Abstract
Cordyceps militaris is a valuable medicinal fungus which has been widely used as a traditional medicine in East Asia. Compared to the well-known medicinal fungus C. sinensis, C. militaris can produce similar fermented metabolites with various biological activities, but it requires a shorter culture time and simpler culture conditions, and therefore, it has attracted increasing attention in recent years. The purpose of this review was to organize the current studies regarding metabolite production from C. militaris relative to their biological functions. We combined findings of metabolite production to correlate with different fermentation modes to obtain a full view of production processes used to yield the product. While research on C. militaris fermentation is not uncommon to date, its high value still highlights the importance of developing more modern fermentation processes for industrial production.
1. Introduction
Cordyceps militaris, which is called Yong Chong Cao in Chinese, is a type of entomopathogenic fungus that belongs to the Cordyceps genus in the phylum Ascomycota and family Cordycipitaceae [1]. It is most abundant in humid temperate and tropical regions, and is widely distributed throughout North America, Europe, and East and Southeast Asia [2]. Cordyceps militaris grows parasitically on the larvae and pupae of arthropods, mainly lepidopteron and coleopteran species. As the cultivation period progresses, the spores of C. militaris develop into hyphae, gradually growing into club-shaped, orange fruiting bodies measuring 2–5 cm in height. The morphology of mature C. militaris consists of two parts, including the stalk (the grass part, also known as the fruiting body) and the sclerotium (the corpse part of the insect) [3]. The basal medium for cultivating C. militaris typically consisted of 40 g of glucose (or other sugars), 10 g of peptone, 5 g of yeast extract, 1 g of K2HPO4, 1 g of KCl, 1 g of MgSO4·7H2O, and 2 g of adenosine per liter [4], without pH control [5]. Niketan and Lakshmi [6] discovered that under the culture conditions of 20 °C temperature, 2.5% (w/v) glucose, and 0.8% (w/v) yeast extract, the maximal yield of the mycelial biomass reached 547 ± 2.09 mg/100 mL. This yield was 1.95-fold higher than that obtained in the basal medium.
All Cordyceps species share a mechanism of invading and growing on their hosts to maintain their life cycles [7], but the main difference occurs in the hosts they infect [8]. The majority of known species in the Cordyceps genus infect insects and other arthropods, with a few parasitizing fungi and plants. Most Cordyceps species exhibit host specificity and are known to infect particular or closely related host species, with only a few having the ability to infect a wide range of hosts [9]. During the parasitic process, Cordyceps has developed a complex survival mechanism to facilitate its attachment to a host and avoid clearance by the host’s immune system. For this mechanism, Cordyceps produces several unique secondary metabolites that aid in its parasitism and reproduction on its hosts [7], showing great promise as potential sources of new drugs.
The Cordyceps genus has a history of several hundred years of use as a traditional Chinese medicine, with C. sinensis being the species with the longest history of use, exhibiting a wide range of biological activities. At present, the main source of C. sinensis is wild collection, with its rarity and high price are due to its wild characteristics. Although artificial cultivation has been achieved in recent years, production costs remain high, and cultivation times are long. In contrast, artificial cultivation of C. militaris yields lower production costs and shorter cultivation times compared to C. sinensis. Cordyceps militaris also has a similar chemical composition and comparable medicinal properties to those of C. sinensis, and even contains higher levels of certain bioactive components. Therefore, it is regarded as a promising substitute for C. sinensis. Figure 1 displays both natural and cultured C. militaris for comparison [10].
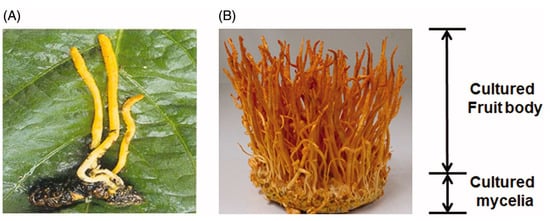
Figure 1.
Natural (A) and cultivated (B) C. militaris. [7].
2. Biological Compounds of C. militaris
The bioactivities of C. militaris are significantly important for both medical and biological applications. Table 1 summarize the physiological functions of various bioactive compounds extracted from C. militaris, which include cordycepin [11], D-mannitol [12], adenosine [13,14], polysaccharides [15], gamma aminobutyric acid (GABA) [16,17], lovastatin [16,17], and carotenoids [18,19], and these are further categorized and explained in the following paragraphs.

Table 1.
Bioactive components of Cordyceps militaris and their biological activities.
2.1. Cordycepin
The molecular formula of cordycepin is C10H13N5O3, with a molecular weight of 251. In 1950, Cunningham et al. [11] discovered 3-deoxyadenosine in the fermentation broth of C. militaris and named it cordycepin. Cordycepin is an adenosine derivative, but it lacks a hydroxyl group at the 3’ position of ribose. It was shown to possess diverse pharmacological properties, in addition to its well-known antioxidant effects [20,21,22], including antiviral activity [24], inhibition of lipopolysaccharide (LPS)-induced inflammation [57], reduction of blood lipids [78], inhibition of platelet aggregation [79], and induction of apoptosis in neuroblastoma and melanoma cells [80].
Cordycepin exhibits significant antioxidant effects by reducing the cellular content of malondialdehyde (MDA) and intracellular reactive oxygen species (ROS), and increasing the activity of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPx) in cells treated with 6-hydroxydopamine (6-OHDA) [22]. Cordycepin extraction using n-hexane led to a significant increase in activities of antioxidant enzymes. GPx was enhanced by 25.6% (5 μM), 55.3% (10 μM), and 98.4% (20 μM), while SOD activity was, respectively, increased by 20.5%, 50.2%, and 77.4% [22].
Cordycepin has shown promise as a therapeutic agent for treating multiple inflammatory conditions, including rheumatoid arthritis [81], Parkinson’s disease (PD) [82], multiple sclerosis (MS) [83], atherosclerosis [84], pneumonia [85], hepatitis [86], and atopic dermatitis (AD) [87,88]. For example, topical application of 2,4-dinitrofluorobenzene on the dorsal skin of mice induces AD-like lesions, which can be alleviated by cordycepin. This natural compound reduces serum levels of histamine, immunoglobulin E (IgE), and inflammatory cytokines; suppresses the infiltration of mast cells and eosinophils; and downregulates expressions of thymic stromal-derived lymphopoietin (TSLP), macrophage inflammatory protein (MIP)-2, intracellular cell adhesion molecule (ICAM)-1, thymus and activation-regulated chemokine (TARC), and C-C chemokine receptor (CCR)-3 [89].
Regarding the antiviral effect of cordycepin, it was found that cordycepin significantly inhibited the transfer of Epstein–Barr virus (EBV) from lymphoblastoid cell line (LCL)-EBV-green fluorescent protein (GFP) cells to AGS cells (a human gastric adenocarcinoma cell line), indicating a significant inhibition of EBV infection in gastric epithelial cells [24].
In terms of its antitumor effects, cordycepin significantly increased expressions of DNA methyl transferases (DNMTs), particularly DNMT3, leading to hypermethylation of the BCL7A tumor-suppressor gene in response to cordycepin treatment [24].
Cordycepin inhibits LPS-induced inflammation by suppressing the activation of nuclear factor (NF)-κB, Akt, and p38 phosphorylation, resulting in the downregulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 gene expressions and subsequent inhibition of NO production [57]. It was shown to prevent hyperlipidemia by activating adenosine monophosphate-activated protein kinase (AMPK), demonstrating its potential as a functional food ingredient for managing lipid metabolism disorders [78].
In a dose-dependent manner, cordycepin inhibited platelet aggregation induced by thapsigargin and significantly decreased levels of ([Ca+]i), which had been increased by thapsigargin (1 mM) or U46619 (a stable analog of the endoperoxide prostaglandin, H2) (3 mM). Furthermore, cordycepin increased cyclic guanosine monophosphate (cGMP) levels that had been reduced by thapsigargin [79].
The 50% inhibitory concentration (IC50) values of cordycepin for human neuroblastoma SK-N-BE(2)-C and human melanoma SK-MEL-2 cells were 120 and 80 mM, respectively, indicating significant inhibition of their proliferation [80].
2.2. D-Mannitol
One of the most important products of C. militaris metabolism is D-mannitol, commonly known as cordycepic acid, which has a chemical formula of C6H14O6, with a molecular weight of 182.172 g/mol, and is classified as a polyhydric alcohol (polyol) [16]. Mannitol has various medical applications due to its osmotic properties, and it is used in the food and pharmaceutical industries. It also stimulates the release of renal prostaglandins, resulting in renal vasodilation and increased urine flow through the tubules, which is thought to prevent renal injury by reducing tubular obstruction [90]. The biological activities of mannitol can be broadly classified into several categories, including antioxidant [27], anti-inflammatory, antitumor, hypolipidemic, and hepatoprotective properties.
As for the antioxidant ability of mannitol, it was shown to possess free radical-scavenging properties in vitro, protecting active substances such as hyaluronic acid from degradation by oxygen-derived free radicals. Moreover, it inhibits the release of ROS caused by the thermal- or photodecomposition of tetracyclines [91].
Mannitol was shown to have anti-inflammatory effects by inhibiting lipid peroxidation and formation of the NF-κB complex [92].
Tumor-bearing animals treated with a high dose of cisplatin (CDDP; 9.5 mg/kg body weight (BW) i.v.) and mannitol survived longer than those given CDDP (7.0 or 9.5 mg/kg BW i.v.) alone, which suggests that mannitol can effectively enhance antitumor ability [29].
Mannitol (10 mg/kg BW) demonstrated hypolipidemic effects in hypercholesterolemic mice that were comparable to those of ezetimibe (10 mg/kg BW), without inducing liver damage or impacting high-density lipoprotein cholesterol (HDL-C) levels [30].
Mannitol administration has the potential to mitigate or alleviate liver injury caused by experimental obstructive jaundice, as elevated levels of platelet-activating factor (PAF) in both plasma and liver tissue were observed, which are thought to contribute to the progression of the injury [31].
2.3. Ergothioneine
Ergothioneine, with a molecular formula of C9H15N3O2S and a molecular weight of 229.3 g/mol, is a water-soluble thiol compound. It is classified as a non-protein amino acid and is naturally synthesized by certain bacteria, plants, and fungi. Notably, mammals do not produce ergothioneine endogenously, underscoring its importance as an essential dietary nutrient. It has gained attention due to its identification as a biogenic key substrate of the organic cation transporter 1 (OCTN1), which has a protective role in monocytes and is associated with autoimmune disorders like rheumatoid arthritis and Crohn’s disease [93]. Cordyceps militaris is a rich source of ergothioneine, as its fruiting bodies contain a concentration of 782.3 mg/kg dry weight (DW), and that of mycelium ranged up to 130.6 mg/kg DW [19]. Another study estimated a concentration of 409.8 mg/kg DW in its fruiting bodies [17].
Ergothioneine exhibits potent antioxidant properties, as it can scavenge hydroxyl radicals and inhibit their generation from hydrogen peroxide in a copper or iron ion-dependent manner. Additionally, it acts as an inhibitor of the copper ion-dependent oxidation of oxyhemoglobin, and of arachidonic acid peroxidation mediated by mixtures of myoglobin (or hemoglobin) and hydrogen peroxide. Furthermore, ergothioneine can protect alpha 1-antiproteinase from inactivation by hypochlorous acid due to its ability to effectively scavenge this reactive molecule [33].
Anti-inflammatory properties, including the modulation of interleukin (IL)-6, were attributed to ergothioneine. Furthermore, it may mitigate peroxyacetyl nitrate (PA)-induced cell death by diminishing the activity of the mitogen-activated protein kinase (MAPK) cascade [35].
The antiaging effect of ergothioneine is based on its ability to inhibit ultraviolet A (UVA)-induced activator protein (AP)-1 translocation, which in turn inhibits collagenolytic matrix metalloproteinase-1 activation and type I procollagen degradation [37].
Ergothioneine, along with its precursor, trimethyl histidine, and trehalose are considered anti-stress compounds that are maintained at high levels during starvation-mediated fasting [38].
Through activation of Nrf2 and FoxO3, ergothioneine exhibited cytoprotective effects, and FoxO3 appeared to play a role in erythroid proliferation and differentiation [94].
The radioprotective effect of ergothioneine is attributed to the reduction of ergothioneine disulfide, which is formed by irradiation-generated hydrogen peroxide, to ergothioneine in a neutral solution. Subsequently, the regenerated ergothioneine can again react with hydrogen peroxide [40].
2.4. Adenosine
Adenosine, with a molecular formula of C10H13N5O4 and a molecular weight of 267.2413 g/mol, is a nucleoside formed by the bonding of ribose and adenine through β-N9-glycosidic bonds [95]. It is identified as one of the primary active components in C. militaris, alongside other nucleosides like guanosine, cytidine, uridine, adenine, and uracil [96]. Its efficacy in treating chronic heart disease and modulating the release of neurotransmitters in the central nervous system was demonstrated by numerous studies [95,97,98].
The activation of cellular antioxidant enzymes is a novel mechanism by which adenosine exerts cytoprotective to the schemic cell injuries [42].
Adenosine inhibits some functions of neutrophils, which are a type of inflammatory cell. By occupying specific adenosine receptors, it modulates leukocyte function through a novel mechanism that “uncouples” chemoattractant receptors from their stimulus-transduction proteins. Adenosine and its agonists are therefore excellent candidates for the development of anti-inflammatory agents [99].
Adenosine accumulates during tumor hypoxia and acts as a strong immunosuppressive agent by modulating the immune system. This can inhibit the antitumor immune responses elicited by standard radiotherapy, presenting ADO-mediated mechanisms that may thwart such responses [100].
The anti-ischemic effect exerted via the development of collateral circulation, at the heart and the brain level, is a therapeutically relevant possibility raised by the available evidence linking adenosine to angiogenesis [101].
Adenosine is involved in various pharmacological actions related to cardiovascular therapies. A2A receptor agonists are being developed by the pharmaceutical industry for this purpose, and methotrexate, an atheroprotective and anti-inflammatory drug, exerts its effects through the release of adenosine and the activation of the A2A receptor. Additionally, antiplatelet agents reduce platelet activation and adhesion and inhibit thrombotic occlusion of the atherosclerotic arteries by blocking adenosine diphosphate-mediated effects on the P2Y12 receptor [47,102,103,104].
By modulating the activity of Gsk3β through adenosine receptor activation, adenosine stimulates the Wnt/β-catenin signaling pathway. The hair growth-promoting effect of adenosine is attributed to the activation of the Gαs/cAMP/protein kinase A (PKA)/mammalian target of the rapamycin (mTOR) cascade, which plays a pivotal role in adenosine-mediated Wnt pathway activation as the underlying molecular mechanism [48].
2.5. Polysaccharides
Polysaccharides are some of the most abundant and important bioactive components in Cordyceps, exhibiting a wide range of pharmacological activities and applications, including antioxidant [105], anti-inflammatory [106], antiviral [107], antiaging [108], and antitumor [107] effects. Polysaccharides can be extracted and isolated from Cordyceps fruiting bodies, mycelia, and fermentation broth, with different physicochemical properties, making them a targeted product for the development and quality control of Cordyceps-related health supplements [108]. In recent years, the isolation, purification, structural identification, and biological activities of Cordyceps polysaccharides have been extensively studied. However, due to variations in raw materials, extraction methods, and purification procedures, the structures and biological activities of polysaccharides obtained from Cordyceps exhibit significant differences, making it difficult to establish a correlation between structure, chain conformation, and biological activity. Therefore, it is necessary to develop a standardized preparation method for Cordyceps polysaccharides.
Microbial polysaccharides can be categorized based on their location within the cell, as follows:
- Structural polysaccharides, such as teichoic acids, LPSs, and peptidoglycans, form the cell wall to provide protection and maintain the microorganism’s structure.
- Intracellular polysaccharides, also known as cytosolic polysaccharides, serve as a source of energy and carbon for the cell.
- Exopolysaccharides (EPSs) are secreted into the extracellular medium either as a secondary metabolite or in the form of a biofilm to protect against environmental stress [109,110].
With respect to the antioxidant activities of polysaccharides from C. militaris, the presence of certain monosaccharides may have a significant impact. In particular, a purified polysaccharide consisting primarily of glucose, mannose, galactose, and arabinose in an α-type glycosidic linkage was found to exhibit in vitro scavenging activities against DPPH, hydroxyl, and superoxide radicals [49,50].
CPS-1, a polysaccharide with a molecular weight of 2.3 × 104 Da, is composed of rhamnose, xylose, mannose, glucose, and galactose, and was shown to have anti-inflammatory and humoral immunity-reducing properties [15].
An acidic polysaccharide extracted from C. militaris cultivated on germinated soybeans was found to possess antiviral properties against the influenza A virus. This effect was believed to be partly attributable to its ability to modulate the immune response of macrophages. The polysaccharide was shown to reduce viral titers in both bronchoalveolar lavage fluid and lung tissues. In addition, it was observed to enhance the production of tumor necrosis factor (TNF)-α and interferon (INF)-γ in vivo, as well as to stimulate nitric oxide (NO) production and induce expressions of inducible NO synthase (iNOS) messenger (m)RNA and protein in vitro. Furthermore, the polysaccharide was shown to modulate mRNA expressions of IL-1β, IL-6, IL-10, and TNF-α [53].
Cultivated fruiting bodies of C. militaris containing polysaccharides were found to have effects on the activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and anti-hydroxyl radicals when assayed in vivo using commercial monitoring kits. These polysaccharides inhibited the mitochondrial injury and swelling induced by Fe2+-L-cysteine in a concentration-dependent manner and had a significant superoxide anion scavenging effect. Additionally, they significantly increased the activities of CAT, SOD, GPx, and anti-hydroxyl radicals in mice liver. These findings indicated that polysaccharides extracted from C. militaris may have pharmaceutical value in protecting mitochondria, scavenging ROS, and increasing the activities of antioxidases, which could potentially contribute to antiaging effects [54].
By orally administering 20 mg/kg BW of the water extract of the fruiting body of C. militaris containing polysaccharides to mice, it was discovered that the extracts could enhance the secretion of IFN-γ by macrophages through IL-18. This suggests that C. militaris has potential use as an immune activator or antitumor agent [56,57].
2.6. GABA
GABA, short for γ-Aminobutyric acid, has a molecular formula of C4H9NO2 and a molecular weight of 103.12 g/mol. It is classified as a non-protein amino acid and is synthesized within the human body from glutamic acid. Concentrations of GABA were determined to be 68.6–180.1 mg/kg and 756.30 mg/kg dry weight in the mycelium and fruiting bodies of C. militaris, respectively [17]. GABA exhibits various biological activities, such as antioxidative, anti-inflammatory, anticonvulsant, antihypertensive, and antidiabetic properties.
GABA, known for its antioxidant properties [59], helps plants cope with various environmental stresses by providing nicotinamide adenine dinucleotide (NADH) and/or succinic acid via GABA shunts in the tricarboxylic acid cycle [111]. In addition to maintaining hormone and mineral nutrition, GABA reduces lipid peroxidation and enhances stress resistance in plants [112].
In relation to the anti-inflammatory ability of GABA, it exerts potent protective effects in models of type 1 diabetes by acting on both T cells and macrophages [60]. Additionally, GABAergic agents directly act on antigen-presenting cells (APCs), reducing MAPK signals and attenuating the following adaptive inflammatory responses to myelin proteins [113].
GABAergic neurons and neurotransmitters modulate various brain circuits to regulate stress and anxiety responses, under both normal and pathological conditions [114]. They also modulate both rapid eye movement (REM) and non-REM, especially slow wave sleep (SWS), through corticomedullary pathways [115]. Additionally, GABAergic neurons and neurotransmitters regulate circadian rhythms by modulating the suprachiasmatic nuclei (SCN) [116].
By activating GABA receptors, central GABA has the potential to lower blood pressure and slow down the heart rate, making it a valuable therapeutic option for preventing and treating cardiovascular diseases resulting from hypertension, particularly in patients with cardiac insufficiency [117].
GABA, an antidiabetic factor coexisting with insulin in pancreatic β-cells [118], exerts its effect by acting on beta cell proliferation and the immune system. In patients with type 2 diabetes, a significant decrease in circulating GABA concentrations was observed, indicating its potential therapeutic value for diabetes [119].
2.7. Lovastatin
Lovastatin, found in the fruiting bodies of C. militaris [17,120], is a type of statin that selectively blocks the synthesis of endogenous cholesterol by inhibiting the rate-limiting enzyme in cholesterol production. Lovastatin features a six-membered lactone ring alongside a hydroxyl group and a partially hydrogenated naphthalene, with a hydroxyl substituent esterified by a 2-methylbutyric acid residue. Its molecular formula is C24H36O5, with a molecular weight of 404.54 g/mol. It is converted from a lactone to a hydroxy acid form during enzymatic reactions, and this form competitively blocks 3-hydroxymethylglutaryl-coenzyme A (HMG-CoA) [121]. The lovastatin concentration in the mycelium of C. militaris ranged from 37.7~57.3 mg/kg DW, which was lower than the concentration found in C. sinensis at 1365.3 mg/kg [17]. Fruiting bodies of C. militaris contain lovastatin at a concentration of 2.76 mg/kg. However, the fruiting bodies of Hericium erinaceus and Ganoderma lucidum exhibit some of the highest lovastatin contents, at 14.38 and 11.54 mg/kg, respectively [16]. Approved for treating hypercholesterolemia and reducing the risk of coronary heart disease, lovastatin also exhibits additional beneficial effects such as anti-inflammatory and antioxidant properties, as well as vascular endothelium protection [122].
Upon oral administration, lovastatin was found to reduce oxidative stress and modulate activities of antioxidant enzymes in the liver and heart tissues of H2O2-treated rats, indicating its potential in mitigating the effects of oxidative stress [65].
Lovastatin inhibits the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR signaling pathway and downregulates histone deacetylase 1 (HDAC1) expression, which prevents cytosolic NF-κB from entering the nuclei of LPS-stimulated RAW264.7 macrophages. Additionally, it reduces expressions of iNOS and TNF-α and induces an anti-inflammatory response [67].
By directly inhibiting hydroxymethylglutaryl coenzyme A reductase, lovastatin inhibits the prenylation pathways in cells. It was found to reduce respiratory syncytial virus (RSV) replication when administered within 24 h after infection, but not vaccinia virus replication. In mice, lovastatin also decreases virus-induced weight loss and illness, indicating its potential as a therapeutic agent for RSV infection [123].
Lovastatin was demonstrated to enhance the antitumor effects of cisplatin and TNF-α, as well as to increase apoptosis induced by cisplatin, 5-fluorouracil, and sulindac in colon cancer cells [124].
Lovastatin plays an important role in inducing the expression of thrombomodulin, a gene with antithrombotic properties. Moreover, it decreases the expressions of prothrombotic factors such as E-selectin, plasminogen activator inhibitor type 1 (PAI-1), vascular cell adhesion molecule (VCAM)-1, intracellular cell adhesion molecule (ICAM)-1, and endothelin [125] and enhances the expression of the endothelial (e)NOS gene. Studies suggested that endothelial NOS expression is closely linked to thrombomodulin expression [126].
Lovastatin is effective as an antiatherosclerotic drug and a cell-cycle blocker due to its ability to induce cell-cycle arrest or retardation in various cell types. This effect is not only limited to the G1 phase but also affects the G2/M transition. Upon incubation of epithelial PtK2, T24, HeLa, and fibroblastic L929 cells with 1.0~60.0 μm lovastatin for 24~48 h, various mitotic perturbations were observed [127].
2.8. Carotenoids
Carotenoids, such as β-carotene, lycopene, lutein, and zeaxanthin, are responsible for the vivid yellow-orange hue of the fruiting bodies of various species of fungi, including C. militaris, in which their presence was confirmed [18,128]. In the aqueous extract of C. militaris, β-carotene was found at a concentration of 24.51 mg/kg, while lycopene was detected at 3.42 mg/kg [88]. Cordyxanthins are a novel class of carotenoids found in the fruiting bodies of C. militaris. They show better solubility in water and have unique chemical structures when compared to traditional carotenoids. Four types of cordyxanthins, including cordyxanthin I, II, III, and IV, have been identified in fruiting bodies of C. militaris, with respective concentrations of 0.289, 0.235, 0.401, and 0.175 mg/g. Using β-carotene as a standard, total Cordyceps carotenoids were directly quantified at 447 nm.
Carotenoids are powerful antioxidants due to their ability to efficiently quench singlet oxygen (1O2) [129,130,131,132], which is attributed to their triplet energy levels being in close proximity to that of 1O2 [133,134]. The 1O2-quenching process is particularly efficient for carotenoids that have 11 conjugated double bonds [135], with an approximate rate constant of 1010 M−1·s−1. In addition to their antioxidant properties, carotenoids were observed to act as protective agents against ROS in eye-related disorders [136]. Lutein and zeaxanthin, two oxygen-containing carotenoids, are present in high concentrations in the eye lens and the macular region of the retina (yellow spot) [137].
Carotenoids possess anti-inflammatory properties by suppressing the activation of NF-κB [138,139,140,141]. Apocarotenals, which are derivatives of carotenoids, possess electrophilic groups that can interact with cysteine residues of IκB kinase (IKK) and NF-κB subunits (p65), leading to inactivation of the NF-κB pathway [142].
Most carotenoids have demonstrated antiaging effects. For instance, an animal study showed that lutein can extend the lifespan and reduce the mortality rate caused by hydrogen peroxide and paraquat in Drosophila melanogaster [143], and further study indicated that lutein (at 0.5, 1.5, 5, and 15 µM) can mitigate the age-related decline in human skin cells [144]. Astaxanthin, another carotenoid, can also prolong the lifespan in wild-type and long-lived mutant age-1 Caenorhabditis elegans when administered at a concentration of 0.1~1 mM [145]. That study suggested that carotenoids partially modulate insulin-like growth factor (IGF)-1 signaling by increasing DAF-16 gene expression and decreasing the mitochondrial production of ROS. Beta-carotene, a commonly found carotenoid derivative, also exerts antiaging effects by downregulating the expression of lysine acetyltransferase 7 (KAT7), while KAT7 is a histone acetyltransferase gene known to be a driver of senescence [146].
Some research examined the antimicrobial effects of carotenoid pigments in Rhodotorula against two food-borne bacterial strains, Staphylococcus aureus ATCC 25,923 and Salmonella typhimurium ST38 [147]. Another study indicated that a wide range of antimicrobial activities against Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Saccharomyces cerevisiae, and Rhizopus oryzae were exhibited by the methanol extract of carotenoids, as evidenced by the disc diffusion method and minimum inhibitory concentration (MIC) determination, with inhibition zones ranging 8.97~19.47 mm and MICs ranging 18.75~140 µg/mL [148].
Antiapoptotic properties were demonstrated in some carotenoids. Lycopene, for instance, is capable of preventing the loss of such antiapoptotic proteins as Bcl-2 and Bcl-xL [149,150], as well as inhibiting proapoptotic proteins like Bax under neurotoxic conditions [149,151]. Lutein, on the other hand, is effective in preventing the loss of Bcl-2 and Bcl-xL, the accumulation of Bax, and the activation of caspases-3 and -8 in cerebral ischemia and Parkinson’s disease models [152,153,154].
3. Cultivation of C. militaris
Wild C. militaris is rarely found in nature due to its extreme growth habitat, i.e., high-elevation areas, and its host specificity for arthropod pupae [155]. Due to great demands for the C. militaris in the therapeutic and cosmeceutical industry, the scaled-up production of C. militaris has drawn the attention of scientists and business sectors [156]. Based on these needs, the artificial cultivation of Cordyceps was developed and optimized to fulfill such a demand–supply gap and to prevent the extinction of natural Cordyceps resulting from over-harvesting. In addition, the mycelial cultivation of C. militaris was also deemed important due to its rapid cultivation time, ease of scaling-up production, and ability to rapidly obtain specific biological compounds.
3.1. Solid-State Fermentation
Solid-state fermentation is commonly used for the artificial cultivation of C. militaris on a solid substrate, comprised of rice or grains, to obtain fruiting bodies [157,158]. However, it requires several months for the cultivation to form fruiting bodies, and it is difficult to control the quality of the final product [159]. Moreover, the large-scale extraction of bioactive compounds from C. militaris fruiting bodies is usually time-consuming and labor-intensive [1]. As a result, solid-state fermentation is unsuitable for obtaining biological ingredients from the large-scale industrial production of C. militaris. However, the fruiting body as the traditional consumption type of C. militaris still possesses high economic value in specific consumer markets, such as those offering Chinese herbs and medicinal diets [159].
In solid-state fermentation, the substrate plays an important role in C. militaris cultivation and the production of specific ingredients. Many substrates can be used for the solid-state fermentation of C. militaris, including wheat, oats, and rice [160]. Among these substrates, rice, which presents various advantages such as abundant nutrients, including amino acids, multiple B vitamins, and mineral elements, is the most commonly used solid-state substrate for C. militaris growth. Additionally, because rice is a cash crop, production costs are also relatively low [49]. In addition, Xiao, et al. [161] inoculated C. militaris on chickpeas to develop a new nutritious food product, and the results showed that fermented chickpeas presented higher contents of crude proteins, true proteins, and essential amino acids. In addition to producing specific products, C. militaris fermentation can also be used to improve the flavor of tea [162] under solid-state fermentation.
3.2. Liquid Culture
Liquid culture is the other option for C. militaris cultivation. It is considered a better procedure for application in industry due to its shorter cultivation times, smaller space requirements, and higher productivities [163]. Cordyceps militaris is allowed to grow in liquid as mycelia, without forming fruiting bodies, and total cultivation times can decrease from 60 to 15 days [164]. Bioactive compounds are released from mycelia and accumulate in the culture broth to simplify the extraction process [156]. Liquid culture can be carried out without the need for light/dark treatment and regulation of a specific relative humidity [158]. Therefore, liquid culture is considered a promising C. militaris cultivation procedure for obtaining useful and potent substances for industrial-scale applications.
In a recent study, the liquid culture of C. militaris was divided into submerged fermentation and surface culture methods. Submerged culture continually provides shaking or agitation during fermentation in order to dissolve nutrients and oxygen in the liquid medium [159]. For surface culture, the system remains in a static condition after the seeding of the inoculum. In surface culture, a biofilm forms on the surface, and some mycelia precipitate to the bottom of the culture flask, while the byproducts are directly secreted and accumulate in the medium [165]. In terms of metabolite yields, higher production can be achieved than that obtained using submerged culture; however, the surface culture time is relatively longer. In addition, surface culture is not easy to perform on an industrial scale [156]. Submerged culture can be carried out in a fermentation tank, and the fermentation time is relatively short and controllable [166]. Consequently, the C. militaris industry has mainly focused on developing the submerged culture method.
4. Production Mode of Cordyceps militaris and Metabolite Yields
4.1. Fed-Batch Culture and Batch Culture
For batch culture, all of the medium and the seed culture are fed iinto the bioreactor during cultivation, and the product remains in the bioreactor until cultivation is complete [167]. The environment of the batch culture process changes greatly with time. In the later stage of culture, the lack of nutrients or the accumulation of inhibitory metabolites often causes difficulty in regards to cell survival [168]. Compared to batch culture, the fed-batch mode, through the continuous addition of nutrients, results in an increase in the total liquid volume to accumulate higher cell densities, which therefore improves the efficiency of the production process [169,170].
Mao and Zhong [171] found that feeding a nitrogen source during fermentation significantly increased the production of cordycepin from 208.8 to 346.1 mg/L. Furthermore, when C. militaris is mutated by UV irradiation, there is an increase in its cordycepin production, with a maximum cordycepin production of about 445 mg/L [172].
4.2. Repeated-Batch Fermentation
Repeated-batch fermentation is an economical, environmentally friendly, and stable method of fermentation, which can even be used as a modified approach for targeted products during production [173]. A repeated batch replaces the fresh medium after collecting the product at the end of each batch. It is expected that repeated-batch culture can increase the cell growth rate, ensuring high cell productivity [174,175]. During repeated-batch fermentation, microbial cells are reutilized for subsequent fermentation runs. Several benefits can be derived from this; for instance, reusing microbial cells for subsequent fermentation runs can achieve higher initial cell concentrations, and shorter operation times are required [174,176].
A previous study reported that the biomass of C. militaris can maintain a production level of >85% of that of the initial cycle for at least four repetitions by shortening the period of the lag phase by repeated-batch fermentation [174]. Another study showed that exopolysaccharide (EPS) production of C. militaris could be enhanced to a maximum of 5.713 g/L, with a productivity of 476 mg/L/day in the second run [165]. Moreover, Zheng et al. [177] combined repeated-batch fermentation and two-stage foam fractionation to develop a strategy to improve EPS production. The foam system can also promote the EPS separation efficiency during fractionation. Table 2 below illustrates the fermentation modes, types, and metabolite yields of different microbiota strains.

Table 2.
Fermentation modes, types, and metabolite yields.
5. Conclusions
In summary, C. militaris offers a promising alternative to C. sinensis in traditional medicine due to its shorter cultivation time and simpler growth requirements. Through fermentation, it produces bioactive compounds like cordycepin, with diverse physiological functions. Liquid culture, including fed-batch and batch culture, shows advantages over solid-state fermentation, including shorter cultivation times and simplified extraction processes. Repeated-batch fermentation holds promise for maintaining high production levels over multiple cycles. Optimizing fermentation conditions, i.e., by the addition of nitrogen sources or UV irradiation-induced mutations, can significantly increase the production of key bioactive compounds. Understanding the production modes and metabolite yields of C. militaris is crucial for realizing its full potential in medicinal and industrial applications. Continued research into optimizing fermentation processes and exploring novel cultivation techniques will further unlock the therapeutic and commercial value of this valuable fungus.
Author Contributions
Y.-C.C.: investigation and writing—original draft. T.-H.S.: resources and writing—original draft. S.-J.H.: resources and writing—original draft. D.K.: writing—review and Editing. S.P.S.: writing—review and editing. S.-P.L.: writing—original draft, writing—review and editing, and supervision. K.-C.C.: writing—review and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D. Cordyceps sinensis: A precious parasitic fungus infecting Tibet. Field Mycol. 2010, 11, 60–67. [Google Scholar] [CrossRef]
- Shrestha, B.; Han, S.K.; Lee, W.H.; Choi, S.K.; Lee, J.O.; Sung, J.M. Distribution and in vitro Fruiting of Cordyceps militaris in Korea. Mycobiology 2005, 33, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Liu, Z.; Wang, Y.; Lin, Q.; Ding, S.; Li, C.; Deng, C. High-level production of cordycepin by the xylose-utilising Cordyceps militaris strain 147 in an optimised medium. Bioresour. Technol. 2023, 388, 129742. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Sung, T.H.; Angkawijaya, A.E.; Go, A.W.; Hsieh, C.W.; Hsu, H.Y.; Santoso, S.P.; Cheng, K.C. Enhanced exopolysaccharide production of Cordyceps militaris via mycelial cell immobilization on plastic composite support in repeated-batch fermentation. Int. J. Biol. Macromol. 2023, 250, 126267. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, N.; Bhaskaran, L. Optimization of cultural and nutritional conditions to enhance mycelial biomass of Cordyceps militaris using statistical approach. Braz. J. Microbiol. 2024, 55, 235–244. [Google Scholar] [CrossRef]
- Hajek, A.E.; St. Leger, R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, C.T. The Hypocrealean Fungi (Ascomycetes, Hypocreales). Mycologia 1970, 62, 865–910. [Google Scholar] [CrossRef]
- Choi, E.; Oh, J.; Sung, G.-H. Antithrombotic and antiplatelet effects of Cordyceps militaris. Mycobiology 2020, 48, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.G.; Manson, W.; Spring, F.S.; Hutchinson, S.A. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xiao, L.; Zheng, B.; Wei, X.; Ellis, A.; Liu, Y.M. Identification of chemical markers in Cordyceps sinensis by HPLC-MS/MS. Anal. Bioanal. Chem. 2015, 407, 8059–8066. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Lindroth, A.M.; Kwon, S.; Park, S.J.; Park, Y.J. Adenosine derivatives from Cordyceps exert antitumor effects against ovarian cancer cells through ENT1-mediated transport, induction of AMPK signaling, and consequent autophagic cell death. Biomed. Pharmacother. 2022, 153, 113491. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhu, J.; Zhang, C.; Zhang, L. Determination of adenosine and 3′-deoxyadenosine in Cordyceps militaris (L.) Link. by HPLC. China J. Chin. Mater. Med. 1998, 23, 236–237, 256. [Google Scholar]
- Yu, R.; Song, L.; Zhao, Y.; Bin, W.; Wang, L.; Zhang, H.; Wu, Y.; Ye, W.; Yao, X. Isolation and biological properties of polysaccharide CPS-1 from cultured Cordyceps militaris. Fitoterapia 2004, 75, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Ho, K.-J.; Hsieh, Y.-J.; Wang, L.-T.; Mau, J.-L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Dong, J.Z.; Wang, S.H.; Ai, X.R.; Yao, L.; Sun, Z.W.; Lei, C.; Wang, Y.; Wang, Q. Composition and characterization of cordyxanthins from Cordyceps militaris fruit bodies. J. Funct. Foods 2013, 5, 1450–1455. [Google Scholar] [CrossRef]
- Chan, J.S.L.; Barseghyan, G.S.; Asatiani, M.D.; Wasser, S.P. Chemical composition and medicinal value of fruiting bodies and submerged cultured mycelia of caterpillar medicinal fungus Cordyceps militaris CBS-132098 (Ascomycetes). Int. J. Med. Mushrooms 2015, 17, 649–659. [Google Scholar] [CrossRef] [PubMed]
- He, Y.T.; Zhang, X.L.; Xie, Y.M.; Xu, Y.X.; Li, J.R. Extraction and antioxidant property in vitro of cordycepin in artificially cultivated Cordyceps militaris. Adv. Mater. Proc. 2013, 750, 1593–1596. [Google Scholar] [CrossRef]
- Ramesh, T.; Yoo, S.K.; Kim, S.W.; Hwang, S.Y.; Sohn, S.H.; Kim, I.W.; Kim, S.K. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp. Gerontol. 2012, 47, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z. Cordycepin protects PC12 cells against 6-hydroxydopamine induced neurotoxicity via its antioxidant properties. Biomed. Pharmacother. 2016, 81, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Jin, C.Y.; Kim, G.Y.; Lee, J.D.; Park, C.; Kim, G.D.; Kim, W.J.; Jung, W.K.; Seo, S.K.; Choi, I.W. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 2010, 10, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.; Son, M.; Lee, M.; Lee, K.; Cho, J.Y.; Cho, S.; Lee, S.K.; Lee, Y.M.; Cho, H.; Sung, G.H.; et al. Cordycepin is a novel chemical suppressor of Epstein-Barr virus replication. Oncoscience 2014, 1, 866–881. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.H.; Qu, K.; Zhu, H.B. Beneficial effects of cordycepin on metabolic profiles of liver and plasma from hyperlipidemic hamsters. J. Asian Nat. Prod. Res. 2011, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, S.; Du, M. Cordycepin from Cordyceps militaris prevents hyperglycemia in alloxan-induced diabetic mice. Nutr. Res. 2015, 35, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Marchyshak, T.; Semernikova, L.; Yakovenko, T.; Tkachuk, Z. Hepatoprotective and antioxidant effects of oligoribonucleotides-D-mannitol complexes against thioacetamide-induced liver fibrosis. In Proceedings of the 4th International Electronic Conference on Medicinal Chemistry. 2018, p. 5624. Available online: https://www.dl.begellhouse.com/journals/708ae68d64b17c52,05ab5a7e40e7ba9a,3c49713c49c6fa55.html (accessed on 1 May 2024).
- Melnichuk, N.; Zarubaev, V.; Iosyk, I.; Andreychyn, M.; Semernikova, L.; Tkachuk, Z. Pre-clinical and clinical efficiency of complexes of oligoribonucleotides with D-Mannitol against respiratory viruses. Pharmaceutics 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Pera, M.F., Jr.; Harder, H.C. Effects of mannitol or furosemide diuresis on cis-dichlorodiammineplatinum (II) antitumor activity and toxicity to host-renewing cell populations in rats. Cancer Res. 1979, 39, 1279–1286. [Google Scholar] [PubMed]
- Castro-Torres, I.G.; De la O-Arciniega, M.; Naranjo-Rodríguez, E.B.; Castro-Torres, V.A.; Domínguez-Ortíz, M.; Martínez-Vázquez, M. The Hypocholesterolemic Effects of Eryngium carlinae F. Delaroche Are Mediated by the Involvement of the Intestinal Transporters ABCG5 and ABCG8. Evid. Based Complement. Altern. Med. 2017, 2017, 3176232. [Google Scholar] [CrossRef] [PubMed]
- Coker, A.; Coker, I.; Huseyinov, A.; Sokmen, S.; Karademir, S. Is mannitol effective against platelet-activating factor (PAF)-induced liver damage in obstructive jaundice? Hepatogastroenterology 2001, 48, 1134–1137. [Google Scholar] [PubMed]
- Dubost, N.J.; Ou, B.; Beelman, R.B. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Akanmu, D.; Cecchini, R.; Aruoma, O.I.; Halliwell, B. The antioxidant action of ergothioneine. Arch. Biochem. Biophys. 1991, 288, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kato, M.; Tsuchida, H.; Harada, E.; Niwa, T.; Osawa, T. Ergothioneine as an anti-oxidative/anti-inflammatory component in several edible mushrooms. Food Sci. Technol. Res. 2011, 17, 103–110. [Google Scholar] [CrossRef]
- Laurenza, I.; Colognato, R.; Migliore, L.; Del Prato, S.; Benzi, L. Modulation of palmitic acid-induced cell death by ergothioneine: Evidence of an anti-inflammatory action. BioFactors 2008, 33, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Apparao, Y.; Phan, C.W.; Kuppusamy, U.R.; Sabaratnam, V. Ergothioneine and its prospects as an anti-ageing compound. Exp. Gerontol. 2022, 170, 111982. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Vudhya Gowrisankar, Y.; Chen, X.Z.; Yang, Y.C.; Yang, H.L. The Antiaging Activity of Ergothioneine in UVA-Irradiated Human Dermal Fibroblasts via the Inhibition of the AP-1 Pathway and the Activation of Nrf2-Mediated Antioxidant Genes. Oxid. Med. Cell. Longev. 2020, 2020, 2576823. [Google Scholar] [CrossRef]
- Pluskal, T.; Hayashi, T.; Saitoh, S.; Fujisawa, A.; Yanagida, M. Specific biomarkers for stochastic division patterns and starvation-induced quiescence under limited glucose levels in fission yeast. FEBS J. 2011, 278, 1299–1315. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. The unusual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010, 17, 1134–1140. [Google Scholar] [CrossRef]
- Motohashi, N.; Mori, I.; Sugiura, Y.; Tanaka, H. Radioprotective Effect of Ergothioneine on γ-Irradiation of Metmyoglobin : Comparison with Cysteine on Sulfmyoglobin-Formation. Chem. Pharm. Bull. 1977, 25, 2516–2523. [Google Scholar]
- Sanchez-Melgar, A.; Albasanz, J.L.; Guixà-González, R.; Saleh, N.; Selent, J.; Martin, M. The antioxidant resveratrol acts as a non-selective adenosine receptor agonist. Free Radic. Biol. Med. 2019, 135, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Maggirwar, S.B.; Dhanraj, D.N.; Somani, S.M.; Ramkumar, V. Adenosine Acts as an Endogenous Activator of the Cellular Antioxidant Defense System. Biochem. Biophys. Res. Commun. 1994, 201, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Cabalín, C.; Villalobos-Labra, R.; Toledo, F.; Sobrevia, L. Involvement of A2B adenosine receptors as anti-inflammatory in gestational diabesity. Mol. Asp. Med. 2019, 66, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Helms, R.S.; Powell, J.D. Rethinking the adenosine-A2AR checkpoint: Implications for enhancing anti-tumor immunotherapy. Curr. Opin. Pharmacol. 2020, 53, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Le, D.-T.E.; Davis, C.; Nagarajan, S.; Scott, K.L.L.; Cao, Z.; Liu, H.; Nabil, A.; Kaul, S. Ranolazine exhibits anti-ischemic properties by increasing cardiac endothelial cell adenosine levels. J. Am. Coll. Cardiol. 2019, 73, 48. [Google Scholar] [CrossRef]
- Reiss, A.B.; Grossfeld, D.; Kasselman, L.J.; Renna, H.A.; Vernice, N.A.; Drewes, W.; Konig, J.; Carsons, S.E.; DeLeon, J. Adenosine and the cardiovascular system. Am. J. Cardiovasc. Drugs. 2019, 19, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.; Schulz, R.; Nylander, S. Adenosine-mediated effects of ticagrelor: Evidence and potential clinical relevance. J. Am. Coll. Cardiol. 2014, 63, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, J.Y.; Choi, Y.-H.; Kang, N.G.; Lee, S. Anti-Hair Loss Effect of Adenosine Is Exerted by cAMP Mediated Wnt/β-Catenin Pathway Stimulation via Modulation of Gsk3β Activity in Cultured Human Dermal Papilla Cells. Molecules 2022, 27, 2184. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, F.; Zhang, Z.; Terry, N. Optimization of Polysaccharide Production from Cordyceps militaris by Solid-State Fermentation on Rice and Its Antioxidant Activities. Foods 2019, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, G.; Huang, Z. Structural analysis and antioxidant activities of polysaccharides from cultured Cordyceps militaris. Int. J. Biol. Macromol. 2013, 58, 18–22. [Google Scholar] [CrossRef]
- Claus-Desbonnet, H.; Nikly, E.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S.; Pierre, G.; Benbassat, N.; Katsarov, P.; Michaud, P.; Lukova, P. Polysaccharides and their derivatives as potential antiviral molecules. Viruses 2022, 14, 426. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Sousa, C.; Lopes, J.A.; Novais, Â.; Peixe, L. A Front Line on Klebsiella pneumoniae Capsular Polysaccharide Knowledge: Fourier Transform Infrared Spectroscopy as an Accurate and Fast Typing Tool. mSystems 2020, 5, e00386-19. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Lee, J.-B.; Hayashi, K.; Fujita, A.; Park, D.K.; Hayashi, T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agric. Food Chem. 2007, 55, 10194–10199. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-T.; Li, H.-C.; Li, C.-B.; Dou, D.-Q.; Gao, M.-B. Protective Effects on Mitochondria and Anti-Aging Activity of Polysaccharides from Cultivated Fruiting Bodies of Cordyceps militaris. Am. J. Chin. Med. 2010, 38, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Liu, S.; Li, X.; He, J.; He, L.; Li, Y.; Yang, C.; Li, Y.; Hua, Y.; Guo, J. The Large Molecular Weight Polysaccharide from Wild Cordyceps and Its Antitumor Activity on H22 Tumor-Bearing Mice. Molecules 2023, 28, 3351. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.Y.; Kang, J.S.; Kim, H.M.; Kim, Y.O.; Hong, I.P.; Lee, M.K.; Hong, J.T.; Kim, Y.; Han, S.B. Cordlan polysaccharide isolated from mushroom Cordyceps militaris induces dendritic cell maturation through toll-like receptor 4 signalings. Food Chem. Toxicol. 2010, 48, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I.; et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, S.; Choi, H.R.; Baek, M.W.; Cheol, L.H.; Kwak, K.W.; Park, D.S.; Solomon, T.; Jeong, C.S. Antioxidant properties, γ-aminobutyric acid (GABA) content, and physicochemical characteristics of tomato cultivars. Agronomy 2021, 11, 1204. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Wang, M.; Sun, M.; Gu, Z.; Yang, R. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 2019, 270, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.; Glinka, Y.; Wang, Q. GABA exerts anti-inflammatory and immunosuppressive effects (P5175). J. Immunol. 2013, 190, 68.15. [Google Scholar] [CrossRef]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Oh, S.; Lee, H.S.; Choi, J.; Lee, B.J.; Park, J.H.; Park, C.H.; Son, K.H.; Byun, K. Gamma-aminobutyric acid-salt attenuated high cholesterol/high salt diet induced hypertension in mice. Korean J. Physiol. Pharmacol. 2021, 25, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Hussian, N.R.; Al-Naimi, M.S.; Al-Gareeb, A.I.; Al-Mamorri, F.; Al-Buhadily, A.K. The potential role of pancreatic γ-aminobutyric acid (GABA) in diabetes mellitus: A critical reappraisal. Int. J. Prev. Med. 2021, 12, 19. [Google Scholar] [PubMed]
- Al-Janabi, A.A.; Alsalami, M.S.; Mohammed, A.B.; Al-Douri, A.A. Lipids Profiles And Antioxidants Status Of Male Rabbits Fed With Chitosan And Lovastatin. J. Surv. Fish. Sci. 2023, 10, 1459–1467. [Google Scholar]
- Kumar, S.; Srivastava, N.; Gomes, J. The effect of lovastatin on oxidative stress and antioxidant enzymes in hydrogen peroxide intoxicated rat. Food Chem. Toxicol. 2011, 49, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Karampoor, S.; Hesamizadeh, K.; Shams, Z.; Novin, A.G.; Farahmand, M.; Zahednasab, H.; Mirzaei, R.; Zamani, F.; Hajibaba, M.; Bouzari, B. The role of lovastatin in the attenuation of COVID-19. Int. Immunopharmacol. 2021, 101, 108192. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-W.; Shin, P.-G.; Lee, J.-H.; Choi, W.-S.; Kang, M.-J.; Kong, W.-S.; Oh, M.-J.; Seo, Y.-B.; Kim, G.-D. Anti-inflammatory effect of lovastatin is mediated via the modulation of NF-κB and inhibition of HDAC1 and the PI3K/Akt/mTOR pathway in RAW264.7 macrophages. Int. J. Mol. Med. 2018, 41, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C.; Cush, J.; Bolster, M.B.; Striebich, C.C.; Dall’era, M.; Mackay, M.; Olech, E.; Frech, T.; Box, J.; Keating, R. A double-blind, placebo-controlled, phase II, randomized study of lovastatin therapy in the treatment of mildly active rheumatoid arthritis. Rheumatology 2020, 59, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z. Effects of Statins on Gut Microbiota (Microbiome). Clin. Med. Rev. 2019, 6, 55–59. [Google Scholar]
- Jiang, C.; Qi, Z.; Tang, Y.; Jia, H.; Li, Z.; Zhang, W.; Liu, J. Rational design of lovastatin-loaded spherical reconstituted high density lipoprotein for efficient and safe anti-atherosclerotic therapy. Mol. Pharm. 2019, 16, 3284–3291. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.d.P.; Gutiérrez, L.-F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Bovier, E.R.; Hammond, B.R. A randomized placebo-controlled study on the effects of lutein and zeaxanthin on visual processing speed in young healthy subjects. Arch. Biochem. Biophys. 2015, 572, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, Q.; Orfila, C.; Zhao, J.; Zhang, L. Systematic Review and Meta-Analysis on the Effects of Astaxanthin on Human Skin Ageing. Nutrients 2021, 13, 2917. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H. Cancer prevention by carotenoids. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1998, 402, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Keceli, T.; Erginkaya, Z.; Turkkan, E.; Kaya, U. Antioxidant and Antibacterial Effects of Carotenoids Extracted from Rhodotorula glutinis Strains. Asian J. Chem. 2013, 25, 42–46. [Google Scholar] [CrossRef]
- Manimala, M.; Murugesan, R. In vitro antioxidant and antimicrobial activity of carotenoid pigment extracted from Sporobolomyces sp. isolated from natural source. J. Appl. Nat. Sci. 2014, 6, 649–653. [Google Scholar] [CrossRef]
- Peng, H.C.; Chen, J.R.; Chen, Y.L.; Yang, S.C.; Yang, S.S. β-Carotene exhibits antioxidant and anti-apoptotic properties to prevent ethanol-induced cytotoxicity in isolated rat hepatocytes. Phytother. Res. 2010, 24, S183–S189. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Kai, Q.; Gao, J.; Lian, Z.Q.; Wu, C.M.; Wu, C.A.; Zhu, H.B. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J. Pharmacol. Sci. 2010, 113, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Cho, J.Y.; Rhee, M.H.; Kim, H.S.; Lee, H.S.; Park, H.J. Inhibitory effects of cordycepin (3′-deoxyadenosine), a component of Cordyceps militaris, on human platelet aggregation induced by thapsigargin. J. Microbiol. Biotechnol. 2007, 17, 1134–1138. [Google Scholar] [PubMed]
- Baik, J.S.; Kwon, H.Y.; Kim, K.S.; Jeong, Y.K.; Cho, Y.S.; Lee, Y.C. Cordycepin induces apoptosis in human neuroblastoma SK-N-BE(2)-C and melanoma SK-MEL-2 cells. Indian J. Biochem. Biophys. 2012, 49, 86–91. [Google Scholar] [PubMed]
- Noh, E.M.; Kim, J.S.; Hur, H.; Park, B.H.; Song, E.K.; Han, M.K.; Kwon, K.B.; Yoo, W.H.; Shim, I.K.; Lee, S.J.; et al. Cordycepin inhibits IL-1beta-induced MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts. Rheumatology 2009, 48, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, W.M.; Tang, P.C.; Zhang, X.; Zhang, X.Y.; Yu, B.C.; Fan, Y.Y.; Ge, X.Q.; Zhang, X.L. Neuroprotective effects of natural cordycepin on LPS-induced Parkinson’s disease through suppressing TLR4/NF-κB/NLRP3-mediated pyroptosis. J. Funct. Foods 2020, 75, 104274. [Google Scholar] [CrossRef]
- Song, Y.-C.; Liu, C.-T.; Lee, H.-J.; Yen, H.-R. Cordycepin prevents and ameliorates experimental autoimmune encephalomyelitis by inhibiting leukocyte infiltration and reducing neuroinflammation. Biochem. Pharmacol. 2022, 197, 114918. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Y.; Zhang, X.; Cao, X.; Wu, C.; Guo, P. Cordycepin stimulates autophagy in macrophages and prevents atherosclerotic plaque formation in ApoE-/- mice. Oncotarget 2017, 8, 94726–94737. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Kan, W.; Bao, H.; Jia, Y.; Yang, J.; Jia, H. Interactions between adenosine receptors and cordycepin (3′-Deoxyadenosine) from Cordyceps militaris: Possible pharmacological mechanisms for protection of the brain and the amelioration of COVID-19 pneumonia. J. Biomed. Biotechnol. 2021, 4, 26–62. [Google Scholar] [CrossRef]
- Ueda, Y.; Mori, K.; Satoh, S.; Dansako, H.; Ikeda, M.; Kato, N. Anti-HCV activity of the Chinese medicinal fungus Cordyceps militaris. Biochem. Biophys. Res. Commun. 2014, 447, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Song, X.; Ren, Y.; Wang, M.; Guo, C.; Guo, D.; Gu, Y.; Li, Y.; Cao, Z.; Deng, Y. Anti-inflammatory effects of cordycepin: A review. Phytother. Res. 2021, 35, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Park, B.; Lee, J.; Kim, J. Anti-atopic dermatitis properties of Cordyceps militaris on TNFα/IFNγ-stimulated HaCaT cells and experimentally induced atopic dermatitis in mice. Phys. Act. Nutr. 2020, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Moon, P.D.; Kim, H.M.; Jeong, H.J. Cordycepin ameliorates skin inflammation in a DNFB-challenged murine model of atopic dermatitis. Immunopharm. Immunot. 2018, 40, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sear, J.W. Kidney dysfunction in the postoperative period. Br. J. Anaesth. 2005, 95, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Häusler, O.; Blouet, E.; Damien, T. Determination of antioxidant effect of polyols in a cell free environment. In Proceedings of the 12th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, Virtual, 11–14 May 2021. [Google Scholar]
- Schreibman, D.L.; Hong, C.M.; Keledjian, K.; Ivanova, S.; Tsymbalyuk, S.; Gerzanich, V.; Simard, J.M. Mannitol and Hypertonic Saline Reduce Swelling and Modulate Inflammatory Markers in a Rat Model of Intracerebral Hemorrhage. Neurocrit. Care 2018, 29, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Ey, J.; Schömig, E.; Taubert, D. Dietary Sources and Antioxidant Effects of Ergothioneine. J. Agric. Food Chem. 2007, 55, 6466–6474. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, V.S.; Torres, F.F.; de Paula, C.P.; da Silva, J.P.M.d.O.; de Almeida, E.A.; da Cunha, A.F.; da Silva, D.G.H. Potential cytoprotective and regulatory effects of ergothioneine on gene expression of proteins involved in erythroid adaptation mechanisms and Redox pathways in K562 cells. Genes 2022, 13, 2368. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, E.A.; White, P.J.; May, L.T. The adenosine A2B G protein-coupled receptor: Recent advances and therapeutic implications. Pharmacol. Ther. 2019, 198, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Singpoonga, N.; Rittiron, R.; Seang-on, B.; Chaiprasart, P.; Bantadjan, Y. Determination of adenosine and cordycepin concentrations in Cordyceps militaris fruiting bodies using near-infrared spectroscopy. ACS Omega 2020, 5, 27235–27244. [Google Scholar] [CrossRef] [PubMed]
- Novotný, J. Adenosine and its role in physiology. Cesk. Fysiol. 2015, 64, 35–44. [Google Scholar] [PubMed]
- Pelleg, A.; Porter, R.S. The Pharmacology of Adenosine. Pharmacotherap 1990, 10, 157–174. [Google Scholar] [CrossRef]
- Cronstein, B.N. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 1994, 76, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.M.A.; Multhoff, G. Adenosine can thwart antitumor immune responses elicited by radiotherapy: Therapeutic strategies alleviating protumor ADO activities. Strahlenther. Onkol. 2016, 192, 279–287. [Google Scholar] [CrossRef]
- Picano, E.; Abbracchio, M.P. Adenosine, the imperfect endogenous anti-ischemic cardio-neuroprotector. Brain Res. Bull. 2000, 52, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Xue, Y.; Chen, J.; Lv, Q. Ticagrelor compared with clopidogrel increased adenosine and cyclic adenosine monophosphate plasma concentration in acute coronary syndrome patients. Basic Clin. Pharmacol. 2017, 120, 610–614. [Google Scholar] [CrossRef]
- Kim, K.; Lee, T.A.; Ardati, A.K.; DiDomenico, R.J.; Touchette, D.R.; Walton, S.M. Comparative effectiveness of oral antiplatelet agents in patients with acute coronary syndrome. Pharmacotherapy 2017, 37, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Summers, C.; Ewart, L.; Nylander, S.; Sidaway, J.E.; Van Giezen, J. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wei, Y.; Liu, C.; Li, H.; Du, X.; Meng, J.; Liu, J.; Li, Q. Elucidation of antioxidant activities of intracellular and extracellular polysaccharides from Cordyceps militaris in vitro and their protective effects on ulcerative colitis in vivo. Int. J. Biol. Macromol. 2024, 267, 131385. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; He, Z.; Chen, W.; Zhao, Y.; Li, J.; Wang, R.; Zong, Y.; Du, R. Network pharmacology and molecular docking analysis on the mechanism of Cordyceps militaris polysaccharide regulating immunity through TLR4/TNF-α pathwayss. J. Biochem. Mol. Toxicol. 2023, 37, e23345. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Dong, J.-J.; Lee, D.H.; Kim, Y.G.; Lee, M.K.; Cho, J.-Y.; Turk, A.; Vishwakarma, P. Therapeutic potential of Cordyceps militaris mushroom against SARS-CoV-2: Virtual screening against Mpro and in vitro validation. Res. Square. 2023. Available online: https://assets.researchsquare.com/files/rs-3598125/v1/f2b117ea-8913-4fad-a3f8-2312ae9dfd35.pdf (accessed on 1 May 2024).
- Kanlayavattanakul, M.; Lourith, N. Cordyceps militaris polysaccharides: Preparation and topical product application. Fungal Biol. Biotechnol. 2023, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Berlemont, R.; Martiny, A.C. Genomic potential for polysaccharide deconstruction in bacteria. Appl. Environ. Microbiol. 2015, 81, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.A. Microbial hydrolysis of polysaccharides. Annu. Rev. Microbiol. 1996, 50, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Bouché, N.; Fait, A.; Bouchez, D.; Møller, S.G.; Fromm, H. Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Alqarawi, A.A.; Hashem, A.; Elsayed, F.A.A.; Al-Huqail, A.A.; Alshahrani, T.S.; Alshalawi, S.a.R.; Egamberdieva, D. Protective role of gamma amminobutyric acid on Cassia italica Mill under salt stress. Legume Res. 2016, 39, 396–404. [Google Scholar] [CrossRef]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.W.; Steinman, L. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [PubMed]
- Luppi, P.-H.; Peyron, C.; Fort, P. Not a single but multiple populations of GABAergic neurons control sleep. Sleep Med. Rev. 2017, 32, 85–94. [Google Scholar] [CrossRef] [PubMed]
- DeWoskin, D.; Myung, J.; Belle, M.D.; Piggins, H.D.; Takumi, T.; Forger, D.B. Distinct roles for GABA across multiple timescales in mammalian circadian timekeeping. Proc. Natl. Acad. Sci. USA 2015, 112, E3911–E3919. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Li, T.; Ji, F.; Wang, H.; Pang, J. Effect of GABA on blood pressure and blood dynamics of anesthetic rats. Int. J. Clin. Exp. Med. 2015, 8, 14296–14302. [Google Scholar]
- Indrowati, M.; Pratiwi, R.; Astuti, P. Levels of blood glucose and insulin expression of beta-cells in streptozotocin-induced diabetic rats treated with ethanolic extract of Artocarpus altilis leaves and GABA. Pak. J. Biol. Sci. PJBS 2017, 20, 28–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, A.L.; Xiang, Y.-Y.; Gui, L.; Kaltsidis, G.; Feng, Q.; Lu, W.-Y. Paracrine GABA and insulin regulate pancreatic alpha cell proliferation in a mouse model of type 1 diabetes. Diabetologia 2017, 60, 1033–1042. [Google Scholar] [CrossRef]
- Chen, F.; Hu, X. Study on red fermented rice with high concentration of monacolin K and low concentration of citrinin. Int. J. Food Microbiol. 2005, 103, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. A tribute to Akira Endo, discoverer of a “Penicillin” for cholesterol. Atherosclerosis 2004, 3, 13–16. [Google Scholar] [CrossRef]
- Aarons, C.B.; Cohen, P.A.; Gower, A.; Reed, K.L.; Leeman, S.E.; Stucchi, A.F.; Becker, J.M. Statins (HMG-CoA reductase inhibitors) decrease postoperative adhesions by increasing peritoneal fibrinolytic activity. Ann. Surg. 2007, 245, 176–184. [Google Scholar] [CrossRef]
- Gower, T.L.; Graham, B.S. Antiviral activity of lovastatin against respiratory syncytial virus in vivo and in vitro. Antimicrob. Agents Chemother. 2001, 45, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Feleszko, W.; Młynarczuk, I.; Bałkowiec-Iskra, E.Z.; Czajka, A.; Świtaj, T.; Stokłosa, T.; Giermasz, A.; Jakóbisiak, M. Lovastatin potentiates antitumor activity and attenuates cardiotoxicity of doxorubicind in three tumor models in mice1. Clin. Cancer Res. 2000, 6, 2044–2052. [Google Scholar] [PubMed]
- Morikawa, S.; Takabe, W.; Mataki, C.; Wada, Y.; Izumi, A.; Saito, Y.; Hamakubo, T.; Kodama, T. Global analysis of RNA expression profile in human vascular cells treated with statins. J. Atheroscler. Thromb. 2004, 11, 62–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, J.; Wang, J.; Zheng, H.; Ling, W.; Joseph, J.; Li, D.; Mehta, J.L.; Ponnappan, U.; Lin, P.; Fink, L.M.; et al. Statins increase thrombomodulin expression and function in human endothelial cells by a nitric oxide-dependent mechanism and counteract tumor necrosis factor alpha-induced thrombomodulin downregulation. Blood Coagul. Fibrinolysis 2003, 14, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, J.; Wójcik, C.; Jakóbisiak, M.; Stoehr, M.; Schrorter, D.; Paweletz, N. Lovastatin induces mitotic abnormalities in various cell lines. Cell Biol. Int. 1999, 23, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Mastej, M.; Sułkowska-Ziaja, K. Biological function of carotenoids and their occurrence in the fruiting bodies of mushrooms. Med. Int. Rev. 2016, 107, 113–122. [Google Scholar]
- Edge, R.; Truscott, T. Properties of Carotenoid Radicals and Excited States and Their Potential Role in Biological Systems; CRC Press: Boca Raton, FL, USA, 2010; pp. 283–308. [Google Scholar]
- Chantrell, S.J.; McAuliffe, C.A.; Munn, R.W.; Pratt, A.C.; Land, E.J. Excited states of protoporphyrin IX dimethyl ester: Reaction on the triplet with carotenoids. JACS 1977, 73, 858–865. [Google Scholar] [CrossRef]
- Fiedor, J.; Fiedor, L.; Haeßner, R.; Scheer, H. Cyclic endoperoxides of β-carotene, potential pro-oxidants, as products of chemical quenching of singlet oxygen. Biochim. Biophys. Acta (BBA) 2005, 1709, 1–4. [Google Scholar] [CrossRef]
- Stratton, S.P.; Schaefer, W.H.; Liebler, D.C. Isolation and identification of singlet oxygen oxidation products of β-carotene. Chem. Res. Toxicol. 1993, 6, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Scheer, H. The pigments. In Light-Harvesting Antennas in Photosynthesis; Springer: Dordrecht, The Netherlands, 2003; pp. 29–81. [Google Scholar]
- Christensen, R.L. The electronic states of carotenoids. In The Photochemistry of Carotenoids; Springer: Dordrecht, The Netherlands, 1999; pp. 137–159. [Google Scholar]
- Matsushita, S.; Terao, J. Singlet oxygen-initiated photooxidation of unsaturated fatty acid esters and inhibitory effects of tocopherols and β-carotene. In Autoxidation in Food and Biological Systems; Springer: Boston, MA, USA, 1980; pp. 27–44. [Google Scholar]
- Sesso, H.D.; Buring, J.E.; Norkus, E.P.; Gaziano, J.M. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am. J. Clin. Nutr. 2004, 79, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. Antioxidant activity of β-carotene-related carotenoids in solution. Lipids 1989, 24, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Serini, S.; Torsello, A.; Di Nicuolo, F.; Piccioni, E.; Ubaldi, V.; Pioli, C.; Wolf, F.I.; Calviello, G. β-Carotene regulates NF-κB DNA-binding activity by a redox mechanism in human leukemia and colon adenocarcinoma cells. J. Nutr. 2003, 133, 381–388. [Google Scholar] [CrossRef]
- Simone, R.E.; Russo, M.; Catalano, A.; Monego, G.; Froehlich, K.; Boehm, V.; Palozza, P. Lycopene inhibits NF-kB-mediated IL-8 expression and changes redox and PPARγ signalling in cigarette smoke–stimulated macrophages. PLoS ONE 2011, 6, e19652. [Google Scholar] [CrossRef] [PubMed]
- Armoza, A.; Haim, Y.; Basiri, A.; Wolak, T.; Paran, E. Tomato extract and the carotenoids lycopene and lutein improve endothelial function and attenuate inflammatory NF-κB signaling in endothelial cells. J. Hypertens. 2013, 31, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.K.; Park, M.K.; Cho, M.L.; Oh, H.J.; Park, E.M.; Lee, D.G.; Lee, J.; Kim, H.Y.; Park, S.H. Retinoic acid attenuates rheumatoid inflammation in mice. J. Immunol. 2012, 189, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Linnewiel-Hermoni, K.; Motro, Y.; Miller, Y.; Levy, J.; Sharoni, Y. Carotenoid derivatives inhibit nuclear factor kappa B activity in bone and cancer cells by targeting key thiol groups. Free Radic. Biol. Med. 2014, 75, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Han, S.; Wang, H.; Wang, T. Lutein extends the lifespan of Drosophila melanogaster. Arch. Gerontol. Geriatr. 2014, 58, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Keller, T.; Hendrix, C.; Hamilton, S.; Arena, R.; Tuason, M.; Gonzalez, S. Regulation of the extracellular matrix remodeling by lutein in dermal fibroblasts, melanoma cells, and ultraviolet radiation exposed fibroblasts. Arch. Dermatol. Res. 2007, 299, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Yoshikoshi, C.; Oshiro, S.; Yanase, S. Supplemental cellular protection by a carotenoid extends lifespan via Ins/IGF-1 signaling in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2011, 2011, 596240. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.V.; Xu, W.; Li, Y.; Qin, J.; Zhou, T.; Li, D.; Xu, Y.; Cheng, X.; Xiong, Y.; Chen, Z. Anti-aging effect of β-carotene through regulating the KAT7-P15 signaling axis, inflammation and oxidative stress process. Cell. Mol. Biol. Lett. 2022, 27, 86. [Google Scholar] [CrossRef] [PubMed]
- Alni, R.H.; Ghorban, K.; Dadmanesh, M. Combined effects of Allium sativumand cuminum cyminumessential oils on planktonic and biofilm forms of Salmonella typhimurium isolates. 3 Biotech 2020, 10, 315. [Google Scholar]
- Tao, N.; Gao, Y.; Liu, Y.; Ge, F. Carotenoids from the peel of Shatian pummelo (Citrus grandis Osbeck) and its antimicrobial activity. Am. Eurasian J. Agric. Environ. Sci. 2010, 7, 110–115. [Google Scholar]
- Hwang, S.; Lim, J.W.; Kim, H. Inhibitory effect of lycopene on amyloid-β-induced apoptosis in neuronal cells. Nutrients 2017, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Soleymaninejad, M.; Joursaraei, S.G.; Feizi, F.; Jafari Anarkooli, I. The effects of lycopene and insulin on histological changes and the expression level of Bcl-2 family genes in the hippocampus of streptozotocin-induced diabetic rats. J. Diabetes Res. 2017, 2017, 4650939. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Gan, D.; Fan, C.; Wen, C.; Li, A.; Li, Q.; Zhao, J.; Wang, Z.; Zhu, L.; Lu, D. The secretion from neural stem cells pretreated with lycopene protects against tert-butyl hydroperoxide-induced neuron oxidative damage. Oxid. Med. Cell. Longev. 2018, 2018, 5490218. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Yang, D.; Fu, Z.J.; Woo, T.; Wong, D.; Lo, A.C.Y. Lutein enhances survival and reduces neuronal damage in a mouse model of ischemic stroke. Neurobiol. Dis. 2012, 45, 624–632. [Google Scholar] [CrossRef]
- Fung, F.K.; Law, B.Y.; Lo, A.C. Lutein attenuates both apoptosis and autophagy upon cobalt (II) chloride-induced hypoxia in rat Műller cells. PLoS ONE 2016, 11, e0167828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.; Zhao, J.; Li, Q.; Huang, C.; Zhu, L.; Lu, D. Neuroprotective effect of lutein on NMDA-induced retinal ganglion cell injury in rat retina. Cell. Mol. Neurobiol. 2016, 36, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Hwang, H.J.; Park, J.P.; Cho, Y.J.; Song, C.H.; Yun, J.W. Mycelial growth and exo-biopolymer production by submerged culture of various edible mushrooms under different media. Lett. Appl. Microbiol. 2002, 34, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. Enrichment of cordycepin for cosmeceutical applications: Culture systems and strategies. Appl. Microbiol. Biotechnol. 2019, 103, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Lee, C.; Chang, E. Optimization of solid state culture conditions for the production of adenosine, cordycepin, and D-mannitol in fruiting bodies of medicinal caterpillar fungus Cordyceps militaris (L.:Fr.) Link (Ascomycetes). Int. J. Med. Mushrooms 2012, 14, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Yoon, D.H.; Shrestha, B.; Choi, H.K.; Sung, G.H. Metabolomic profiling reveals enrichment of cordycepin in senescence process of Cordyceps militaris fruit bodies. J. Microbiol. 2019, 57, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D. Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Crit. Rev. Biotechnol. 2015, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Ashraf, S.A.; Khan, S.; Alshammari, E.; Awadelkareem, A.M. Effect of pH, temperature and incubation time on cordycepin production from Cordyceps militaris using solid-state fermentation on various substrates. CyTA-J. Food 2017, 15, 617–621. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Effect of solid-state fermentation with Cordyceps militaris SN-18 on physicochemical and functional properties of chickpea (Cicer arietinum L.) flour. LWT—Food Sci. Technol. 2015, 63, 1317–1324. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zhang, P.; Le, M.-M.; Qi, Y.; Yang, Z.; Hu, F.-L.; Ling, T.-J.; Bao, G.-H. Improving flavor of summer Keemun black tea by solid-state fermentation using Cordyceps militaris revealed by LC/MS-based metabolomics and GC/MS analysis. Food Chem. 2023, 407, 135172. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-R.; Zhang, W.-R. Optimization of submerged culture conditions involving a developed fine powder solid seed for exopolysaccharide production by the medicinal mushroom Ganoderma lucidum. Food Sci. Biotechnol. 2018, 28, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Hatashita, M.; Sakurai, A.; Sakakibara, M. A new approach for improving cordycepin productivity in surface liquid culture of Cordyceps militaris using high-energy ion beam irradiation. Lett. Appl. Microbiol. 2008, 47, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Wu, J.Y.; Chang, C.Y.; Yu, S.T.; Liu, Y.C. Enhanced exopolysaccharide production by Cordyceps militaris using repeated batch cultivation. J. Biosci. Bioeng. 2019, 127, 499–505. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, H.J.; Xu, C.P.; Sung, J.M.; Choi, J.W.; Yun, J.W. Optimization of submerged culture process for the production of mycelial biomass and exo-polysaccharides by Cordyceps militaris C738. J. Appl. Microbiol. 2003, 94, 120–126. [Google Scholar] [CrossRef]
- Blaby, I.K.; de Crécy-Lagard, V.; Lyons, T.J. 1.22—Modes of Culture/Microbial. In Comprehensive Biotechnology, 2nd ed; 2011; pp. 303–314. Available online: https://books.google.com.tw/books?hl=zh-TW&lr=&id=uyWqDwAAQBAJ&oi=fnd&pg=PP1&dq=Comprehensive+Biotechnology&ots=nlwkQWRXxe&sig=KFVRuzrc2g9lq2hHJb0_qLTrIZM&redir_esc=y#v=onepage&q=Comprehensive%20Biotechnology&f=false (accessed on 1 May 2024).
- Ghomi Avili, M.; Fazaelipoor, M.H.; Jafari, S.A.; Ataei, S.A. Comparison between batch and fed-batch production of rhamnolipid by Pseudomonas aeruginosa. Iran. J. Biotechnol. 2012, 10, 263–269. [Google Scholar]
- Joshi, R.; Sharma, V.; Kuila, A. Fermentation Technology: Current status and future prospects. In Principles and Applications of Fermentation Technology; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Sharma, R.; Joshi, R.; Kumar, D. Present status and future prospect of genetic and metabolic engineering for biofuels production from lignocellulosic biomass. In Genetic and Metabolic Engineering for Improved Biofuel Production from Lignocellulosic Biomass; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Mao, X.-B.; Zhong, J.-J. Significant effect of NH4+ on cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris. Enzyme Microb. Technol. 2006, 38, 343–350. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.H.; Kim, H.R.; Chun, Y.; Lee, J.H.; Yoo, H.Y.; Park, C.; Kim, S.W. Improved Cordycepin Production by Cordyceps militaris KYL05 Using Casein Hydrolysate in Submerged Conditions. Biomolecules 2019, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Setlhaku, M.; Brunberg, S.; Villa Edel, A.; Wichmann, R. Improvement in the bioreactor specific productivity by coupling continuous reactor with repeated fed-batch reactor for acetone-butanol-ethanol production. J. Biotechnol. 2012, 161, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Das, S.K.; Fujihara, S.; Hatashita, M.; Sakurai, A. Production of cordycepin by a repeated batch culture of a Cordyceps militaris mutant obtained by proton beam irradiation. J. Biosci. Bioeng. 2011, 111, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, Z.; Chen, N.; Xu, Q. Enhancing the efficiency of L-tyrosine by repeated batch fermentation. Bioengineered 2020, 11, 852–886. [Google Scholar] [CrossRef] [PubMed]
- Ganjali Dashti, M.; Abdeshahian, P.; Wan Yusoff, W.M.; Kalil, M.S.; Abdul Hamid, A. Repeated batch fermentation biotechnology for the biosynthesis of lipid and gamma-linolenic acid by Cunninghamella bainieri 2A1. Biomed. Res. Int. 2014, 2014, 831783. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Hao, M.; Liu, W.; Zheng, W.; Fan, S.; Wu, Z. Foam fractionation for the concentration of exopolysaccharides produced by repeated batch fermentation of Cordyceps militaris. Sep. Purif. Technol. 2019, 210, 682–689. [Google Scholar] [CrossRef]
- Liu, J.-M.; Yu, T.-C.; Lin, S.-P.; Hsu, R.-J.; Hsu, K.-D.; Cheng, K.-C. Evaluation of kojic acid production in a repeated-batch PCS biofilm reactor. J. Biotechnol. 2016, 218, 41–48. [Google Scholar] [CrossRef]
- Shih, I.-L.; Tsai, K.-L.; Hsieh, C. Effects of culture conditions on the mycelial growth and bioactive metabolite production in submerged culture of Cordyceps militaris. Biochem. Eng. J. 2007, 33, 193–201. [Google Scholar] [CrossRef]
- Lin, R.; Liu, H.; Wu, S.; Pang, L.; Jia, M.; Fan, K.; Jia, S.; Jia, L. Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int. J. Biol. Macromol. 2012, 51, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.-D.; Jia, S.-R. Optimization of medium on exopolysaccharides production in submerged culture of Cordyceps militaris. Food Sci. Biotechnol. 2010, 19, 1567–1571. [Google Scholar] [CrossRef]
- Kang, C.; Wen, T.-C.; Kang, J.-C.; Meng, Z.-B.; Li, G.-R.; Hyde, K.D. Optimization of large-scale culture conditions for the production of cordycepin with Cordyceps militaris by liquid static culture. Sci. World J. 2014, 2014, 510627. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).