Influence of Kluyveromyces lactis and Enterococcus faecalis on Obtaining Lactic Acid by Cheese Whey Fermentation
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Biochemical and Molecular Identification
2.2. Determination of Optimal Microorganism Growth Conditions
2.3. Batch Fermentation
2.3.1. Batch Fermentation with Strains in Suspension Using Enterococcus faecalis and Kluyveromyces lactis
2.3.2. Batch Fermentation with Strains Immobilized Using Enterococcus faecalis and Kluyveromyces lactis
2.4. Fermentation Monitoring
2.4.1. Acidity Analysis
2.4.2. Lactose Consumption
2.5. Lactic Acid Purification and Characterization
3. Results and Discussion
3.1. Characteristics of the Strains Isolated
3.2. Determination of Optimal Bacterial Growth Conditions
3.3. Batch Fermentation with Strains in Suspension and Immobilized
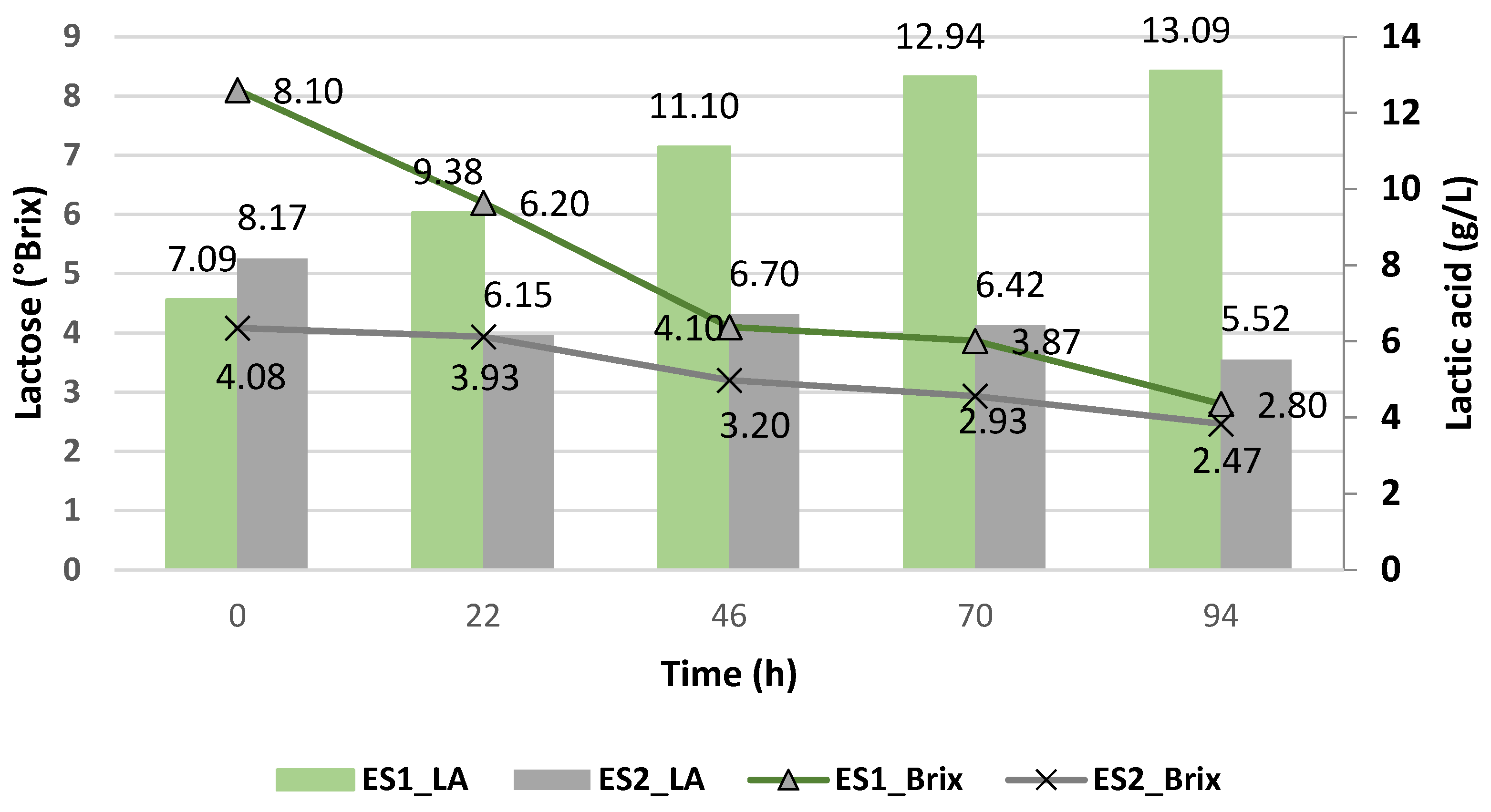
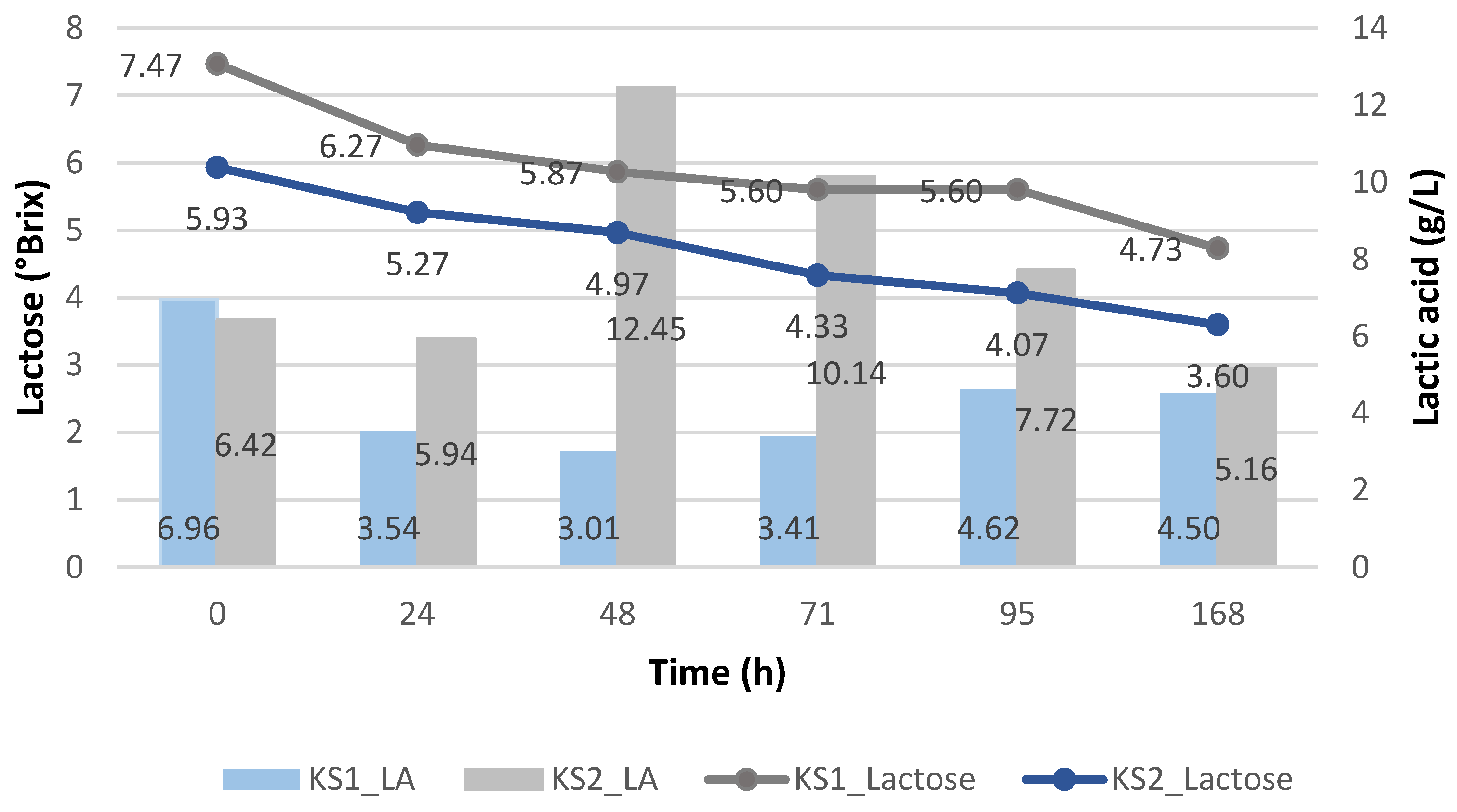
3.3.1. Batch Fermentation with Strains in Suspension Using Enterococcus faecalis and Kluyveromyces lactis
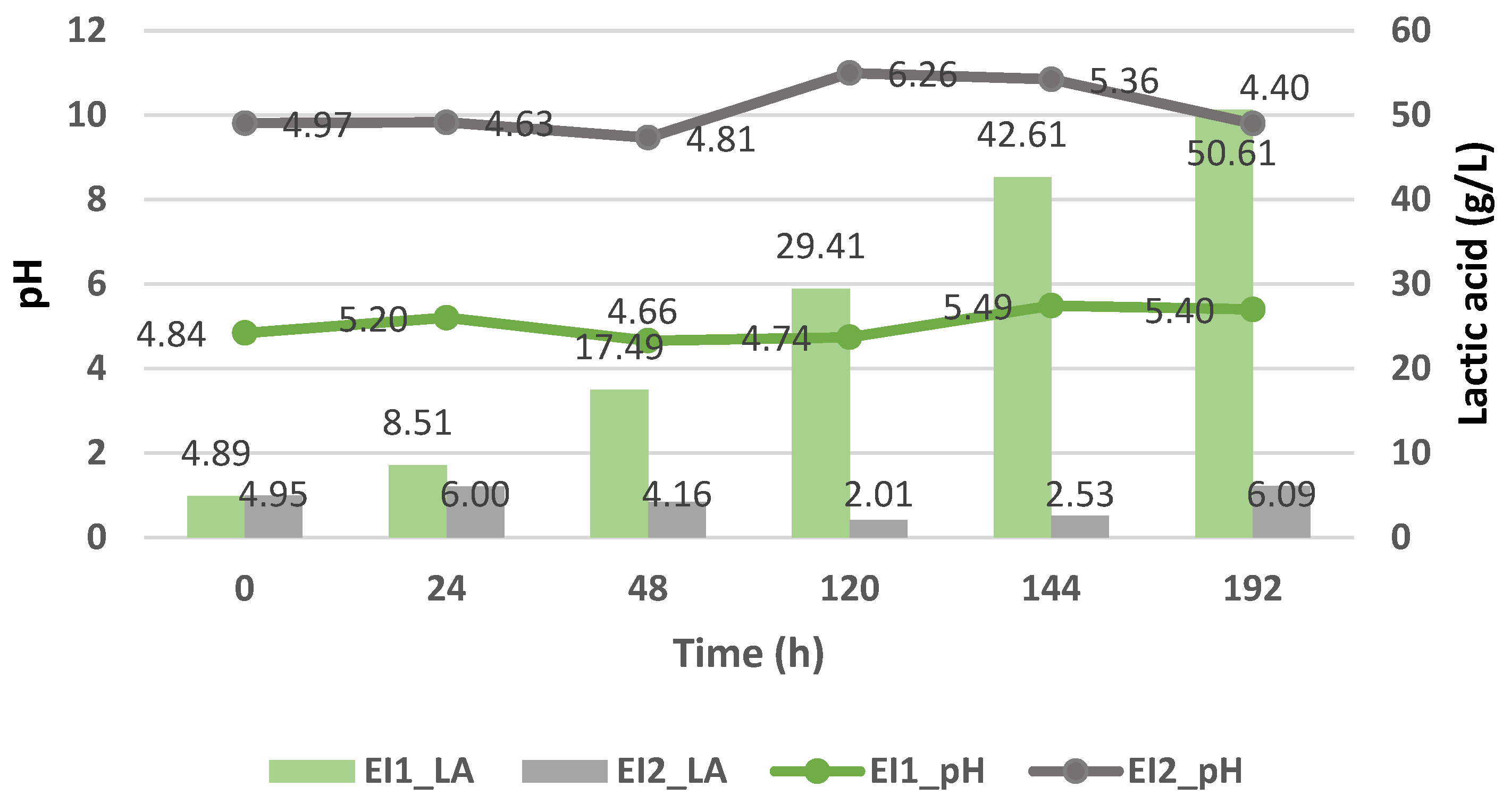
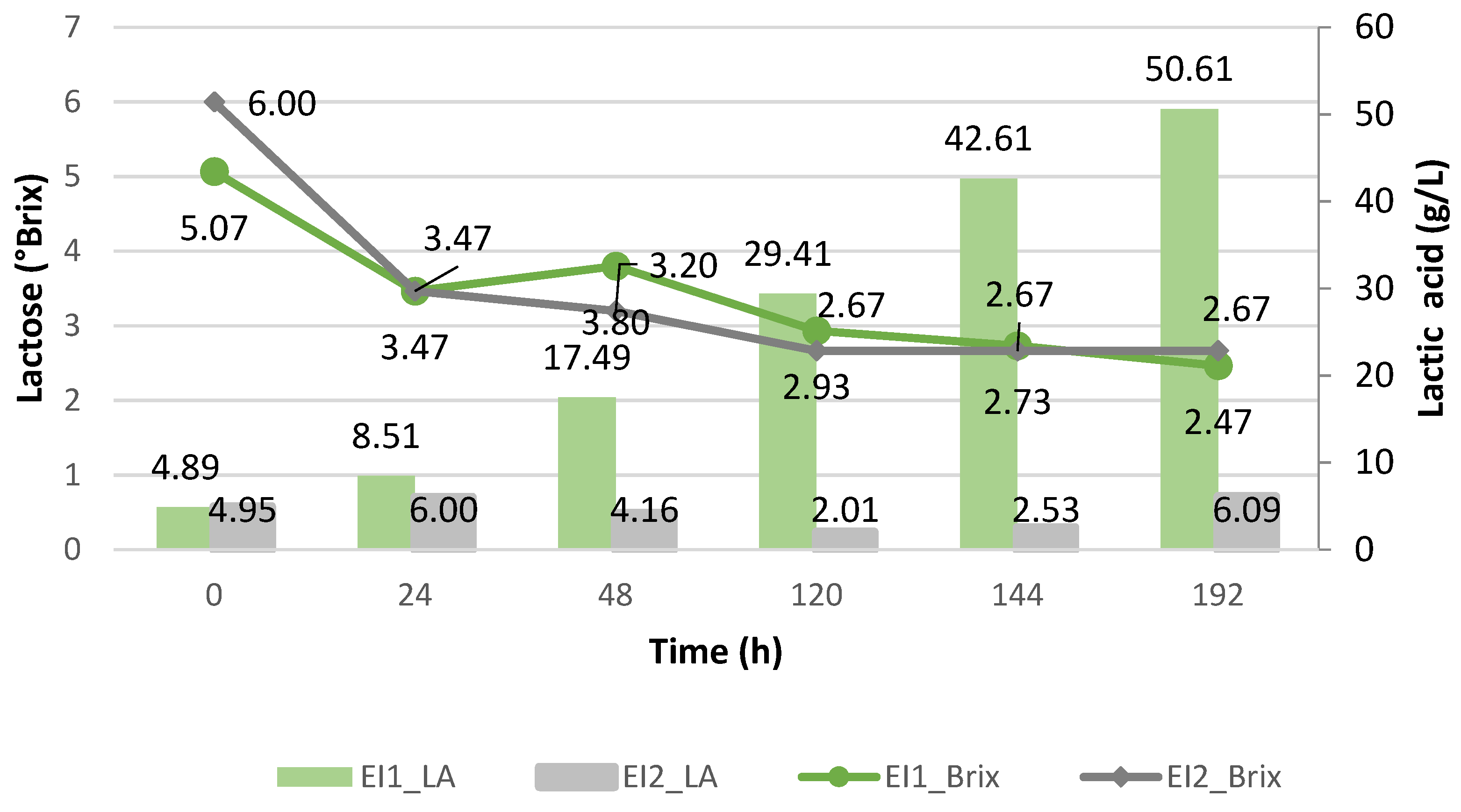
3.3.2. Batch Fermentation with Strains Immobilized Using Enterococcus faecalis and Kluyveromyces lactis
3.4. Lactic Acid Purification and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Treu, L.; Tsapekos, P.; Peprah, M.; Campanaro, S.; Giacomini, A.; Corich, V.; Kougias, P.G.; Angelidaki, I. Microbial profiling during anaerobic digestion of cheese whey in reactors operated at different conditions. Bioresour. Technol. 2019, 275, 375–385. [Google Scholar] [CrossRef]
- Eş, I.; Khaneghah, A.M.; Barba, F.J.; Saraiva, J.A.; Sant’Ana, A.S.; Hashemi, S.M.B. Recent advancements in lactic acid production—A review. Food Res. Int. 2018, 107, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Komesu, A.; de Oliveira, J.A.R.; da Silva Martins, L.H.; Maciel, M.R.W.; Filho, R.M. Lactic Acid Production to Purification: A Review. Bioresources 2017, 12, 4364–4383. [Google Scholar] [CrossRef]
- Tixicuro, J.M.F.; Chanfrau, J.M.P.; de Céspedes, I.S.S.; Fiallos, M.V.L.; Pérez, J.N. Optimización estadística de un bioproceso de ácido láctico a partir de lactosuero. Cienc. Lat. Rev. Científica Multidiscip. 2021, 5, 3259–3274. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production–producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Veerapagu, M.; Jeya, K.R. Evaluation of probiotic characteristics of bacteria isolated from fermented foods. Pharma Innov. J. 2017, 6, 322–325. [Google Scholar]
- Dosuky, A.S.; Elsayed, T.R.; Yousef, E.T.; Barakat, O.S.; Nasr, N.F. Isolation, identification, and application of lactic acid-producing bacteria using salted cheese whey substrate and immobilized cells technology. J. Genet. Eng. Biotechnol. 2022, 20, 26. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ghosh, A.R. Characterization of Functional, Safety, and Probiotic Properties of Enterococcus faecalis AG5 Isolated From Wistar Rat, Demonstrating Adherence to HCT 116 Cells and Gastrointestinal Survivability. Probiotics Antimicrob. Proteins 2018, 10, 435–445. [Google Scholar] [CrossRef]
- Sayed, W.F.; Salem, W.M.; Sayed, Z.A.; Abdalla, A.K. Production of lactic acid from whey permeates using lactic acid bacteria isolated from cheese. SVU-Int. J. Vet. Sci. 2020, 3, 78–95. [Google Scholar] [CrossRef]
- Marcus, J.F.; Demarsh, T.A.; Alcaine, S.D. Upcycling of whey permeate through yeast-and mold-driven fermentations under anoxic and oxic conditions. Fermentation 2021, 7, 16. [Google Scholar] [CrossRef]
- Spohner, S.C.; Schaum, V.; Quitmann, H.; Czermak, P. Kluyveromyces lactis: An emerging tool in biotechnology. J. Biotechnol. 2016, 222, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.V.; Netto, J.H.C.M.; Carvalho, L.S.; Parachin, N.S. Heterologous Hyaluronic Acid Production in Kluyveromyces lactis. Microorganisms 2019, 7, 294. [Google Scholar] [CrossRef]
- Yeo, I.S.; Yoon, Y.J.; Seo, N.; An, H.J.; Kim, J.H. Biopurification of oligosaccharides by immobilized Kluyveromyces lactis. Appl. Sci. 2019, 9, 2845. [Google Scholar] [CrossRef]
- ASTM E1252-98; Standard Practice for General Techniques for Obtaining Infra-red Spectra for Qualitative Analysis. ASTM: West Conshohocken, PA, USA, 2021.
- Yuan, S.F.; Hsu, T.C.; Wang, C.A.; Jang, M.F.; Kuo, Y.C.; Alper, H.S.; Guo, G.L.; Hwang, W.S. Production of optically pure l(+)-lactic acid from waste plywood chips using an isolated thermotolerant Enterococcus faecalis SI at a pilot scale. J. Ind. Microbiol. Biotechnol. 2018, 45, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Hun, C.H.; Sueb, M.; Abd Malek, R.; Othman, Z.; Elsayed, E.A.; Ramili, S.; Elmarzugi, N.A.; Sarmidi, M.R.; Aziz, R.; El Enshasy, H.A. Bioprocess Development for High Cell Mass Production of the Probiotic Yeast-Kluyveromyces lactis. IOSR J. Pharm. Biol. Sci. 2013, 8, 49–59. [Google Scholar] [CrossRef]
- Bahry, H.; Abdalla, R.; Pons, A.; Taha, S.; Vial, C. Optimization of lactic acid production using immobilized Lactobacillus Rhamnosus and carob pod waste from the Lebanese food industry. J. Biotechnol. 2019, 306, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Orozco, F. Producción de Ácido Láctico por Medio de Fermentación Anaerobia y su Polimerización a Partir de Reacciones de Apertura de Anillo. Master’s Thesis, Centro de Investigación Científica de Yucata, Mérida, Mexico, 2011. Available online: https://cicy.repositorioinstitucional.mx/jspui/bitstream/1003/1333/1/PMP_M_Tesis_2011_Fatima_Orozco_Olivarez.pdf (accessed on 22 April 2024).
- Karande, R.D.; Abitha, V.; Rane, A.V.; Mishra, R.K. Preparation of Polylactide from Synthesized Lactic Acid and Effect of reaction Parameters on Conversion. J. Mater. Sci. Eng. Adv. Technol. 2015, 12, 1–37. [Google Scholar] [CrossRef]
- Mazo, P.; Rios, L.A.; Restrepo, G. Síntesis de poli ácido láctico y poli ricinoleato empleando calentamiento por microondas y su utilización en la producción de termoplasticos de poliuretano. Polimeros 2011, 21, 83–89. [Google Scholar] [CrossRef][Green Version]
- Vargas, M.; Gordillo-Andia, C.; Tupayachy-Quispe, D.; Almirón, J.; Roudet, F. Influence of Kluyveromyces lactis Arranged in Suspension and Immobilized on Obtaining Lactic Acid by Cheese Whey Fermentation. In Proceedings of the 9th World Congress on New Technologies, London, UK, 9–11 August 2023. [Google Scholar]
- Nikolic, L.; Ristic, I.; Adnadjevic, B.; Nikolic, V.; Jovanovic, J.; Stankovic, M. Novel microwave-assisted synthesis of poly(D,L-lactide): The influence of monomer/initiator molar ratio on the product properties. Sensors 2010, 10, 5063. [Google Scholar] [CrossRef] [PubMed]






| Code | Strain | Strain | Culture Medium | |
|---|---|---|---|---|
| Disposition | DCW 1 | CW 2 | ||
| ES1 | E. faecalis | Suspended | X | |
| ES2 | E. faecalis | Suspended | X | |
| KS1 | K. lactis | Suspended | X | |
| KS2 | K. lactis | Suspended | X | |
| EI1 | E. faecalis | Immobilized | X | |
| EI2 | E. faecalis | Immobilized | X | |
| KI1 | K. lactis | Immobilized | X | |
| KI2 | K. lactis | Immobilized | X | |
| Code | Strain | Strain | Culture | LA Purification Conditions | |
|---|---|---|---|---|---|
| Disposition | Medium | Temperature [°C] | Time [h] | ||
| ES1_P11 | E. faecalis | Suspended | DCW 3 | 60 | 45 min |
| ES1_P22 | E. faecalis | Suspended | DCW | 40 | 1 h |
| KS2_P1 | K. lactis | Suspended | CW 4 | 40 | 5 h |
| KS2_P2 | K. lactis | Suspended | CW | 45 | 5 h |
| EI1_P1 | E. faecalis | Immobilized | DCW | 40 | 5 h |
| EI1_P2 | E. faecalis | Immobilized | DCW | 45 | 5 h |
| KI1_P1 | K. lactis | Immobilized | DCW | 40 | 5 h |
| KI1_P2 | K. lactis | Immobilized | DCW | 45 | 5 h |
| Characteristics | E. faecalis | K. lactis |
|---|---|---|
| Pigmentation | White | White |
| Size | 0.1–1 mm | 0.5–4 mm |
| Form | Circular | Circular |
| Elevation | Flat | Convex |
| Margin | Entire | Entire |
| Texture | Viscose | Dry |
| Gram | + | + |
| Catalase | − | + |
| Microorganism | Cocci | Yeast |
| Temperature (°C) | E. faecalis | Media Blank | K. lactis | Media Blank |
|---|---|---|---|---|
| 4 | − | − | − | − |
| 8 | − | − | − | − |
| Room temperature (21 °C) | ++ | − | ++ | − |
| 25 | ++ | − | +++ | − |
| 28 | ++ | − | +++ | − |
| 29 | ++ | − | ++ | − |
| 32 | +++ | − | +++ | − |
| 34 | ++ | − | +++ | − |
| 35 | ++ | − | + | − |
| 36 | −+ | − | −+ | − |
| 37 | − | − | − | − |
| 45 | − | − | − | − |
| Salinity (%) | E. faecalis | Media Blank | K. lactis | Media Blank |
| 1 | +++ | − | +++ | − |
| 2 | +++ | − | +++ | − |
| 4 | +++ | − | +++ | − |
| 6 | − | − | ++ | − |
| 8 | − | − | + | − |
| 10 | − | − | +− | − |
| 11 | − | − | +− | − |
| 12 | − | − | +− | − |
| 13 | − | − | − | − |
| pH | E. faecalis | Media Blank | K. lactis | Media Blank |
| 4 | − | − | +− | − |
| 7 | +++ | − | +++ | − |
| 9 | +++ | − | +++ | − |
| Code | Strain | Culture Medium | LA Purification Conditions | Characteristics | |||
|---|---|---|---|---|---|---|---|
| T [°C] | t [h] | Yield [%] | Density [g/mL] | Colour | |||
| ES1_P1 | E. faecalis | DCW 1 | 60 | 45 min | 6.15 | 0.72 | Yellowish |
| ES1_P2 | E. faecalis | DCW | 40 | 1 h | 16.92 | 0.77 | Yellowish |
| KS2_P1 | K. lactis | CW 2 | 40 | 5 h | 3.89 | 0.97 | Whitish |
| KS2_P2 | K. lactis | CW | 45 | 5 h | 2.5 | 1.21 | Whitish |
| EI1_P1 | E. faecalis | DCW | 40 | 5 h | 5.13 | 0.81 | Whitish |
| EI1_P2 | E. faecalis | DCW | 45 | 5 h | 8.33 | 0.69 | Yellowish |
| KI1_P1 | K. lactis | DCW | 40 | 5 h | 8.44 | 0.71 | Yellowish |
| KI1_P2 | K. lactis | DCW | 45 | 5 h | 3.26 | 0.98 | Whitish |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordillo-Andia, C.; Almirón, J.; Barreda-Del-Carpio, J.E.; Roudet, F.; Tupayachy-Quispe, D.; Vargas, M. Influence of Kluyveromyces lactis and Enterococcus faecalis on Obtaining Lactic Acid by Cheese Whey Fermentation. Appl. Sci. 2024, 14, 4649. https://doi.org/10.3390/app14114649
Gordillo-Andia C, Almirón J, Barreda-Del-Carpio JE, Roudet F, Tupayachy-Quispe D, Vargas M. Influence of Kluyveromyces lactis and Enterococcus faecalis on Obtaining Lactic Acid by Cheese Whey Fermentation. Applied Sciences. 2024; 14(11):4649. https://doi.org/10.3390/app14114649
Chicago/Turabian StyleGordillo-Andia, Carlos, Jonathan Almirón, Jaime E. Barreda-Del-Carpio, Francine Roudet, Danny Tupayachy-Quispe, and María Vargas. 2024. "Influence of Kluyveromyces lactis and Enterococcus faecalis on Obtaining Lactic Acid by Cheese Whey Fermentation" Applied Sciences 14, no. 11: 4649. https://doi.org/10.3390/app14114649
APA StyleGordillo-Andia, C., Almirón, J., Barreda-Del-Carpio, J. E., Roudet, F., Tupayachy-Quispe, D., & Vargas, M. (2024). Influence of Kluyveromyces lactis and Enterococcus faecalis on Obtaining Lactic Acid by Cheese Whey Fermentation. Applied Sciences, 14(11), 4649. https://doi.org/10.3390/app14114649







