Evaluation of Water Sorption and Solubility and FTIR Spectroscopy of Thermoplastic Orthodontic Retainer Materials Subjected to Thermoforming and Thermocycling
Abstract
Featured Application
Abstract
1. Introduction
- 1.
- There are no differences in the water sorption and solubility and surface molecular composition of the tested thermoplastic retainers before thermoforming.
- 2.
- There are no differences in the water sorption, water solubility, and surface molecular composition of the tested thermoplastic retainers after thermoforming.
- 3.
- There are no differences in the water sorption, water solubility, and surface molecular composition of the tested thermoplastic retainers after thermoforming and subsequent thermocycling.
- 4.
- Thermoforming has no statistically significant effect on the water sorption and solubility and surface molecular composition of the tested thermoplastic retainers.
- 5.
- Thermocycling has no statistically significant effect on the water sorption and solubility and surface molecular composition of the tested thermoplastic retainers.
2. Materials and Methods
2.1. Thermoforming
2.2. Thermocycling
2.3. FTIR Analysis
2.4. Water Sorption and Solubility
2.4.1. Specimen Preparation
2.4.2. Water Sorption Evaluation
2.4.3. Water Solubility Evaluation
2.5. Statistical Analysis
3. Results
3.1. Water Sorption and Solubility
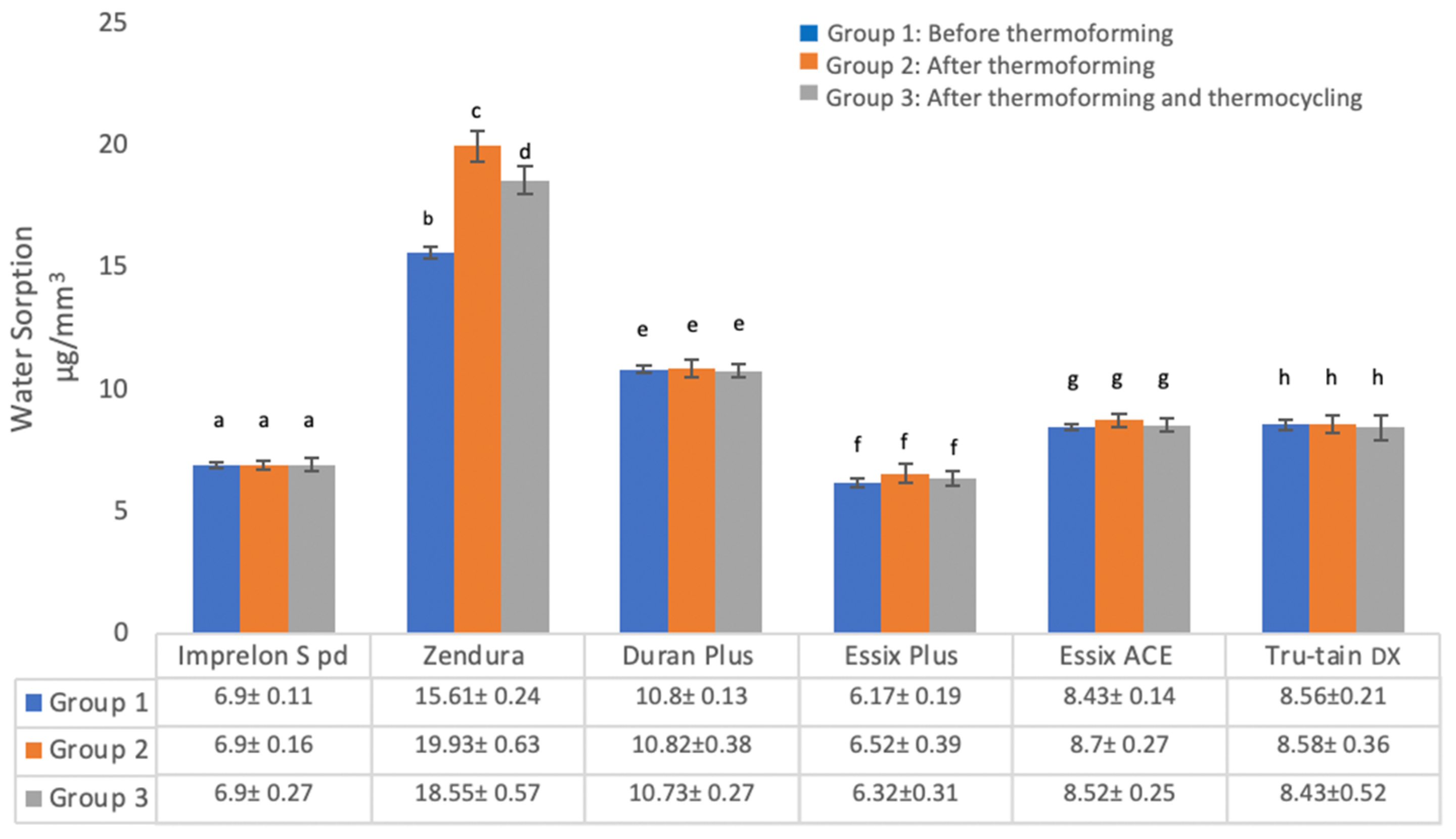
3.1.1. Water Sorption
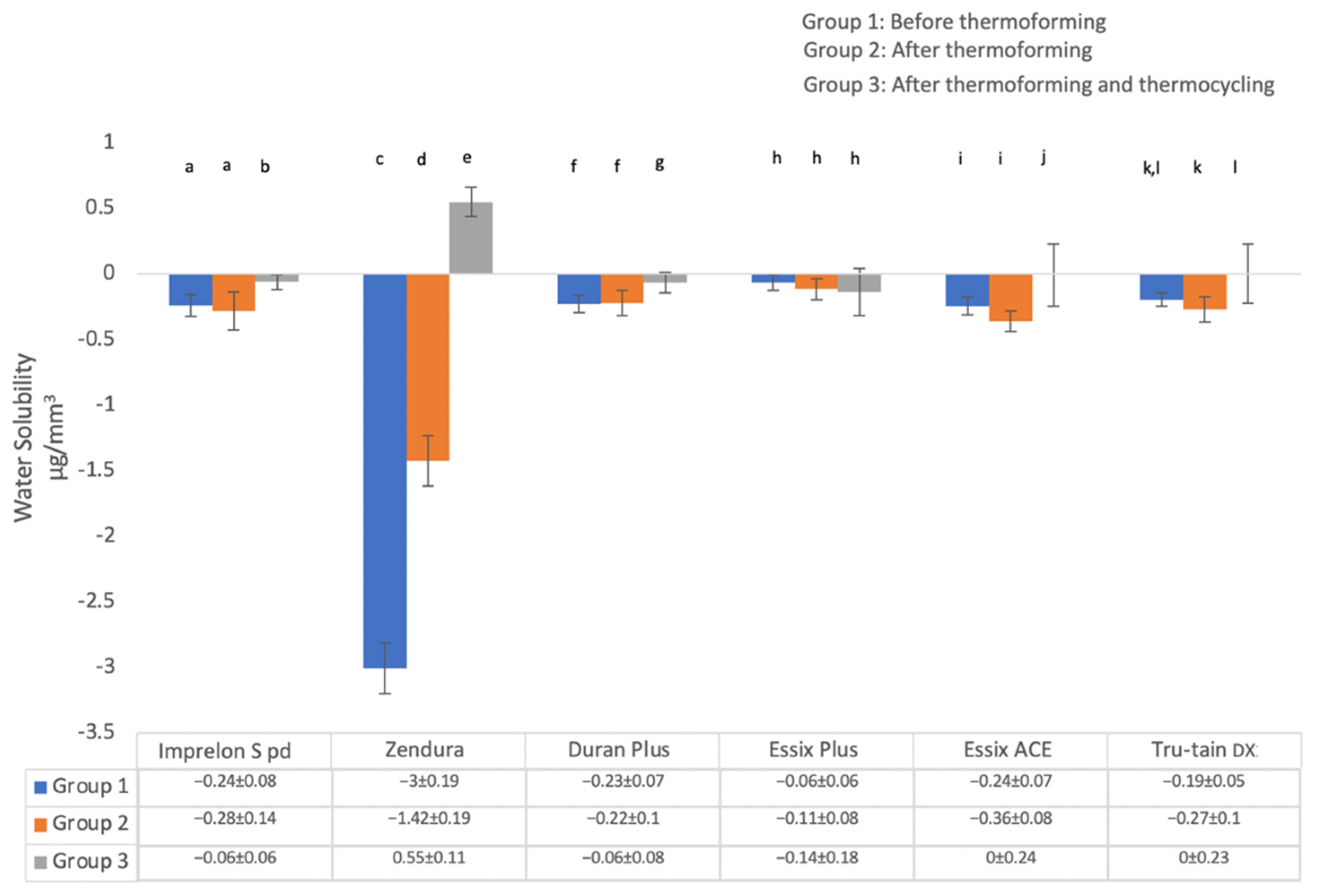
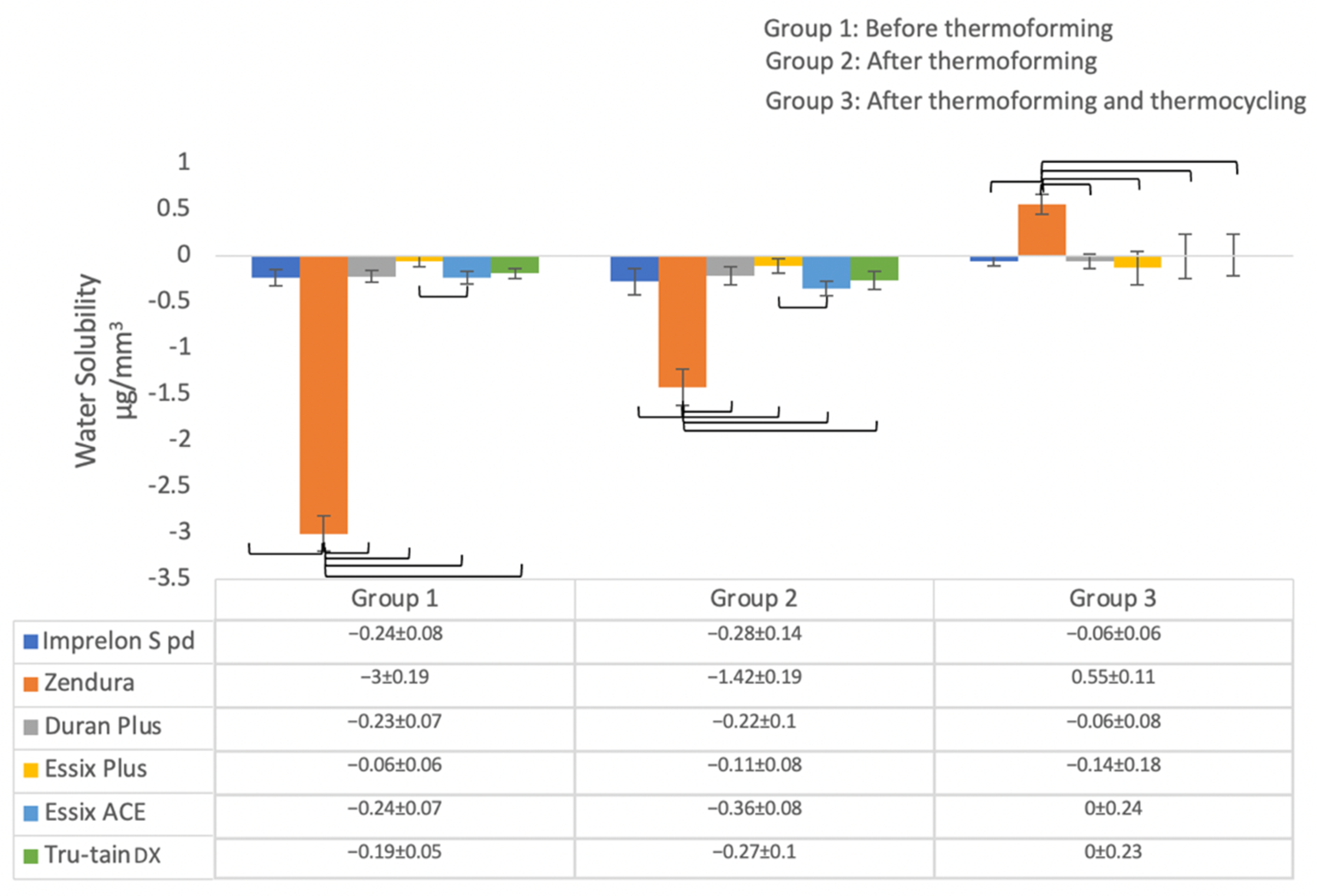
3.1.2. Water Solubility
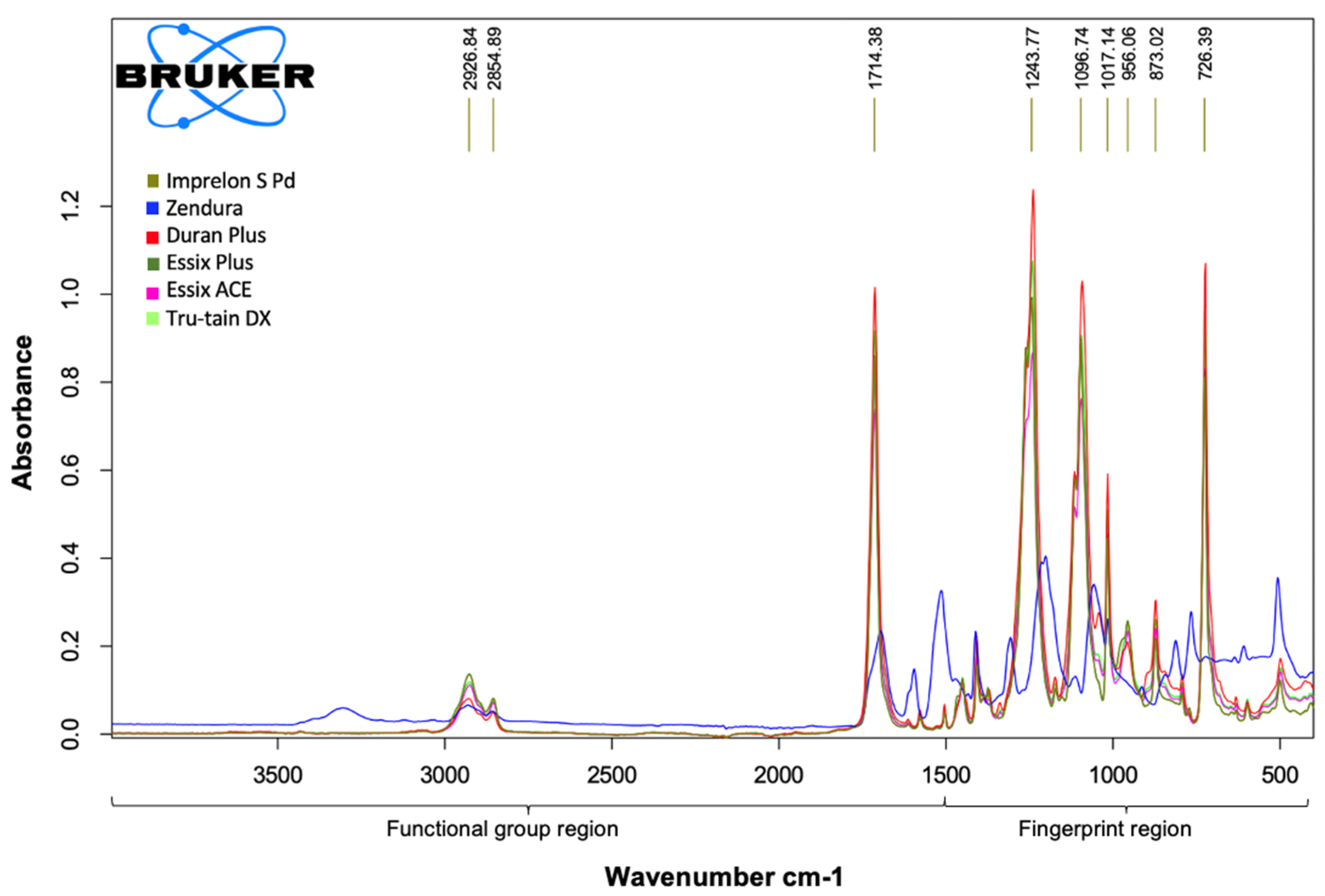
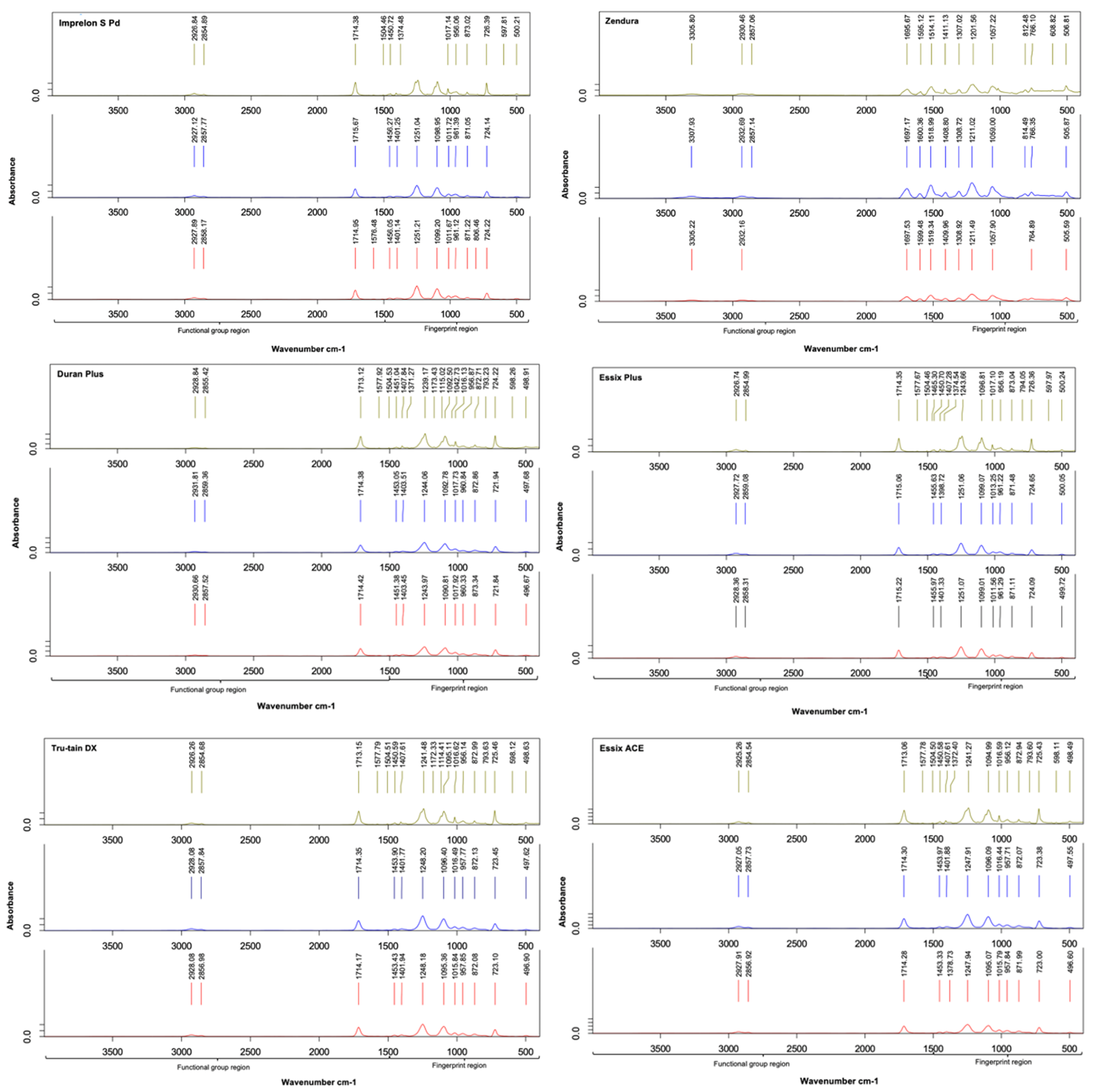
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
4. Discussion
4.1. Water Sorption and Solubility
4.2. FTIR Spectroscopy
4.3. Limitations and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Little, R.M.; Riedel, R.A.; Artun, J. An evaluation of changes in mandibular anterior alignment from 10 to 20 years postretention. Am. J. Orthod. Dentofac. Orthop. 1988, 93, 423–428. [Google Scholar] [CrossRef]
- Al-Moghrabi, D.; Johal, A.; O’Rourke, N.; Donos, N.; Pandis, N.; Gonzales-Marin, C.; Fleming, P.S. Effects of fixed vs removable orthodontic retainers on stability and periodontal health: 4-year follow-up of a randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 167–174.e1. [Google Scholar] [CrossRef]
- Kearney, M.K.; Pandis, N.; Fleming, P.S. Mixed-methods assessment of perceptions of mandibular anterior malalignment and need for orthodontic retreatment. Am. J. Orthod. Dentofac. Orthop. 2016, 150, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, S.J.; Millett, D.T.; Doubleday, B.; Bearn, D.R.; Worthington, H.V. Retention procedures for stabilising tooth position after treatment with orthodontic braces. Cochrane Database Syst. Rev. 2016, 1, CD002283. [Google Scholar] [CrossRef]
- Sheridan, J.J.; LeDoux, W.; McMinn, R. Essix retainers: Fabrication and supervision for permanent retention. J. Clin. Orthod. 1993, 27, 37–45. [Google Scholar] [PubMed]
- Hichens, L.; Rowland, H.; Williams, A.; Hollinghurst, S.; Ewings, P.; Clark, S.; Ireland, A.; Sandy, J. Cost-effectiveness and patient satisfaction: Hawley and vacuum-formed retainers. Eur. J. Orthod. 2007, 29, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Pratt, M.C.; Kluemper, G.T.; Lindstrom, A.F. Patient compliance with orthodontic retainers in the postretention phase. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Rowland, H.; Hichens, L.; Williams, A.; Hills, D.; Killingback, N.; Ewings, P.; Clark, S.; Ireland, A.J.; Sandy, J.R. The effectiveness of Hawley and vacuum-formed retainers: A single-center randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 730–737. [Google Scholar] [CrossRef]
- Horowitz, S.L.; Hixon, E.H. Physiologic recovery following orthodontic treatment. Am. J. Orthod. 1969, 55, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Little, R.M. Stability and relapse of dental arch alignment. Br. J. Orthod. 1990, 17, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Case, C. Principles of retention in orthodontia. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 352–361. [Google Scholar] [CrossRef]
- Valiathan, M.; Hughes, E. Results of a survey-based study to identify common retention practices in the United States. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 170–177; discussion 177. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Eliades, G.; Zinelis, S.; Eliades, T.; Bradley, T.G. Structural conformation and leaching from in vitro aged and retrieved Invisalign appliances. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Lindauer, S.J.; Shoff, R.C. Comparison of Essix and Hawley retainers. J. Clin. Orthod. 1998, 32, 95–97. [Google Scholar]
- Sheridan, J.J.; Armbruster, P.; Moskowitz, E.; Nguyen, P. Avoiding demineralization and bite alteration from full-coverage plastic appliances. J. Clin. Orthod. 2001, 35, 444–448. [Google Scholar] [PubMed]
- Proffit, W.; Fields, H.W.; Larson, B.; Sarver, D.M. Contemporary Orthodontics, 6th ed.; Elsevier: Philadelphia, PA, USA, 2019. [Google Scholar]
- Johal, A.; Sharma, N.R.; McLaughlin, K.; Zou, L.-F. The reliability of thermoform retainers: A laboratory-based comparative study. Eur. J. Orthod. 2015, 37, 503–507. [Google Scholar] [CrossRef]
- Gale, M.S.; Darvell, B.W. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Boubakri, A.; Haddar, N.; Elleuch, K.; Bienvenu, Y. Impact of aging conditions on mechanical properties of thermoplastic polyurethane. Mater. Des. 2010, 31, 4194–4201. [Google Scholar] [CrossRef]
- DèNève, B.; Shanahan, M.E.R. Water absorption by an epoxy resin and its effect on the mechanical properties and infra-red spectra. Polymer 1993, 34, 5099–5105. [Google Scholar] [CrossRef]
- Boubakri, A.; Elleuch, K.; Guermazi, N.; Ayedi, H. Investigations on hygrothermal aging of thermoplastic polyurethane material. Mater. Des. 2009, 30, 3958–3965. [Google Scholar] [CrossRef]
- Borges, C.S.P.; Akhavan-Safar, A.; Marques, E.A.S.; Carbas, R.J.C.; Ueffing, C.; Weißgraeber, P.; da Silva, L.F.M. Effect of Water Ingress on the Mechanical and Chemical Properties of Polybutylene Terephthalate Reinforced with Glass Fibers. Materials 2021, 14, 1261. [Google Scholar] [CrossRef] [PubMed]
- Meade, M.J.; Millett, D. Retention protocols and use of vacuum-formed retainers among specialist orthodontists. J. Orthod. 2013, 40, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Jaggy, F.; Zinelis, S.; Polychronis, G.; Patcas, R.; Schätzle, M.; Eliades, G.; Eliades, T. ATR-FTIR Analysis and One-Week Stress Relaxation of Four Orthodontic Aligner Materials. Materials 2020, 13, 1868. [Google Scholar] [CrossRef]
- ISO 20795-2:2013; Dentistry-base polymers-Part 2:orthodontic base polymers. ISO: Geneva, Switzerland, 2013.
- Daniele, V.; Macera, L.; Taglieri, G.; Di Giambattista, A.; Spagnoli, G.; Massaria, A.; Messori, M.; Quagliarini, E.; Chiappini, G.; Campanella, V.; et al. Thermoplastic Disks Used for Commercial Orthodontic Aligners: Complete Physicochemical and Mechanical Characterization. Materials 2020, 13, 2386. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Kwon, J.-S.; Jiang, H.B.; Cha, J.-Y.; Kim, K.-M. Effects of thermoforming on the physical and mechanical properties of thermoplastic materials for transparent orthodontic aligners. Korean J. Orthod. 2018, 48, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, B.A.; Willmann, J.H.; Nimer, A.; Drescher, D. Effect of in vitro aging by water immersion and thermocycling on the mechanical properties of PETG aligner material. J. Orofac. Orthop. 2019, 80, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Zhang, N.; Chen, H.; Bai, Y. Dynamic stress relaxation of orthodontic thermoplastic materials in a simulated oral environment. Dent. Mater. J. 2013, 32, 946–951. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]




| Material Name | Manufacturer | Composition |
|---|---|---|
| Zendura Item #9169 | Bay Materials LLC, Fremont, CA, USA | Polyurethane (PU) |
| Essix Plus | Dentsply Raintree Essix, Bradenton, FL, USA | Copolyester |
| Tru-Tain DX | Tru-Tain, Minnesota, USA | Copolyester |
| Duran Plus | Scheu-Dental GmbH, Iserlohn, Germany | Polyethylene terephthalate glycol (PETG) |
| Essix ACE | Dentsply Raintree Essix, Bradenton, Fla, USA | Copolyester (of polyethylene terephthalate) |
| Imprelon S pd | Scheu-Dental GmbH, Iserlohn, Germany | Copolyester |
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected Model | 1720.8 a | 17 | 101.23 | 907.82 | 0 |
| Intercept | 10,485 | 1 | 10,484.98 | 94,032.67 | 0 |
| Group | 12.56 | 2 | 6.28 | 56.3 | 0 |
| Material | 1661.6 | 5 | 332.32 | 2980.35 | 0 |
| Group × Material | 46.7 | 10 | 4.67 | 41.87 | 0 |
| Error | 10 | 90 | 0.112 | ||
| Total | 12,215.85 | 108 | |||
| Corrected Total | 1730.87 | 107 |
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected Model | 58.25 a | 17 | 3.43 | 199.14 | 0 |
| Intercept | 13.47 | 1 | 13.47 | 782.92 | 0 |
| Group | 9.53 | 2 | 4.76 | 276.78 | 0 |
| Material | 19.21 | 5 | 3.84 | 223.23 | 0 |
| Group × Material | 29.52 | 10 | 2.95 | 171.57 | 0 |
| Error | 1.55 | 90 | 0.02 | ||
| Total | 73.27 | 108 | |||
| Corrected Total | 59.8 | 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albilali, A.T.; Baras, B.H.; Aldosari, M.A. Evaluation of Water Sorption and Solubility and FTIR Spectroscopy of Thermoplastic Orthodontic Retainer Materials Subjected to Thermoforming and Thermocycling. Appl. Sci. 2023, 13, 5165. https://doi.org/10.3390/app13085165
Albilali AT, Baras BH, Aldosari MA. Evaluation of Water Sorption and Solubility and FTIR Spectroscopy of Thermoplastic Orthodontic Retainer Materials Subjected to Thermoforming and Thermocycling. Applied Sciences. 2023; 13(8):5165. https://doi.org/10.3390/app13085165
Chicago/Turabian StyleAlbilali, Alaa T., Bashayer H. Baras, and Mohammad A. Aldosari. 2023. "Evaluation of Water Sorption and Solubility and FTIR Spectroscopy of Thermoplastic Orthodontic Retainer Materials Subjected to Thermoforming and Thermocycling" Applied Sciences 13, no. 8: 5165. https://doi.org/10.3390/app13085165
APA StyleAlbilali, A. T., Baras, B. H., & Aldosari, M. A. (2023). Evaluation of Water Sorption and Solubility and FTIR Spectroscopy of Thermoplastic Orthodontic Retainer Materials Subjected to Thermoforming and Thermocycling. Applied Sciences, 13(8), 5165. https://doi.org/10.3390/app13085165





