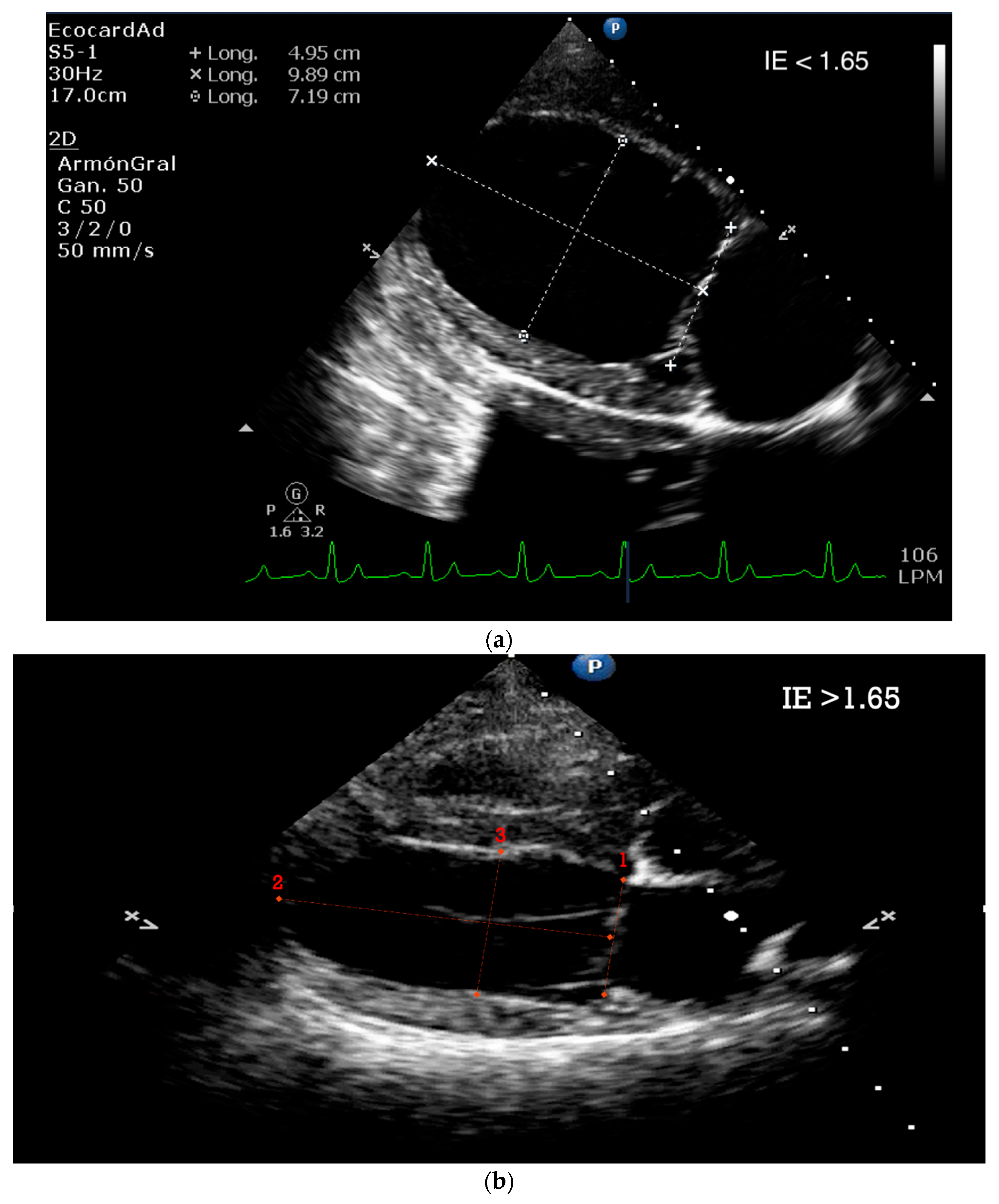
| Technique | |
|---|---|
| Echocardiographic positioning | Dogs are usually imaged in right and left lateral position. The human patient should be supine or left lateral decubitus. This will bring the heart away from the sternum. |
| Normal Basic Echocardiographic Views | Human: Parasternal long axis, parasternal short axis, apical four chamber, apical four-chamber view, subxiphoid (subcostal), suprasternal view, and IVC views. Canines: Four-chamber right-sided parasternal long-axis view, five-chamber right-sided parasternal long-axis view, right-sided short-axis view of the left ventricle at the level of the papillary muscles, right-sided short-axis view at the level of the left atrium and aorta. |
| Quantitative assessment of mitral regurgitation | Commonly used in human patients, but seldom utilized in canine patients and practical application is limited (defining EROA and flow convergence shape can be challenging) [60]. |
| Flow convergence area measurement by PISA | A standard gold method in humans, not routinely performed in cardiologic evaluation in dogs (PISA quantification showed a wide range of RF in a clinical study) [61,62]. |
| Color flow imaging of the mitral regurgitation jet area | The most commonly used technique for assessing severity in dogs. The former method is not used in humans as it is not considered reliable for determining the severity of mitral insufficiency. |
| Measurement of Left Ventricular Volume | M-mode echocardiography is widely used in canine cardiology, but its utility is debated. American Society of Echocardiography recommends against using linear measurements in the human patient. SMOD is recommended [100,102,103]. |
| Quantification of the severity of left ventricular remodeling | Canine: AI: Ao ratio, AI diameter indexed to weight, VI: Ao ratio, the normalized internal diameter of the VI at the end of normal diastole. Widely used. Human: Cardiovascular magnetic resonance is the gold standard and more accurate than echocardiography [118,119]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores Dueñas, C.A.; Cordero Yañez, I.A.; González, R.M.; Herrera Ramírez, J.C.; Montaño Gómez, M.F.; Gaxiola Camacho, S.M.; García Reynoso, I.C. Translational Echocardiography: The Dog as a Clinical Research Model of Cardiac Dysfunction. Appl. Sci. 2023, 13, 4437. https://doi.org/10.3390/app13074437
Flores Dueñas CA, Cordero Yañez IA, González RM, Herrera Ramírez JC, Montaño Gómez MF, Gaxiola Camacho SM, García Reynoso IC. Translational Echocardiography: The Dog as a Clinical Research Model of Cardiac Dysfunction. Applied Sciences. 2023; 13(7):4437. https://doi.org/10.3390/app13074437
Chicago/Turabian StyleFlores Dueñas, Cesar Augusto, Ignacio Alonso Cordero Yañez, Roberto Mujica González, José Carlomán Herrera Ramírez, Martín Francisco Montaño Gómez, Soila Maribel Gaxiola Camacho, and Issa Carolina García Reynoso. 2023. "Translational Echocardiography: The Dog as a Clinical Research Model of Cardiac Dysfunction" Applied Sciences 13, no. 7: 4437. https://doi.org/10.3390/app13074437
APA StyleFlores Dueñas, C. A., Cordero Yañez, I. A., González, R. M., Herrera Ramírez, J. C., Montaño Gómez, M. F., Gaxiola Camacho, S. M., & García Reynoso, I. C. (2023). Translational Echocardiography: The Dog as a Clinical Research Model of Cardiac Dysfunction. Applied Sciences, 13(7), 4437. https://doi.org/10.3390/app13074437





