Synergistic Antimicrobial Effect of Photodynamic Inactivation and SWEEPS in Combined Treatment against Enterococcus faecalis in a Root Canal Biofilm Model: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photosensitizer and Light Source
2.2. Preparation of Tooth Specimens
2.3. Root Canal Infection with E. faecalis
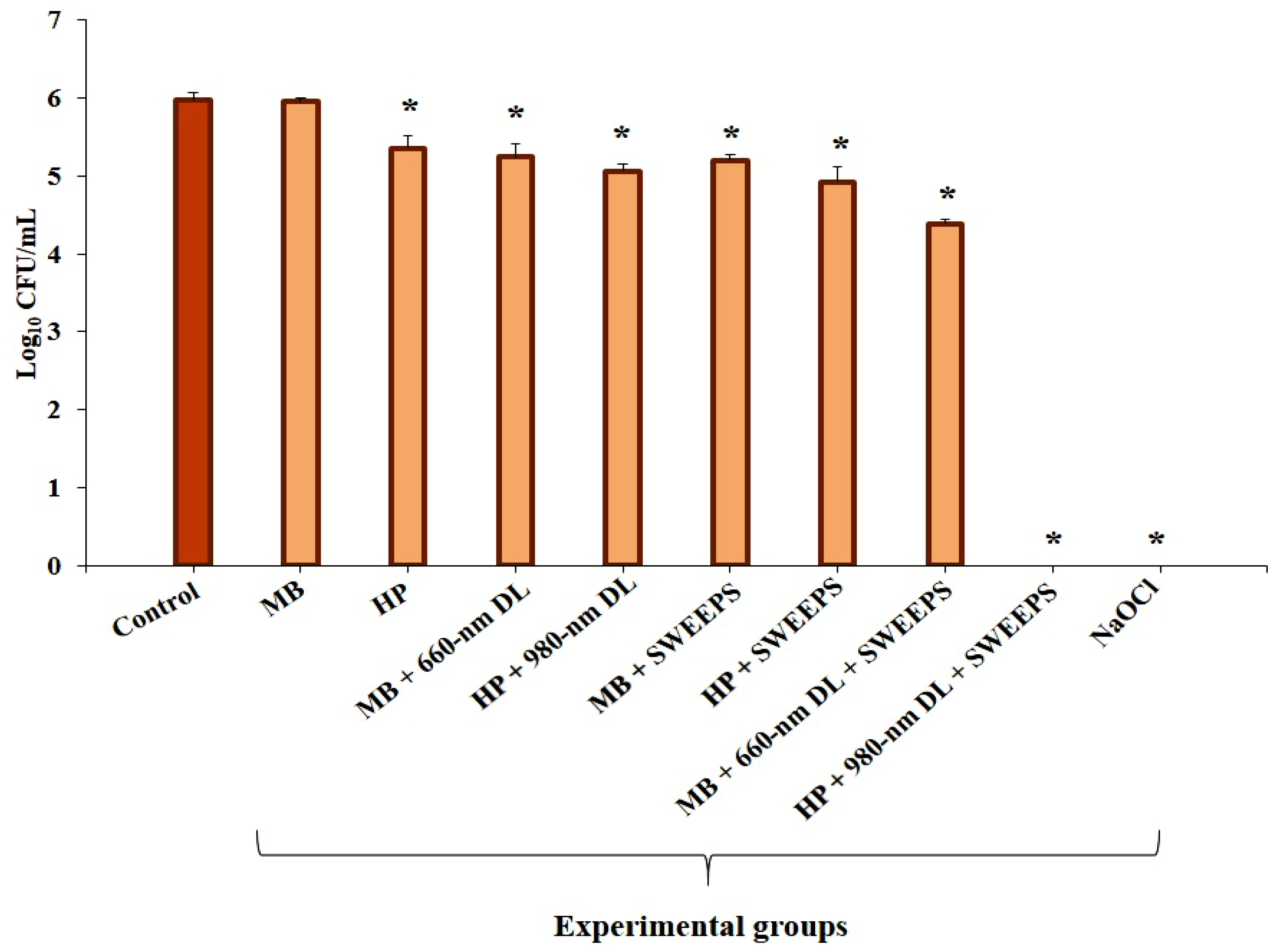
2.4. Experimental Design
- A.
- MB (0.1 mg/mL): The root canals were filled with 5 μL MB, followed by dark incubation for 5 min at 25 °C.
- B.
- 3% HP: The root canals were filled with 5 μL HP, followed by dark incubation for 2 min at 25 °C [30].
- C.
- MB (0.1 μg/mL) + 660 nm diode laser: The root canals were treated with MB similar to group A, and the test samples were then exposed to a 660 nm diode laser for 60 s.
- D.
- 3% HP + 980 nm diode laser: The root canals were treated by HP similar to group B and the test samples were then exposed to a 980 nm diode laser for 30 s.
- E.
- MB (100 μg/mL) + SWEEPS: The root canals were treated by MB similar to group A, and the test samples were then irradiated with Er:YAG laser with 2940 nm wavelength. The device tip (SWEEPS600, Fotona, Slovenia) was placed in the pulp chamber and activated for 90 s (30 s of activation, 30 s of rest, and 30 s of activation again).
- F.
- 3% HP + SWEEPS: The root canals were treated by HP similar to group B and the test samples were then subjected to SWEEPS technique similar to group E.
- G.
- MB (100 μg/mL) + 660 nm diode laser + SWEEPS: The root canals were treated by MB-mediated SWEEPS similar to group E, and then after 5 min, 660 nm diode laser irradiation was performed similar to group C.
- H.
- 3% HP + 980 nm diode laser + SWEEPS: The root canals were treated by HP-mediated SWEEPS similar to group F, and then after 5 min, 980 nm diode laser irradiation was performed similar to group D.
- I.
- 5.25% NaOCl (positive control): The root canals were filled with 5 μL NaOCl, followed by incubation for 5 min at 25 °C.
- J.
- Normal saline (negative control): The root canals were filled with 5 μL normal saline, followed by incubation for 5 min at 25 °C.

3. Evaluation of Antibacterial Activity
Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siqueira, J.F., Jr.; Rôças, I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 2008, 34, 1291–1301.e3. [Google Scholar] [CrossRef] [PubMed]
- Al Yahya, R.S.; Al Attas, M.H.; Javed, M.Q.; Khan, K.I.; Atique, S.; Abulhamael, A.M.; Bahammam, H.A. Root Canal Configuration and Its Relationship with Endodontic Technical Errors and Periapical Status in Premolar Teeth of a Saudi Sub-Population: A Cross-Sectional Observational CBCT Study. Int. J. Environ Res. Public Health 2023, 20, 1142. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, T.; Yahata, Y.; Handa, K.; Nakano, M.; Suzuki, S.; Kakiuchi, Y.; Tanaka, T.; Kanehira, M.; Venkataiah, V.S.; Saito, M. Er: YAG laser-induced cavitation can activate irrigation for the removal of intraradicular biofilm. Sci. Rep. 2022, 12, 4897. [Google Scholar] [CrossRef] [PubMed]
- Ørstavik, D. Endodontic Treatment of Apical Periodontitis. In Essential Endodontology, 3rd ed.; Ørstavik, D., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 313–343. [Google Scholar] [CrossRef]
- Duggan, J.M.; Sedgley, C.M. Biofilm formation of oral and endodontic Enterococcus faecalis. J. Endod. 2007, 33, 815–818. [Google Scholar] [CrossRef]
- Kishen, A.; George, S.; Kumar, R. Enterococcus faecalis-mediated biomineralized biofilm formation on root canal dentine in vitro. J. Biomed. Mater. Res. A 2006, 77, 406–415. [Google Scholar] [CrossRef]
- Mozo, S.; Llena, C.; Forner, L. Review of ultrasonic irrigation in endodontics: Increasing action of irrigating solutions. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e512. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef]
- Khan, A.M.; Gangoo, I.K.A.; Ali, N.A.; Khan, M.; Javed, M.Q.; AlAttas, M.H.; Abulhamael, A.M.; Bahammam, H.A.; Alsofi, L.; Yahya, R.S.A. The Effect of Calcium Hydroxide, Triple Antibiotic Paste and Chlorhexidine on Pain in Teeth with Symptomatic Apical Periodontitis: A Randomised Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 3091. [Google Scholar] [CrossRef]
- Garcez, A.S.; Ribeiro, M.S.; Tegos, G.P.; Nunez, S.C.; Jorge, A.O.; Hamblin, M.R. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg. Med. 2007, 39, 59–66. [Google Scholar] [CrossRef]
- George, S.; Kishen, A. Photophysical, photochemical, and photobiological characterization of methylene blue formulations for light-activated root canal disinfection. J. Biomed. Opt. 2007, 12, 034029. [Google Scholar] [CrossRef]
- Garcez, A.S.; Hamblin, M.R. Methylene blue and hydrogen peroxide for photodynamic inactivation in root canal-a new protocol for use in endodontics. Eur. Endod. J. 2017, 2, 29. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Kishen, A. Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochem. Photobiol. Sci. 2009, 8, 788–795. [Google Scholar] [CrossRef]
- Komine, C.; Tsujimoto, Y. A small amount of singlet oxygen generated via excited methylene blue by photodynamic therapy induces the sterilization of Enterococcus faecalis. J. Endod. 2013, 39, 411–414. [Google Scholar] [CrossRef]
- Karaoğlu, G.E.; Erdönmez, D.; Göl, C.; Durmuş, M. Efficacy of antimicrobial photodynamic therapy administered using methylene blue, toluidine blue and tetra 2-mercaptopyridine substituted zinc phthalocyanine in root canals contaminated with Enterococcusaecalis. Photodiagn. Photodyn. Ther. 2020, 32, 102038. [Google Scholar] [CrossRef]
- Costa, L.M.; De Souza Matos, F.; De Oliveira Correia, A.M.; Carvalho, N.C.; Faria-e-Silva, A.L.; Paranhos, L.R.; Ribeiro, M.A.G. Tooth color change caused by photosensitizers after photodynamic therapy: An in vitro study. J. Photochem. Photobiol. B 2016, 160, 225–228. [Google Scholar] [CrossRef]
- Ramalho, K.M.; Cunha, S.R.; Mayer-Santos, E.; De Paula Eduardo, C.; De Freitas, P.M.; Aranha, A.C.C.; Moura-Netto, C. In vitro evaluation of methylene blue removal from root canal after Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2017, 20, 248–252. [Google Scholar] [CrossRef]
- Uekubo, A.; Hiratsuka, K.; Aoki, A.; Takeuchi, Y.; Abiko, Y.; Izumi, Y. Effect of antimicrobial photodynamic therapy using rose bengal and blue light-emitting diode on Porphyromonas gingivalis in vitro: Influence of oxygen during treatment. Laser Ther. 2016, 25, 299–308. [Google Scholar] [CrossRef]
- Nie, M.; Silva, R.C.e.; De Oliveira, K.T.; Bagnato, V.S.; De Souza Rastelli, A.N.; Crielaard, W.; Yang, J.; Deng, D. Synergetic antimicrobial effect of chlorin e6 and hydrogen peroxide on multi-species biofilms. Biofouling 2021, 37, 656–665. [Google Scholar] [CrossRef]
- Caccianiga, G.; Rey, G.; Caccianiga, P.; Leonida, A.; Baldoni, M.; Baldoni, A.; Ceraulo, S. Rough Dental Implant Surfaces and Peri-Implantitis: Role of Phase-Contrast Microscopy, Laser Protocols, and Modified Home Oral Hygiene in Maintenance. A 10-Year Retrospective Study. Appl. Sci. 2021, 11, 4985. [Google Scholar] [CrossRef]
- Cai, Z.; Li, Y.; Wang, Y.; Chen, S.; Jiang, S.; Ge, H.; Lei, L.; Huang, X. Antimicrobial effects of photodynamic therapy with antiseptics on Staphylococcus aureus biofilm on titanium surface. Photodiagn. Photodyn. Ther. 2019, 25, 382–388. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Chiniforush, N. An in vitro study on the efficacy of hydrogen peroxide mediated high-power photodynamic therapy affecting Enterococcus faecalis biofilm formation and dispersal. Photodiagn. Photodyn. Ther. 2023, 41, 103310. [Google Scholar] [CrossRef] [PubMed]
- Sales, L.S.; Guimaraes, G.N.; Wijesinghe, G.K.; Moreira, K.M.S.; Joia, F.; Stipp, R.N.; Rodrigues, L.; Nobre-Dos-Santos, M.; Steiner-Oliveira, C. Addition of hydrogen peroxide to methylene blue conjugated to β-cyclodextrin in photodynamic antimicrobial chemotherapy in S. mutans biofilm. Photodiagn. Photodyn. Ther. 2019, 28, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Caccianiga, G.; Rey, G.; Caccianiga, P.; Leonida, A.; Baldoni, M.; Baldoni, A.; Ceraulo, S. Laser management of peri-implantitis: A comparison between photodynamic therapy combined with hydrogen peroxide (OHLLT) and OHLLT+ Er: YAG laser. A retrospective controlled study. Appl. Sci. 2021, 11, 6771. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirato, M.; Tenkumo, T.; Kanno, T.; Westerlund, A.; Örtengren, U.; Sasaki, K.; Niwano, Y. Hydroxyl radicals generated by hydrogen peroxide photolysis recondition biofilm-contaminated titanium surfaces for subsequent osteoblastic cell proliferation. Sci. Rep. 2019, 9, 4688. [Google Scholar] [CrossRef]
- Foschi, F.; Fontana, C.R.; Ruggiero, K.; Riahi, R.; Vera, A.; Doukas, A.G.; Pagonis, T.C.; Kent, R.; Stashenko, P.P.; Soukos, N.S. Photodynamic inactivation of Enterococcus faecalis in dental root canals in vitro. Lasers Surg. Med. 2007, 39, 782–787. [Google Scholar] [CrossRef]
- Lukac, N.; Muc, B.T.; Jezersek, M.; Lukac, M. Photoacoustic endodontics using the novel SWEEPS Er: YAG laser modality. J. Laser Health Acad. 2017, 1, 1–7. [Google Scholar]
- Matsumoto, H.; Yoshimine, Y.; Akamine, A. Visualization of irrigant flow and cavitation induced by Er: YAG laser within a root canal model. J. Endod. 2011, 37, 839–843. [Google Scholar] [CrossRef]
- Arıcıoğlu, B.; Çıkman, A.Ş.; Babacan, M. The comparison of cleaning efficacy and apical extrusion of advanced irrigation activation methods with a novel Er: YAG laser modality: Sweeps. Lasers Dent. Sci. 2021, 5, 43–52. [Google Scholar] [CrossRef]
- Caccianiga, G.; Rey, G.; Baldoni, M.; Caccianiga, P.; Baldoni, A.; Ceraulo, S. Periodontal decontamination induced by light and not by heat: Comparison between oxygen high level laser therapy (OHLLT) and LANAP. Appl. Sci. 2021, 11, 4629. [Google Scholar] [CrossRef]
- Korkut, E.; Torlak, E.; Gezgin, O.; Özer, H.; Şener, Y. Antibacterial and smear layer removal efficacy of Er: YAG laser irradiation by photon-induced photoacoustic streaming in primary molar root canals: A preliminary study. Photomed. Laser Surg. 2018, 36, 480–486. [Google Scholar] [CrossRef]
- Zhu, X.; Yin, X.; Chang, J.W.; Wang, Y.; Cheung, G.S.; Zhang, C. Comparison of the antibacterial effect and smear layer removal using photon-initiated photoacoustic streaming aided irrigation versus a conventional irrigation in single-rooted canals: An in vitro study. Photomed. Laser Surg. 2013, 31, 371–377. [Google Scholar] [CrossRef]
- Dewi, A.; Upara, C.; Chaiariyakul, D.; Louwakul, P. Smear Layer Removal from Root Canal Dentin and Antimicrobial Effect of Citric Acid-modified Chlorhexidine. Eur. Endod. J. 2020, 5, 257–263. [Google Scholar] [CrossRef]
- Sebbane, N.; Steinberg, D.; Keinan, D.; Sionov, R.V.; Farber, A.; Sahar-Helft, S. Antibacterial Effect of Er:YAG Laser Irradiation Applied by a New Side-Firing Spiral Tip on Enterococcus faecalis Biofilm in the Tooth Root Canal—An Ex Vivo Study. Appl. Sci. 2022, 12, 12656. [Google Scholar] [CrossRef]
- Kasić, S.; Knezović, M.; Beader, N.; Gabrić, D.; Malčić, A.I.; Baraba, A. Efficacy of three different lasers on eradication of Enterococcus faecalis and Candida albicans biofilms in root canal system. Photomed. Laser Surg. 2017, 35, 372–377. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Murray, P.E.; Ordinola-Zapata, R.; Peters, O.A.; Rôças, I.N.; Siqueira, J.F., Jr.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Camilleri, J.; et al. PRILE 2021 guidelines for reporting laboratory studies in Endodontology: A consensus-based development. Int. Endod. J. 2021, 54, 1482–1490. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N.; Favieri, A.; Lima, K.C. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J. Endod. 2000, 26, 331–334. [Google Scholar] [CrossRef]
- Van Der Waal, S.; Connert, T.; Crielaard, W.; De Soet, J. In mixed biofilms Enterococcus faecalis benefits from a calcium hydroxide challenge and culturing. Int. Endod. J. 2016, 49, 865–873. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr. Biofilms and apical periodontitis: Study of prevalence and association with clinical and histopathologic findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef]
- Ensafi, F.; Fazlyab, M.; Chiniforush, N.; Akhavan, H. Comparative effects of SWEEPS technique and antimicrobial photodynamic therapy by using curcumin and nano-curcumin on Enterococcus faecalis biofilm in root canal treatment. Photodiagn. Photodyn. Ther. 2022, 40, 103130. [Google Scholar] [CrossRef]
- Müller, P.; Guggenheim, B.; Schmidlin, P.R. Efficacy of gasiform ozone and photodynamic therapy on a multispecies oral biofilm in vitro. Eur. J. Oral Sci. 2007, 115, 77–80. [Google Scholar] [CrossRef]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, J.; Huang, S.; Wang, S.; Wang, Y.; Cai, Z.; Lei, L.; Huang, X. Hydrogen peroxide potentiates antimicrobial photodynamic therapy in eliminating Candida albicans and Streptococcus mutans dual-species biofilm from denture base. Photodiagn. Photodyn. Ther. 2022, 37, 102691. [Google Scholar] [CrossRef] [PubMed]
- Caccianiga, G.; Baldoni, M.; Ghisalberti, C.A.; Paiusco, A. A preliminary in vitro study on the efficacy of high-power photodynamic therapy (HLLT): Comparison between pulsed diode lasers and superpulsed diode lasers and impact of hydrogen peroxide with controlled stabilization. Biomed. Res. Int. 2016, 2016, 1386158. [Google Scholar] [CrossRef] [PubMed]
- Caccianiga, G.; Rey, G.; Paiusco, A.; Lauritano, D.; Cura, F.; Ormianer, Z.; Carinci, F. Oxygen high level laser therapy is efficient in treatment of chronic periodontitis: A clinical and microbiological study using PCR analysis. J. Biol. Regul. Homeost. Agents 2016, 30, 87–97. [Google Scholar]
- Caccianiga, G.; Rey, G.; Fumagalli, T.; Cambini, A.; Denotti, G.; Giacomello, M. Photodynamic therapy (association diode laser/hydrogen peroxide): Evaluation of bactericidal effects on periodontopathy bacteria: An in vitro study. Eur. J. Inflamm. 2012, 10, 101–106. [Google Scholar] [CrossRef]
- Jezeršek, M.; Lukač, N.; Lukač, M. Measurement of simulated debris removal rates in an artificial root canal to optimize laser-activated irrigation parameters. Lasers Surg. Med. 2021, 53, 411–417. [Google Scholar] [CrossRef]
- Wang, X.-N.; Shi, J. Shock wave-enhanced emission photoacoustic streaming versus photon-induced photoacoustic streaming modes for clearing root canal bacteria using erbium-doped yttrium aluminum garnet lasers: An in vitro study. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Chen, X.; Xu, R.-Q.; Chen, J.-P.; Shen, Z.-H.; Jian, L.; Ni, X.-W. Shock-wave propagation and cavitation bubble oscillation by Nd: YAG laser ablation of a metal in water. Appl. Opt. 2004, 43, 3251–3257. [Google Scholar] [CrossRef]

| Experiments | Log10 CFU/mL Reduction | p Value | |
|---|---|---|---|
| MB | vs. Control | −0.02 | 1.00 |
| HP | −0.61 | <0.001 | |
| MB + 660 nm DL | −0.73 | <0.001 | |
| HP + 980 nm DL | −0.91 | <0.001 | |
| MB + SWEEPS | −0.77 | <0.001 | |
| HP + SWEEPS | −1.05 | <0.001 | |
| MB + 660 nm DL + SWEEPS | −1.58 | <0.001 | |
| HP + 980 nm DL + SWEEPS | −5.97 | <0.001 | |
| NaOCl | −5.97 | <0.001 | |
| MB + 660 nm DL | vs. MB | −0.71 | <0.001 |
| MB + SWEEPS | −0.75 | <0.001 | |
| MB + 660 nm DL + SWEEPS | −1.56 | <0.001 | |
| NaOCl | −5.95 | <0.001 | |
| MB + SWEEPS | vs. MB + 660 nm DL | −0.04 | 0.982 |
| MB + 660 nm DL + SWEEPS | −0.85 | <0.001 | |
| NaOCl | −5.24 | <0.001 | |
| MB + 660 nm DL + SWEEPS | vs. MB + SWEEPS | −0.81 | <0.001 |
| NaOCl | −5.20 | <0.001 | |
| HP + 980 nm DL | vs. HP | −0.30 | 0.014 |
| HP + SWEEPS | −0.44 | 0.002 | |
| HP + 980 nm DL + SWEEPS | −5.36 | <0.001 | |
| NaOCl | −5.36 | <0.001 | |
| HP + SWEEPS | vs. HP + 980 nm DL | −0.14 | 0.982 |
| HP + 980 nm DL + SWEEPS | −5.06 | <0.001 | |
| NaOCl | −5.06 | <0.001 | |
| HP + 980 nm DL + SWEEPS | vs. HP + SWEEPS | −4.92 | <0.001 |
| NaOCl | −4.92 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrasiabi, S.; Parker, S.; Chiniforush, N. Synergistic Antimicrobial Effect of Photodynamic Inactivation and SWEEPS in Combined Treatment against Enterococcus faecalis in a Root Canal Biofilm Model: An In Vitro Study. Appl. Sci. 2023, 13, 5668. https://doi.org/10.3390/app13095668
Afrasiabi S, Parker S, Chiniforush N. Synergistic Antimicrobial Effect of Photodynamic Inactivation and SWEEPS in Combined Treatment against Enterococcus faecalis in a Root Canal Biofilm Model: An In Vitro Study. Applied Sciences. 2023; 13(9):5668. https://doi.org/10.3390/app13095668
Chicago/Turabian StyleAfrasiabi, Shima, Steven Parker, and Nasim Chiniforush. 2023. "Synergistic Antimicrobial Effect of Photodynamic Inactivation and SWEEPS in Combined Treatment against Enterococcus faecalis in a Root Canal Biofilm Model: An In Vitro Study" Applied Sciences 13, no. 9: 5668. https://doi.org/10.3390/app13095668






