Impacts of Land-Use Change from Primary Forest to Farmland on the Storage of Soil Organic Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Data Processing
2.3. Meta Analysis
- (i)
- Calculate the absolute change of SOC in each sample of a single study as follows:where m represents the i-th sample of the m-th study.
- (ii)
- Calculate the mean of the absolute change in SOC of a single study as follows:where n is the sample size of each study.
- (iii)
- Calculate the variance of the absolute change in SOC of a single study (intra-study variance) as follows:
- (iv)
- Calculate the standard error of the absolute change in SOC in a single study as follows:
- (v)
- Estimate the variance between studies as follows:where k is the number of included studies.
- (vi)
- Thus, the total variance of the absolute change in SOC of a single study is estimated as follows:
- (vii)
- Thus, MD, the absolute change in SOC is estimated as the weighted average as follows:
- (viii)
- Similarly, ln(RR), the response ratio of the change in SOC after the log is estimated as the weighted average as follows:
- (ix)
- Accordingly, the change in SOC ratio is calculated as follows:exp[ln(RR) − 1] × 100%
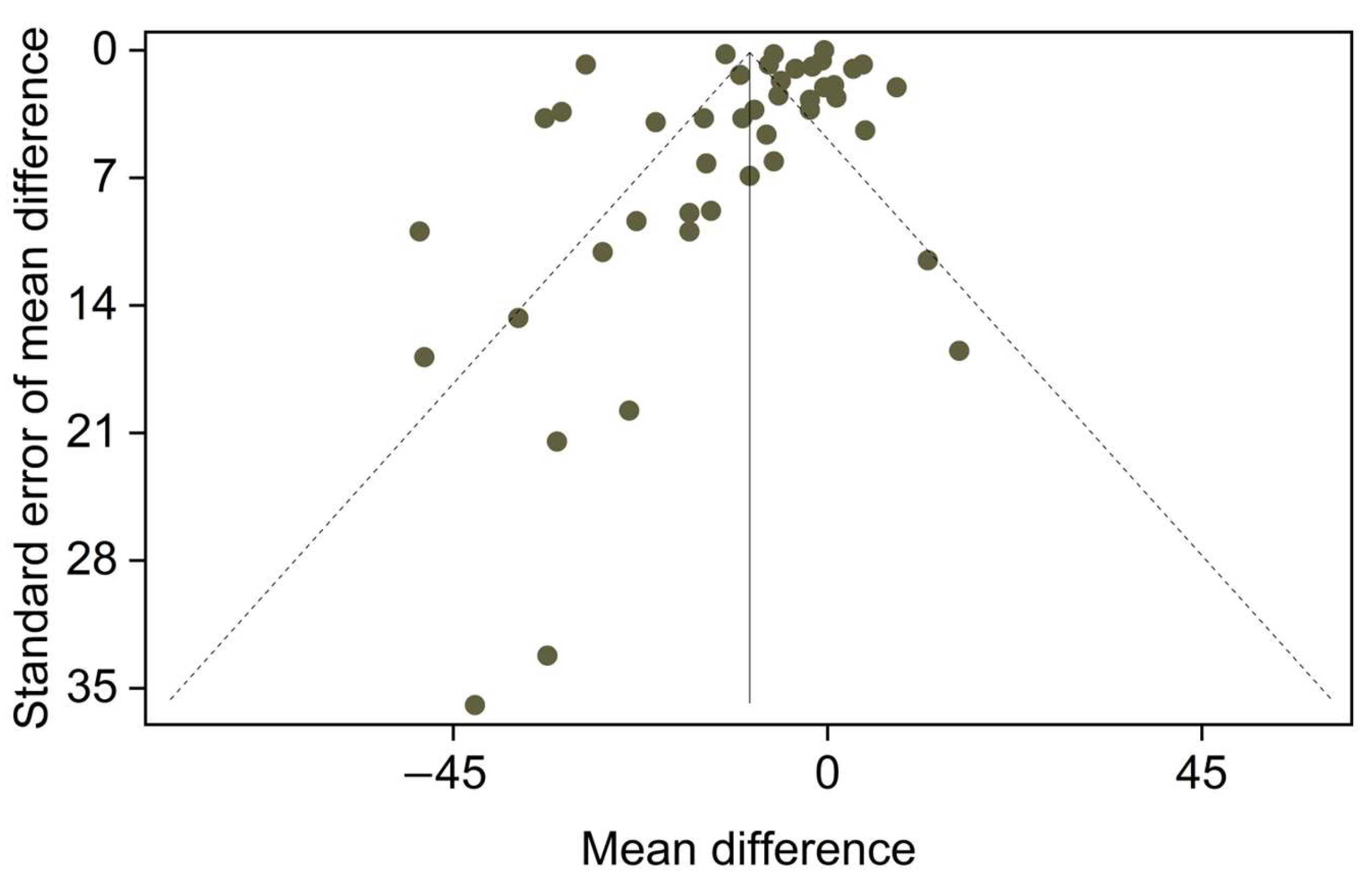
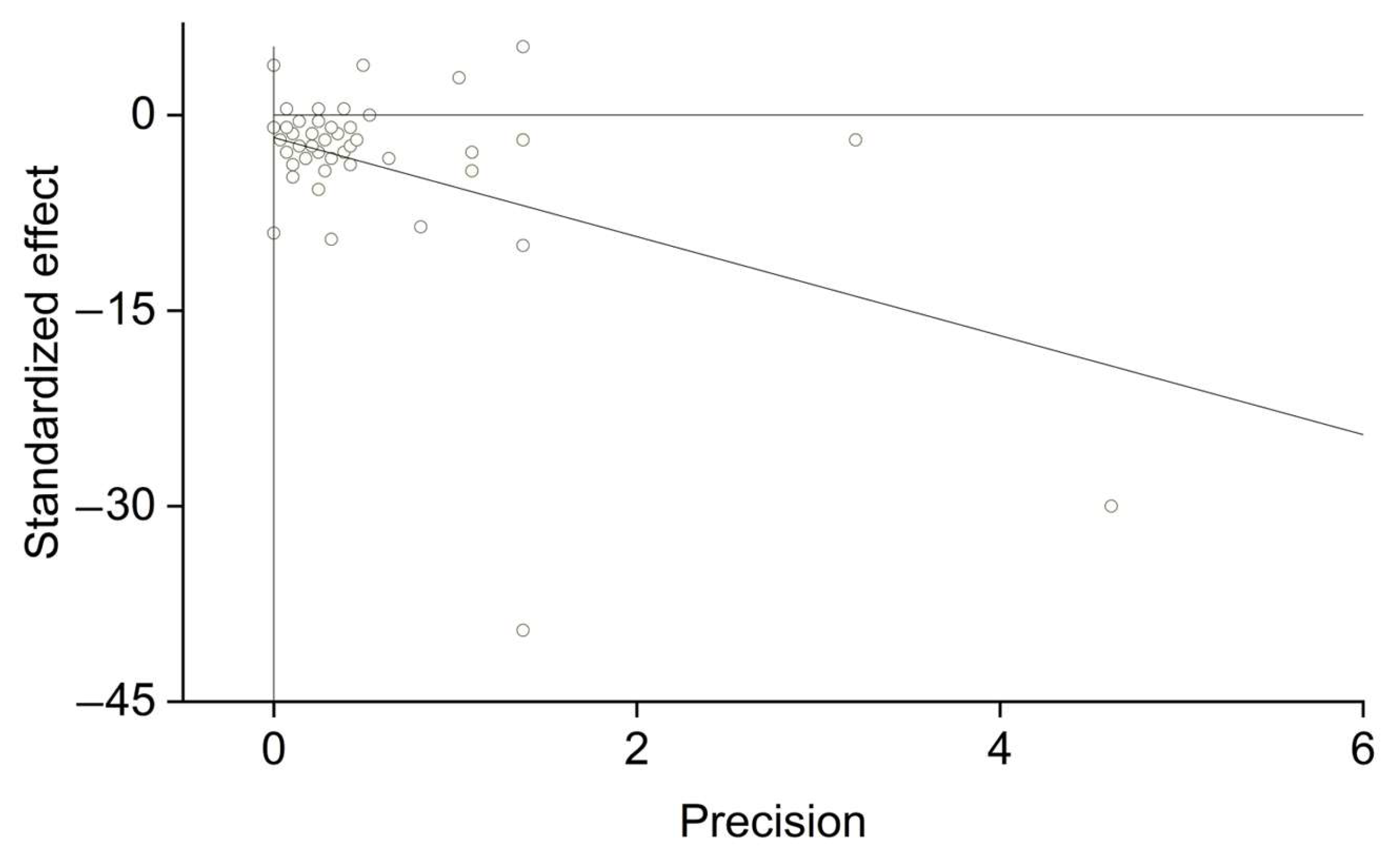
2.4. Publication Bias
3. Results
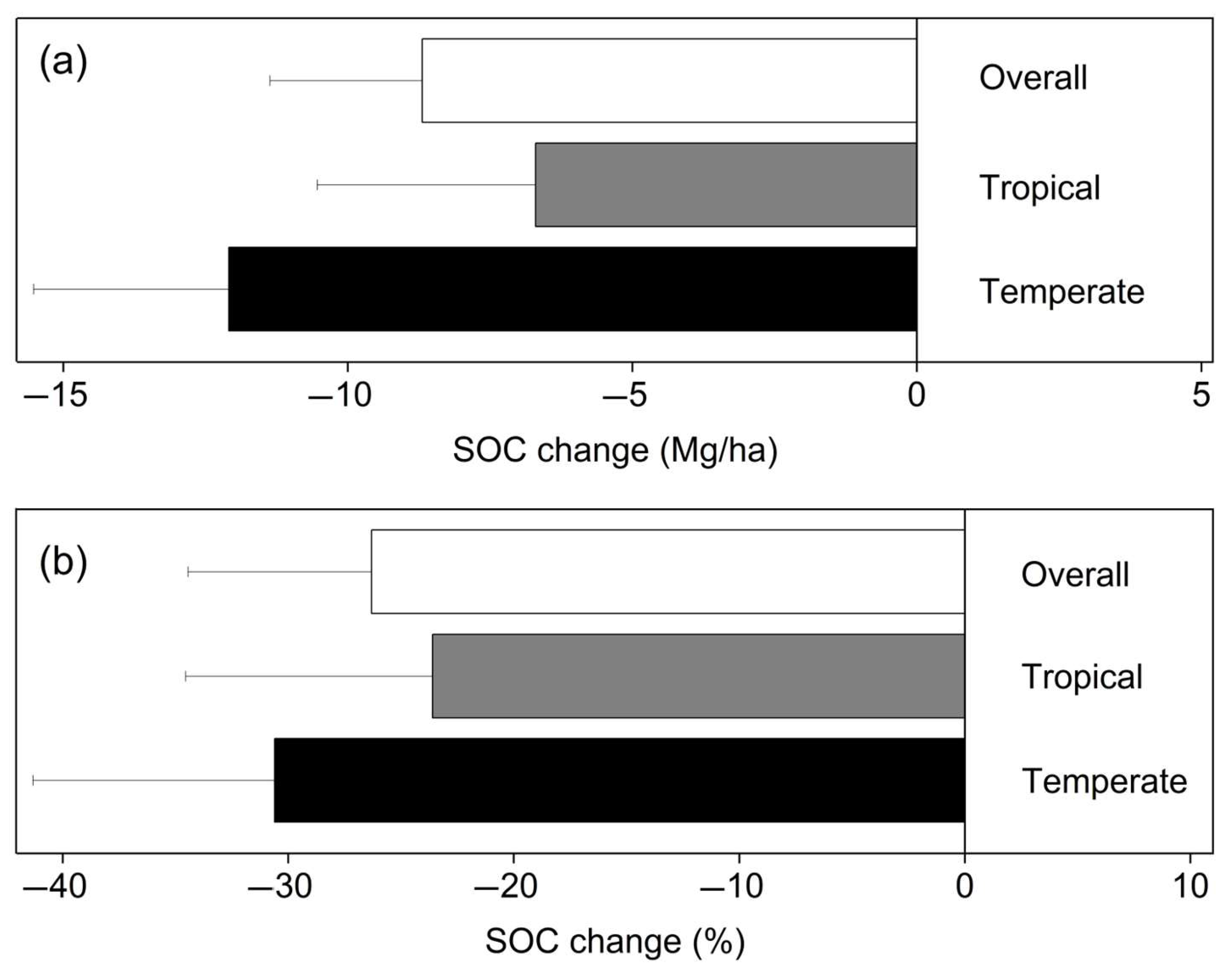
3.1. Changes in SOC Storage after Primary Forest Converted to Farmland
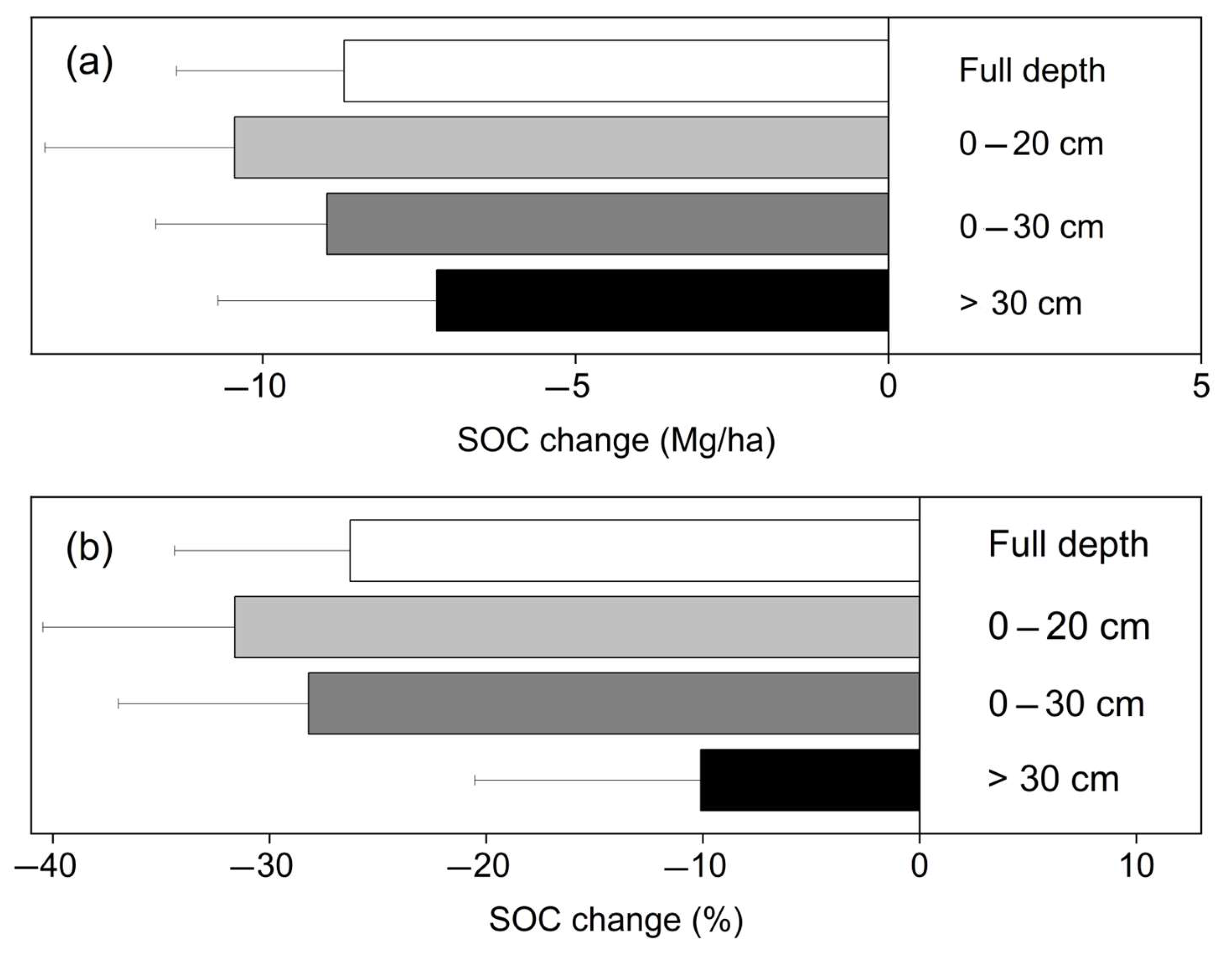
3.2. Influencing Factors of SOC Storage Change
4. Discussion
4.1. The Relationship between SOC Storage and Climate
4.2. The Correlation of Soil Sample Depth and SOC Storage
4.3. Effect of Carbon Input and Output on SOC Storage
4.4. Effect of Soil Structure on SOC Storage
4.5. Dynamic Effects of the Growth Environment on SOC
4.6. Effects of Tillage and No-Tillage on SOC Reserves in Cultivated Land
4.7. Effects of Continuous Cropping and Fallow on SOC Stocks in Cultivated Land
4.8. Differences in SOC Storage among Different Cultivating Systems
5. Conclusions
- After the conversion of primary forests into farmland, there is a significant decrease in the storage of soil organic carbon (SOC). This indicates that land use change has a significant impact on SOC content. Primary forests are complex and stable ecosystems that contain a large amount of organic carbon in the soil. However, once the primary forests are cleared for agriculture, the soil is exposed to the atmosphere and human activities, resulting in an accelerated decomposition rate of SOC and a sharp decline in SOC storage.
- When primary forests are converted into farmland, temperate forests experience greater loss of SOC compared to tropical forests, while the loss of SOC in deeper soil layers is relatively smaller. This suggests that the extent of SOC loss after land use change depends on the type of forest and the soil depth. Temperate forests and tropical forests differ in soil characteristics, climate conditions, and vegetation composition. Therefore, after temperate forests are converted into farmland, the influence of climate change and soil characteristics leads to a more significant loss of SOC. Additionally, SOC decomposition rates are relatively slower in deeper soil layers, resulting in less severe SOC loss compared to shallow soil layers.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LUC | Land-Use Change |
| SOC | Soil Organic Carbon |
| SOM | Soil Organic Matter |
| BD | Soil Bulk Density |
| MD | Weighted Mean Difference |
| RR | Response Ratio |
| REM | Random Effect Model |
References
- Harrison, R.B.; Footen, P.W.; Strahm, B.D. Deep Soil Horizons: Contribution and Importance to Soil Carbon Pools and in Assessing Whole-Ecosystem Response to Management and Global Change. For. Sci. 2011, 57, 67–76. [Google Scholar] [CrossRef]
- Tan, W.-F.; Zhang, R.; Cao, H.; Huang, C.-Q.; Yang, Q.-K.; Wang, M.; Koopal, L.K. Soil Inorganic Carbon Stock under Different Soil Types and Land Uses on the Loess Plateau Region of China. CATENA 2014, 121, 22–30. [Google Scholar] [CrossRef]
- Li, W.; Jia, S.; He, W.; Raza, S.; Zamanian, K.; Zhao, X. Analysis of the Consequences of Land-Use Changes and Soil Types on Organic Carbon Storage in the Tarim River Basin from 2000 to 2020. Agric. Ecosyst. Environ. 2022, 327, 107824. [Google Scholar] [CrossRef]
- Krogh, L.; Noergaard, A.; Hermansen, M.; Greve, M.H.; Balstroem, T.; Breuning-Madsen, H. Preliminary Estimates of Contemporary Soil Organic Carbon Stocks in Denmark Using Multiple Datasets and Four Scaling-up Methods. Agric. Ecosyst. Environ. 2003, 96, 19–28. [Google Scholar] [CrossRef]
- Woodbury, P.B.; Heath, L.S.; Smith, J.E. Land Use Change Effects on Forest Carbon Cycling Throughout the Southern United States. J. Environ. Qual. 2006, 35, 1348–1363. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Dai, J.; He, F.; Pan, Y.; Wang, M. Land Use Changes and Their Relations with Carbon Cycles over the Past 300 a in China. Sci. China Ser. D-Earth Sci. 2008, 51, 871–884. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of Tropical Land-Use Change on Soil Organic Carbon Stocks–a Meta-Analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Zhu, G.; Shangguan, Z.; Hu, X.; Deng, L. Effects of Land Use Changes on Soil Organic Carbon, Nitrogen and Their Losses in a Typical Watershed of the Loess Plateau, China. Ecol. Indic. 2021, 133, 108443. [Google Scholar] [CrossRef]
- Iticha, B.; Mohammed, M.; Kibret, K. Impact of Deforestation and Subsequent Cultivation on Soil Fertility in Komto, Western Ethiopia. J. Soil Sci. Environ. Manag. 2016, 7, 212–221. [Google Scholar]
- Ferreiro-Domínguez, N.; Palma, J.H.N.; Paulo, J.A.; Rigueiro-Rodríguez, A.; Mosquera-Losada, M.R. Assessment of Soil Carbon Storage in Three Land Use Types of a Semi-Arid Ecosystem in South Portugal. CATENA 2022, 213, 106196. [Google Scholar] [CrossRef]
- Xu, E.; Zhang, H.; Xu, Y. Exploring Land Reclamation History: Soil Organic Carbon Sequestration Due to Dramatic Oasis Agriculture Expansion in Arid Region of Northwest China. Ecol. Indic. 2020, 108, 105746. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Sun, C. Soil Organic Carbon and Total Nitrogen Storage as Affected by Land Use in a Small Watershed of the Loess Plateau, China. Eur. J. Soil Biol. 2013, 54, 16–24. [Google Scholar] [CrossRef]
- John, B.; Yamashita, T.; Ludwig, B.; Flessa, H. Storage of Organic Carbon in Aggregate and Density Fractions of Silty Soils under Different Types of Land Use. Geoderma 2005, 128, 63–79. [Google Scholar] [CrossRef]
- Mann, L.K. Changes in soil carbon storage after cultivation. Soil Sci. 1986, 142, 279. [Google Scholar] [CrossRef]
- Fang, X.; Xue, Z.; Li, B.; An, S. Soil Organic Carbon Distribution in Relation to Land Use and Its Storage in a Small Watershed of the Loess Plateau, China. CATENA 2012, 88, 6–13. [Google Scholar] [CrossRef]
- Cook, R.L.; Binkley, D.; Stape, J.L. Eucalyptus Plantation Effects on Soil Carbon after 20 Years and Three Rotations in Brazil. For. Ecol. Manag. 2016, 359, 92–98. [Google Scholar] [CrossRef]
- Kishchuk, B.E.; Morris, D.M.; Lorente, M.; Keddy, T.; Sidders, D.; Quideau, S.; Thiffault, E.; Kwiaton, M.; Maynard, D. Disturbance Intensity and Dominant Cover Type Influence Rate of Boreal Soil Carbon Change: A Canadian Multi-Regional Analysis. For. Ecol. Manag. 2016, 381, 48–62. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Ren, C.J.; Feng, X.X.; Zhang, L.; Doughty, R.; Zhao, F.Z. Temperature Sensitivity of Soil Carbon Decomposition Due to Shifts in Soil Extracellular Enzymes after Afforestation. Geoderma 2020, 374, 114426. [Google Scholar] [CrossRef]
- Dersch, G.; Böhm, K. Effects of Agronomic Practices on the Soil Carbon Storage Potential in Arable Farming in Austria. Nutr. Cycl. Agroecosyst. 2001, 60, 49–55. [Google Scholar] [CrossRef]
- Sainju, U.M.; Lenssen, A.; Caesar-Thonthat, T.; Waddell, J. Dryland Plant Biomass and Soil Carbon and Nitrogen Fractions on Transient Land as Influenced by Tillage and Crop Rotation. Soil Tillage Res. 2007, 93, 452–461. [Google Scholar] [CrossRef]
- Moritz, L.K.; Liang, C.; Wagai, R.; Kitayama, K.; Balser, T.C. Vertical Distribution and Pools of Microbial Residues in Tropical Forest Soils Formed from Distinct Parent Materials. Biogeochemistry 2009, 92, 83–94. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Wu, L.; Liu, C.; Wang, L.; Chen, F.; Li, Z. Soil Aggregate-Associated Organic Carbon Dynamics Subjected to Different Types of Land Use: Evidence from 13C Natural Abundance. Ecol. Eng. 2018, 122, 295–302. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.; Gong, Q.; Zhai, B.; Li, Z. Responses of Fungal–Bacterial Community and Network to Organic Inputs Vary among Different Spatial Habitats in Soil. Soil Biol. Biochem. 2018, 125, 54–63. [Google Scholar] [CrossRef]
- Zheng, C.; Yu, Z.; Shi, Y.; Cui, S.; Wang, D.; Zhang, Y.; Zhao, J. Effects of Tillage Practices on Water Consumption, Water Use Efficiency and Grain Yield in Wheat Field. J. Integr. Agric. 2014, 13, 2378–2388. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate Structure and Carbon, Nitrogen, and Phosphorus in Native and Cultivated Soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Zheng, F.; Wu, X.; Zhang, M.; Liu, X.; Song, X.; Lu, J.; Wang, B.; Jan van Groenigen, K.; Li, S. Linking Soil Microbial Community Traits and Organic Carbon Accumulation Rate under Long-Term Conservation Tillage Practices. Soil Tillage Res. 2022, 220, 105360. [Google Scholar] [CrossRef]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation Tillage Impacts on Soil, Crop and the Environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, W.; Wang, Y.; Cheng, P.; Hou, Y.; Xiong, X.; Du, H.; Yang, L.; Wang, Y. Effects of Land Use and Cultivation Time on Soil Organic and Inorganic Carbon Storage in Deep Soils. J. Geogr. Sci. 2020, 30, 921–934. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Reicosky, D.C.; Wilts, A.R.; McGowan, J.A. Charcoal Carbon in U.S. Agricultural Soils. Soil Sci. Soc. Am. J. 2002, 66, 1249–1255. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Führböter, M.; Solomon, D.; Zech, W. Carbon and Nitrogen Mineralization in Cultivated and Natural Savanna Soils of Northern Tanzania. Biol. Fertil. Soils 2001, 33, 301–309. [Google Scholar] [CrossRef]
- Studdert, G.A.; Echeverría, H.E. Crop Rotations and Nitrogen Fertilization to Manage Soil Organic Carbon Dynamics. Soil Sci. Soc. Am. J. 2000, 64, 1496–1503. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A History of Research on the Link between (Micro)Aggregates, Soil Biota, and Soil Organic Matter Dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Smith, P.; Powlson, D.S.; Glendining, M.J.; Smith, J.U. Preliminary Estimates of the Potential for Carbon Mitigation in European Soils through No-till Farming. Glob. Chang. Biol. 1998, 4, 679–685. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Kundu, S.; Shanker, A.K.; Naik, R.P.; Vanaja, M.; Venkanna, K.; Maruthi Sankar, G.R.; Rao, V.U.M. Continuous Cropping under Elevated CO2: Differential Effects on C4 and C3 Crops, Soil Properties and Carbon Dynamics in Semi-Arid Alfisols. Agric. Ecosyst. Environ. 2016, 218, 73–86. [Google Scholar] [CrossRef]
- Bona, F.D.D.; Bayer, C.; Dieckow, J.; Bergamaschi, H. Soil Quality Assessed by Carbon Management Index in a Subtropical Acrisol Subjected to Tillage Systems and Irrigation. Soil Res. 2008, 46, 469–475. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Vittal, K.P.R.; Venkateswarlu, B.; Wani, S.P.; Sahrawat, K.L.; Marimuthu, S.; Kundu, S. Carbon Stocks in Different Soil Types under Diverse Rainfed Production Systems in Tropical India. Commun. Soil Sci. Plant Anal. 2009, 40, 2338–2356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, C.; Gong, Y.; Pei, X.; Chen, H.; Li, S.; Lu, C.; Chen, L.; Zheng, X.; Zheng, J.; Yan, X. Impacts of Land-Use Change from Primary Forest to Farmland on the Storage of Soil Organic Carbon. Appl. Sci. 2024, 14, 4736. https://doi.org/10.3390/app14114736
Xiao C, Gong Y, Pei X, Chen H, Li S, Lu C, Chen L, Zheng X, Zheng J, Yan X. Impacts of Land-Use Change from Primary Forest to Farmland on the Storage of Soil Organic Carbon. Applied Sciences. 2024; 14(11):4736. https://doi.org/10.3390/app14114736
Chicago/Turabian StyleXiao, Changgui, Yaoqi Gong, Xiaolei Pei, Hanyue Chen, Sheng Li, Chengwen Lu, Li Chen, Xuhui Zheng, Jiaxin Zheng, and Xie Yan. 2024. "Impacts of Land-Use Change from Primary Forest to Farmland on the Storage of Soil Organic Carbon" Applied Sciences 14, no. 11: 4736. https://doi.org/10.3390/app14114736
APA StyleXiao, C., Gong, Y., Pei, X., Chen, H., Li, S., Lu, C., Chen, L., Zheng, X., Zheng, J., & Yan, X. (2024). Impacts of Land-Use Change from Primary Forest to Farmland on the Storage of Soil Organic Carbon. Applied Sciences, 14(11), 4736. https://doi.org/10.3390/app14114736




