Agroecological Transformation: Implementation of an Agroforestry System in a Construction Debris Area Focusing on Vegetables Development through Microbial Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Area Preparation
2.2. Experimental Design
2.3. Inoculum Preparation
2.4. Data Collecting and Analysis
2.5. Statistical Analysis
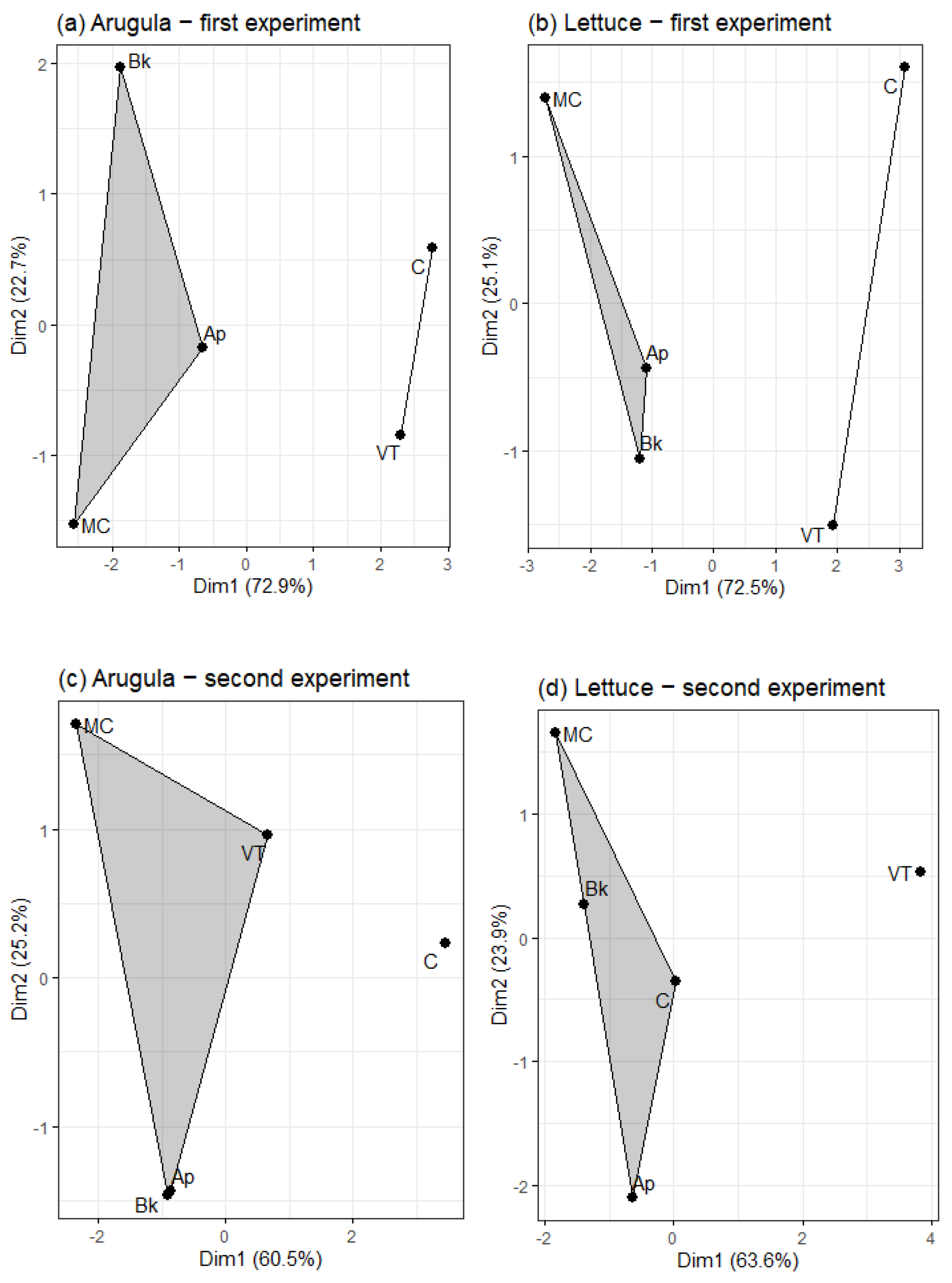
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.-H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for Feeding the World More Sustainably with Organic Agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Ho, M.D.; Phosphorus, L. Rhizoeconomics: Carbon Costs of Phosphorus Acquisition. Plant Soil 2005, 269, 45–56. [Google Scholar] [CrossRef]
- Beltran-Peña, A.; Rosa, L.; D’Odorico, P. Global Food Self-Sufficiency in the 21st Century under Sustainable Intensification of Agriculture. Environ. Res. Lett. 2020, 15, 095004. [Google Scholar] [CrossRef]
- Miccolis, A.; Peneireiro, F.M.; Vieira, D.L.M.; Marques, H.R.; Hoffmann, M.R.M. Restoration through Agroforestry: Options for Reconciling Livelihoods with Conservation in the Cerrado and Caatinga Biomes in Brazil. Exp. Agric. 2019, 55, 208–225. [Google Scholar] [CrossRef]
- Matos, P.S.; Cherubin, M.R.; Damian, J.M.; Rocha, F.I.; Pereira, M.G.; Zonta, E. Short-Term Effects of Agroforestry Systems on Soil Health in Southeastern Brazil. Agrofor. Syst. 2022, 96, 897–908. [Google Scholar] [CrossRef]
- Beule, L.; Vaupel, A.; Moran-Rodas, V.E. Abundance, Diversity, and Function of Soil Microorganisms in Temperate Alley-Cropping Agroforestry Systems: A Review. Microorganisms 2022, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K.; et al. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Bacteria as Inoculants in Agricultural Soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Lopes, M.J.d.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Bomfim, C.A.; Coelho, L.G.F.; do Vale, H.M.M.; de Carvalho Mendes, I.; Megías, M.; Ollero, F.J.; dos Reis Junior, F.B. Brief History of Biofertilizers in Brazil: From Conventional Approaches to New Biotechnological Solutions. Braz. J. Microbiol. 2021, 52, 2215–2232. [Google Scholar] [CrossRef]
- Hungria, M.; Barbosa, J.Z.; Rondina, A.B.L.; Nogueira, M.A. Improving Maize Sustainability with Partial Replacement of N Fertilizers by Inoculation with Azospirillum Brasilense. Agron. J. 2022, 114, 2969–2980. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial Inoculants: Reviewing the Past, Discussing the Present and Previewing an Outstanding Future for the Use of Beneficial Bacteria in Agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef]
- Mira, W.V.W.; Esposito, E.; Vilarraga, C.O.; Anatriello, E. APLICAÇÃO DE BIOINOCULANTES DE SOLO COMPOSTADO PARA PROMOÇÃO DO CRESCIMENTO DE RÚCULA (Eruca sativa), CENOURA (Daucus carota sativus) e RABANETE (Raphanus sativus). Rev. Bras. Agroecol. 2021, 16, 117–122. [Google Scholar] [CrossRef]
- Kuraganti, G.; Edla, S.; Pallaval, V.B. Cyanobacteria as Biofertilizers: Current Research, Commercial Aspects, and Future Challenges. In Advances in Plant Microbiome and Sustainable Agriculture; Yadav, A., Rastegari, A., Yadav, N., Kour, D., Eds.; Springer: Singapore, 2020; pp. 259–278. [Google Scholar]
- Zapata, D.; Arroyave, C.; Cardona, L.; Aristizábal, A.; Poschenrieder, C.; Llugany, M. Phytohormone Production and Morphology of Spirulina Platensis Grown in Dairy Wastewaters. Algal Res. 2021, 59, 102469. [Google Scholar] [CrossRef]
- Rossi, F.; Li, H.; Liu, Y.; De Philippis, R. Cyanobacterial Inoculation (Cyanobacterisation): Perspectives for the Development of a Standardized Multifunctional Technology for Soil Fertilization and Desertification Reversal. Earth Sci. Rev. 2017, 171, 28–43. [Google Scholar] [CrossRef]
- Alobwede, E.; Leake, J.R.; Pandhal, J. Circular Economy Fertilization: Testing Micro and Macro Algal Species as Soil Improvers and Nutrient Sources for Crop Production in Greenhouse and Field Conditions. Geoderma 2019, 334, 113–123. [Google Scholar] [CrossRef]
- Manjunath, M.; Kanchan, A.; Ranjan, K.; Venkatachalam, S.; Prasanna, R.; Ramakrishnan, B.; Hossain, F.; Nain, L.; Shivay, Y.S.; Rai, A.B.; et al. Beneficial Cyanobacteria and Eubacteria Synergistically Enhance Bioavailability of Soil Nutrients and Yield of Okra. Heliyon 2016, 2, e00066. [Google Scholar] [CrossRef]
- Churilova, E.; Midmore, D. Vermiliquer (Vermicompost Leachate) as a Complete Liquid Fertilizer for Hydroponically-Grown Pak Choi (Brassica chinensis L.) in the Tropics. Horticulturae 2019, 5, 26. [Google Scholar] [CrossRef]
- Helaly, A.; El-Dakak, R. Effect of Organic Liquid Vermicompost as a Substitute for Chemical Fertilizer on Morphological and Biochemical Characteristics in Lettuce. Assiut J. Agric. Sci. 2021, 52, 69–81. [Google Scholar] [CrossRef]
- Quiroz, M.; Céspedes, C. Bokashi as an Amendment and Source of Nitrogen in Sustainable Agricultural Systems: A Review. J. Soil. Sci. Plant Nutr. 2019, 19, 237–248. [Google Scholar] [CrossRef]
- Restrepo, J.; Hensel, J. El ABC de La Agricultura Orgánica, Fosfitos y Panes de Piedra; Impressora Feriva S.A.: Santiago de Cali, Colombia, 2013; 396p. [Google Scholar]
- Wilmer, J.; Rodriguez, W.; Rosas, G. Caracterización Física y Química de Bokashi y Lombricompost y su Evaluación Agronómica en Plantas de Maíz. Ing. Amazon. 2014, 7, 5–16. [Google Scholar]
- Theodoro, S.H.; de Paula Medeiros, F.; Ianniruberto, M.; Baiocchi Jacobson, T.K. Soil Remineralization and Recovery of Degraded Areas: An Experience in the Tropical Region. J. South. Am. Earth Sci. 2021, 107, 103014. [Google Scholar] [CrossRef]
- Gris, D.; Temponi, L.G.; Marcon, T.R. Native Species Indicated for Degraded Area Recovery in Western Paraná, Brazil. Rev. Árvore 2012, 36, 113–125. [Google Scholar] [CrossRef][Green Version]
- Alori, E.T.; Gabasawa, A.I.; Elenwo, C.E.; Agbeyegbe, O.O. Bioremediation Techniques as Affected by Limiting Factors in Soil Environment. Front. Soil Sci. 2022, 2, 937186. [Google Scholar] [CrossRef]
- Bicca, J.M.; Nunes Arduin, R.L.; Spinelli Pinto, L.F.; Bamberg, A.L.; Miguel, P.; Stumpf, L. Clay Stabilization and Recovery of Soil Functions of a Degraded Solodic Planosol through Incorporation of Agrominerals: A Case Study in Southern Brazil. J. S. Am. Earth Sci. 2023, 122, 104177. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo-Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Brazilian Soil Classification System, 5th ed.; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Morais, I.P.A.; Tóth, I.V.; Rangel, A.O.S.S. Turbidimetric and Nephelometric Flow Analysis: Concepts and Applications. Spectrosc. Lett. 2006, 39, 547–579. [Google Scholar] [CrossRef]
- Muliterno, A.; Mosele, P.C.; Costa, J.A.V.; Hemkemeier, M.; Bertolin, T.E.; Colla, L.M. Cultivo Mixotrófico Da Microalga Spirulina Platensis Em Batelada Alimentada. Ciência Agrotecnologia 2005, 29, 1132–1138. [Google Scholar] [CrossRef][Green Version]
- Tennant, D. A Test of a Modified Line Intersect Method of Estimating Root Length. J. Ecol. 1975, 63, 995. [Google Scholar] [CrossRef]
- Zangaro, W.; Rostirola, L.V.; de Souza, P.B.; de Almeida Alves, R.; Lescano, L.E.A.M.; Rondina, A.B.L.; Nogueira, M.A.; Carrenho, R. Root Colonization and Spore Abundance of Arbuscular Mycorrhizal Fungi in Distinct Successional Stages from an Atlantic Rainforest Biome in Southern Brazil. Mycorrhiza 2013, 23, 221–233. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and Functional Redundancy in Microbial Systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of Soil Microbial Diversity through Sustainable Agricultural Practices and Its Evaluation by -Omics Approaches: A Perspective for the Environment, Food Quality and Human Safety. Microorganisms 2021, 9, 1400. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a Key Player in Sustainable Agriculture and Human Health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Polyanskaya, L.M.; Vedina, O.T.; Lysak, L.V.; Zvyagintsev, D.G. The Growth-Promoting Effect of Beijerinckia and Clostridium Sp. Cultures on Some Agricultural Crops. Microbiology 2002, 71, 109–115. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Ayangbenro, A.S.; Babalola, O.O. Elucidating the Rhizosphere Associated Bacteria for Environmental Sustainability. Agriculture 2021, 11, 75. [Google Scholar] [CrossRef]
- Fritz, J.I.; Franke-Whittle, I.H.; Haindl, S.; Insam, H.; Braun, R. Microbiological Community Analysis of Vermicompost Tea and Its Influence on the Growth of Vegetables and Cereals. Can. J. Microbiol. 2012, 58, 836–847. [Google Scholar] [CrossRef]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential Applications of Cyanobacteria: Spirulina Platensis Filtrates and Homogenates in Agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80. [Google Scholar] [CrossRef]
- Prasanna, R.; Kanchan, A.; Ramakrishnan, B.; Ranjan, K.; Venkatachalam, S.; Hossain, F.; Shivay, Y.S.; Krishnan, P.; Nain, L. Cyanobacteria-Based Bioinoculants Influence Growth and Yields by Modulating the Microbial Communities Favourably in the Rhizospheres of Maize Hybrids. Eur. J. Soil Biol. 2016, 75, 15–23. [Google Scholar] [CrossRef]
- Molina-Favero, C.; Creus, C.M.; Simontacchi, M.; Puntarulo, S.; Lamattina, L. Aerobic Nitric Oxide Production by Azospirillum Brasilense Sp245 and Its Influence on Root Architecture in Tomato. Mol. Plant-Microbe Interact. 2008, 21, 1001–1009. [Google Scholar] [CrossRef]
- Cassán, F.; Vanderleyden, J.; Spaepen, S. Physiological and Agronomical Aspects of Phytohormone Production by Model Plant-Growth-Promoting Rhizobacteria (PGPR) Belonging to the Genus Azospirillum. J. Plant Growth Regul. 2014, 33, 440–459. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits That Go Far beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Comas, L.H.; Mueller, K.E.; Taylor, L.L.; Midford, P.E.; Callahan, H.S.; Beerling, D.J. Evolutionary Patterns and Biogeochemical Significance of Angiosperm Root Traits. Int. J. Plant Sci. 2012, 173, 584–595. [Google Scholar] [CrossRef]
- Rondina, A.B.L.; dos Santos Sanzovo, A.W.; Guimarães, G.S.; Wendling, J.R.; Nogueira, M.A.; Hungria, M. Changes in Root Morphological Traits in Soybean Co-Inoculated with Bradyrhizobium Spp. and Azospirillum Brasilense or Treated with A. Brasilense Exudates. Biol. Fertil. Soils 2020, 56, 537–549. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; de Vries, F.T. Root Functional Traits Explain Root Exudation Rate and Composition across a Range of Grassland Species. J. Ecol. 2022, 110, 21–33. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates: From Plant to Rhizosphere and Beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Ryser, P. The Importance of Tissue Density for Growth and Life Span of Leaves and Roots: A Comparison of Five Ecologically Contrasting Grasses. Funct. Ecol. 1996, 10, 717. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root Traits Are Multidimensional: Specific Root Length Is Independent from Root Tissue Density and the Plant Economic Spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- McPherson, D.C. Cortical Air Spaces in the Roots of Zea Mays L. New Phytol. 1939, 38, 190–202. [Google Scholar] [CrossRef]
- Dias, G.A.; Rocha, R.H.C.; Araújo, J.L.; Lima, J.F.; Guedes, W.A. Growth, Yield, and Postharvest Quality in Eggplant Produced under Different Foliar Fertilizer (Spirulina platensis) Treatments. Semin. Cienc. Agrar. 2016, 37, 3893. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Arumugam, A.; Ahamed Rasheeq, A.; Sampathkumar, P. Exploring the Microalgae Biofertilizer Effect on Onion Cultivation by Field Experiment. Waste Biomass Valorization 2020, 11, 77–87. [Google Scholar] [CrossRef]
- Siringi, J.O.; Turoop, L.; Njonge, F. Biostimulant Effect of Spirulina (Arthrospira platensis) on Lettuce (Lactuca sativa) Cultivated under Aquaponic System. SCIREA J. Biol. 2022, 7, 23–40. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina Biomass Produced from Treatment of Aquaculture Wastewater as Agricultural Fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Prasanna, R.; Bidyarani, N.; Babu, S.; Hossain, F.; Shivay, Y.S.; Nain, L. Cyanobacterial Inoculation Elicits Plant Defense Response and Enhanced Zn Mobilization in Maize Hybrids. Cogent Food Agric. 2015, 1, 998507. [Google Scholar] [CrossRef]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, Y.S.; Nain, L. Biofortification of Wheat through Inoculation of Plant Growth Promoting Rhizobacteria and Cyanobacteria. Eur. J. Soil. Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.-R. How Effective Are ‘Effective Microorganisms® (EM)’? Results from a Field Study in Temperate Climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of Animal Manures and Chemical Criteria for Compost Maturity Assessment. A Review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Zucconi, F.; Monaco, A.; Forte, M. Phytotoxins during the Stabilization of Organic Matter; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]

| pH (CaCl2) | OM | P-res | K | Ca | Mg | H + Al | Al | SB | CEC | V | m | S | B | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g dm−3 | mg dm−3 | mmolc/dm3 | % | mg/dm3 | |||||||||||||
| 6.5 | 17 | 26 | 3.1 | 52 | 9 | 14 | <0.7 | 64.1 | 78 | 82 | 1 | 10.0 | 0.22 | 2.50 | 27.0 | 1.90 | 6.30 |
| Shoot Dry Weight | Root Dry Weight | Root Volume | Root System Length | Root Mean Diameter | Root Surface Area | Root Specific Length | Root Density | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | cm3 | cm | mm | cm2 | m/g | mg/cm3 | ||||||||||
| First Experiment | C | 1.33 | b | 0.10 | b | 1.33 | b | 190.66 | b | 0.94 | 56.38 | b | 22.68 | ab | 72.72 | bc |
| VT | 1.48 | b | 0.10 | b | 1.67 | b | 235.71 | b | 0.93 | 70.12 | b | 25.67 | a | 60.20 | c | |
| Ap | 2.82 | ab | 0.21 | a | 2.21 | b | 293.84 | ab | 0.99 | 88.49 | b | 15.45 | bc | 94.57 | ab | |
| MC | 3.97 | a | 0.26 | a | 3.68 | a | 348.13 | a | 1.15 | 125.96 | a | 13.70 | bc | 73.38 | bc | |
| Bk | 3.42 | a | 0.26 | a | 2.41 | b | 209.03 | b | 1.25 | 78.18 | b | 8.58 | c | 105.97 | a | |
| C.V. (%) | 44.55 | 39.94 | 37.20 | 28.49 | 18.51 | 26.75 | 38.95 | 23.87 | ||||||||
| Second Experiment | C | 1.16 | c | 0.05 | b | 0.83 | b | 197.73 | b | 0.71 | 44.64 | b | 44.60 | 120.16 | ||
| VT | 1.83 | bc | 0.08 | ab | 1.43 | ab | 467.49 | a | 0.69 | 90.57 | a | 46.40 | 107.79 | |||
| Ap | 2.76 | ab | 0.11 | a | 1.63 | a | 375.04 | a | 0.76 | 87.13 | a | 35.33 | 144.55 | |||
| MC | 3.15 | a | 0.13 | a | 2.10 | a | 459.90 | a | 0.63 | 101.37 | a | 58.40 | 142.25 | |||
| Bk | 2.45 | ab | 0.12 | a | 1.73 | a | 406.99 | a | 0.80 | 98.27 | a | 37.69 | 133.00 | |||
| C.V. (%) | 39.70 | 34.70 | 34.68 | 18.19 | 21.63 | 17.32 | 36.46 | 22.72 | ||||||||
| Shoot Dry Weigh | Root Dry Weigh | Root Volume | Root System Length | Root Mean Diameter | Root Surface Area | Root Specific Length | Root Density | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | cm3 | cm | mm | cm2 | m/g | mg/cm3 | ||||||||||
| First Experiment | C | 3.56 | b | 0.48 | b | 4.80 | b | 556.70 | b | 1.08 | 181.81 | c | 11.62 | b | 494.21 | |
| VT | 3.32 | b | 0.44 | b | 4.67 | b | 662.35 | b | 0.94 | 193.49 | c | 15.81 | ab | 269.30 | ||
| Ap | 5.58 | ab | 0.58 | b | 6.33 | b | 1083.88 | a | 0.86 | 292.74 | b | 18.87 | a | 365.36 | ||
| MC | 7.34 | a | 0.85 | a | 8.47 | a | 1396.03 | a | 0.91 | 380.65 | a | 16.41 | ab | 408.67 | ||
| Bk | 5.00 | b | 0.60 | b | 6.43 | b | 1133.92 | a | 0.85 | 301.84 | b | 18.85 | a | 301.19 | ||
| C.V. (%) | 33.02 | 23.78 | 21.14 | 30.86 | 15.49 | 21.16 | 22.67 | 6.54 * | ||||||||
| Second Experiment | C | 2.01 | 0.25 | 3.17 | 719.44 | 0.75 | a | 168.82 | 32.35 | a | 129.85 | |||||
| VT | 1.89 | 0.18 | 2.40 | 503.10 | 1.03 | b | 148.50 | 17.88 | b | 132.56 | ||||||
| Ap | 1.84 | 0.23 | 3.20 | 854.84 | 0.68 | a | 184.74 | 38.24 | a | 122.55 | ||||||
| MC | 2.79 | 0.30 | 3.27 | 743.27 | 0.66 | a | 182.80 | 44.21 | a | 138.58 | ||||||
| Bk | 2.32 | 0.32 | 3.67 | 856.41 | 0.69 | a | 148.20 | 35.05 | a | 129.65 | ||||||
| C.V. (%) | 40.39 | 36.69 | 31.42 | 26.93 | 19.19 | 22.3 | 30.26 | 11.33 | ||||||||
| N | P | K | Ca | Mg | S | B | Cu | Fe | Mn | Zn | Proteins | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/Plant | µg/Plant | mg/Plant | |||||||||||||||||||||||
| Arugula | C | 49.68 | c | 3.30 | b | 66.72 | c | 42.85 | d | 5.36 | d | 23.21 | c | 35.38 | d | 13.52 | c | 1672.35 | c | 35.42 | c | 150.74 | c | 310.54 | c |
| VT | 50.94 | c | 4.26 | b | 74.74 | c | 49.94 | d | 5.89 | d | 24.37 | c | 42.74 | d | 15.21 | c | 955.67 | c | 39.98 | c | 171.83 | c | 318.36 | c | |
| Ap | 111.92 | b | 9.83 | a | 146.33 | b | 94.73 | c | 10.87 | c | 38.61 | b | 78.53 | c | 30.15 | b | 3359.18 | b | 76.09 | b | 243.86 | bc | 699.55 | b | |
| MC | 136.48 | a | 11.10 | a | 201.62 | a | 126.99 | a | 15.15 | a | 62.66 | a | 110.72 | a | 44.84 | a | 7202.48 | a | 112.17 | a | 467.73 | a | 853.05 | a | |
| Bk | 115.45 | b | 11.87 | a | 184.31 | ab | 109.33 | b | 13.07 | b | 55.59 | a | 95.20 | b | 33.80 | b | 3416.98 | b | 104.66 | a | 355.55 | ab | 721.59 | b | |
| C.V. (%) | 9.18 | 25.95 | 18.52 | 5.35 | 10.50 | 10.65 | 10.51 | 9.53 | 20.77 * | 11.51 | 21.54 | 9.18 | |||||||||||||
| Lettuce | C | 85.36 | b | 14.77 | c | 191.99 | c | 44.75 | b | 9.41 | c | 12.59 | b | 67.08 | c | 42.43 | b | 474.05 | b | 62.10 | b | 235.01 | b | 533.42 | b |
| VT | 91.10 | b | 13.02 | c | 194.39 | c | 45.90 | b | 9.48 | c | 11.84 | b | 69.34 | c | 39.21 | b | 470.76 | b | 60.04 | b | 224.63 | b | 569.45 | b | |
| Ap | 138.70 | a | 22.74 | b | 306.38 | b | 70.57 | a | 13.40 | b | 17.97 | a | 113.12 | b | 69.96 | a | 837.25 | a | 106.98 | a | 347.37 | a | 866.83 | a | |
| MC | 154.48 | a | 28.04 | a | 400.93 | a | 88.94 | a | 18.60 | a | 22.61 | a | 140.88 | a | 75.61 | a | 1113.28 | a | 143.14 | a | 431.12 | a | 965.74 | a | |
| Bk | 110.04 | b | 21.23 | b | 283.13 | b | 67.51 | a | 14.35 | b | 18.37 | a | 104.08 | b | 68.44 | a | 1089.10 | a | 97.59 | a | 318.74 | a | 687.77 | b | |
| CV (%) | 9.82 | 15.00 | 9.98 | 17.67 | 17.62 | 14.39 | 7.11 | 15.47 | 18.15 | 11.17 * | 17.11 | 9.82 | |||||||||||||
| Beetroot | Carrot | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot Fresh Weight | Root Fresh Weight | Shoot Fresh Weight | Root Fresh Weight | Lengh | |||||
| g | g | cm | |||||||
| First Experiment | C | 4.30 | b | 1.34 | b | 34.07 | 53.22 | 16.62 | |
| CT | 7.27 | ab | 3.51 | ab | 43.79 | 63.65 | 16.18 | ||
| Ap | 9.21 | a | 5.70 | a | 52.90 | 68.60 | 16.69 | ||
| MC | 17.43 | a | 6.71 | a | 39.20 | 61.95 | 16.50 | ||
| Bk | 10.32 | a | 6.50 | a | 49.26 | 63.72 | 15.57 | ||
| C.V. (%) | 47.35 * | 26.38 ** | 25.24 ** | 34.5 | 10.55 | ||||
| Second Experiment | C | 17.77 | 4.37 | 8.53 | 36.13 | ab | 14.43 | ||
| CT | 25.77 | 6.91 | 13.63 | 57.56 | a | 14.93 | |||
| Ap | 19.85 | 5.67 | 10.05 | 44.55 | ab | 14.28 | |||
| MC | 21.45 | 6.98 | 13.22 | 57.53 | a | 15.25 | |||
| Bk | 19.63 | 6.62 | 6.99 | 28.72 | b | 13.68 | |||
| C.V. (%) | 28.24 ** | 23.83 ** | 37.84 | 35.46 | 17.46 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, T.F.; Itkes, M.P.M.; Brogiato, G.; Marques, V.A.R.; Martins, V.; Villarraga, C.O.; Esposito, E. Agroecological Transformation: Implementation of an Agroforestry System in a Construction Debris Area Focusing on Vegetables Development through Microbial Treatments. Appl. Sci. 2024, 14, 4648. https://doi.org/10.3390/app14114648
Rodrigues TF, Itkes MPM, Brogiato G, Marques VAR, Martins V, Villarraga CO, Esposito E. Agroecological Transformation: Implementation of an Agroforestry System in a Construction Debris Area Focusing on Vegetables Development through Microbial Treatments. Applied Sciences. 2024; 14(11):4648. https://doi.org/10.3390/app14114648
Chicago/Turabian StyleRodrigues, Thiago Fernandes, Marina Paes Machado Itkes, Giovanne Brogiato, Victor Augusto Reis Marques, Valdir Martins, Carlos Orlando Villarraga, and Elisa Esposito. 2024. "Agroecological Transformation: Implementation of an Agroforestry System in a Construction Debris Area Focusing on Vegetables Development through Microbial Treatments" Applied Sciences 14, no. 11: 4648. https://doi.org/10.3390/app14114648
APA StyleRodrigues, T. F., Itkes, M. P. M., Brogiato, G., Marques, V. A. R., Martins, V., Villarraga, C. O., & Esposito, E. (2024). Agroecological Transformation: Implementation of an Agroforestry System in a Construction Debris Area Focusing on Vegetables Development through Microbial Treatments. Applied Sciences, 14(11), 4648. https://doi.org/10.3390/app14114648








