Environmental Effects of Using Ammonium Sulfate from Animal Manure Scrubbing Technology as Fertilizer
Abstract
:1. Introduction
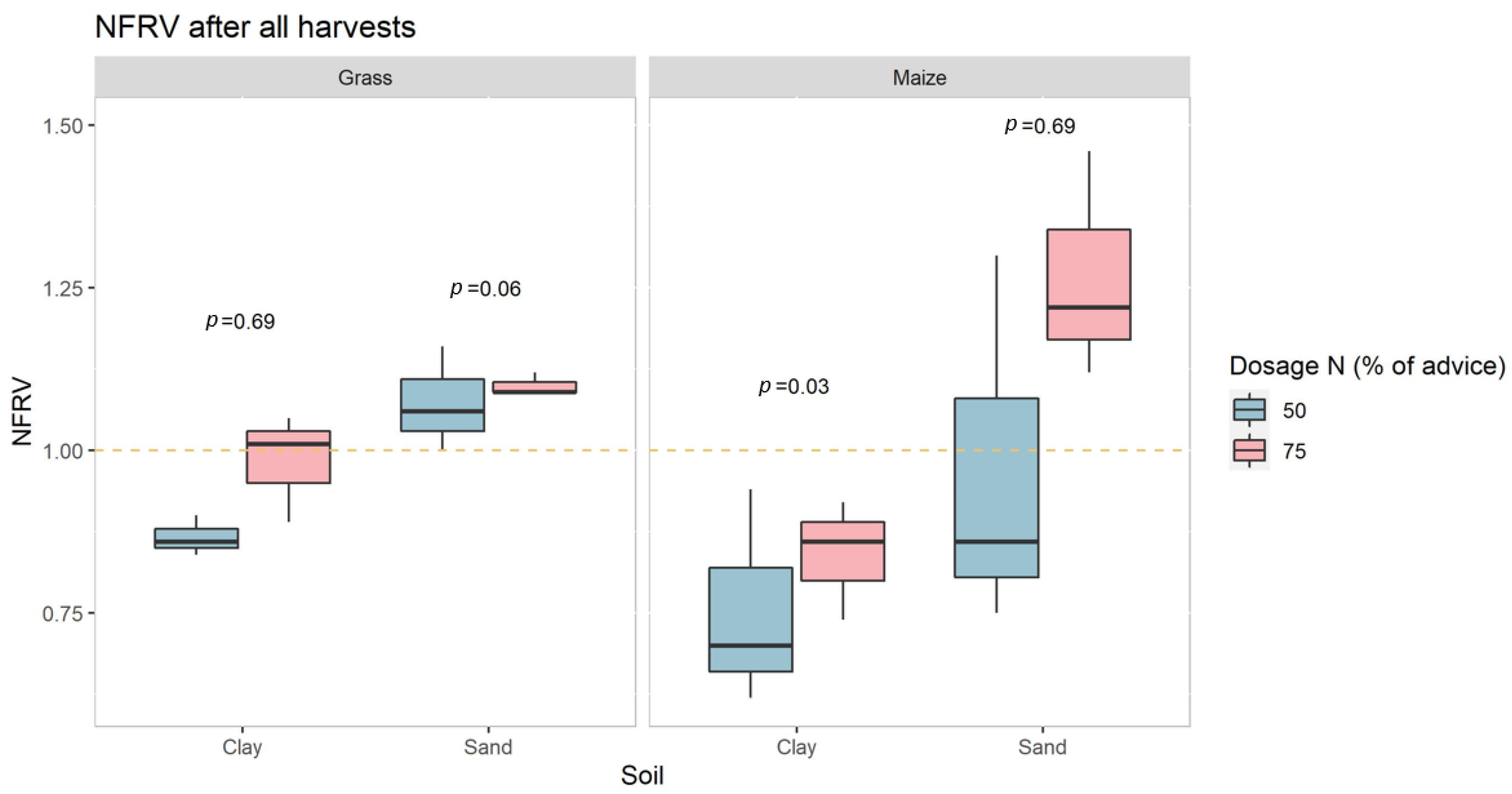
- Ammonium sulfate from the manure stripping process (AS) has the same nitrogen use efficiency as mineral nitrogen fertilizer in a clay soil with a relative high pH (pH > 7) (20), and a sandy soil with a normal pH (pH < 7) (20), for maize and grass. Maize and grass are the relevant crops for livestock farming in The Netherlands.
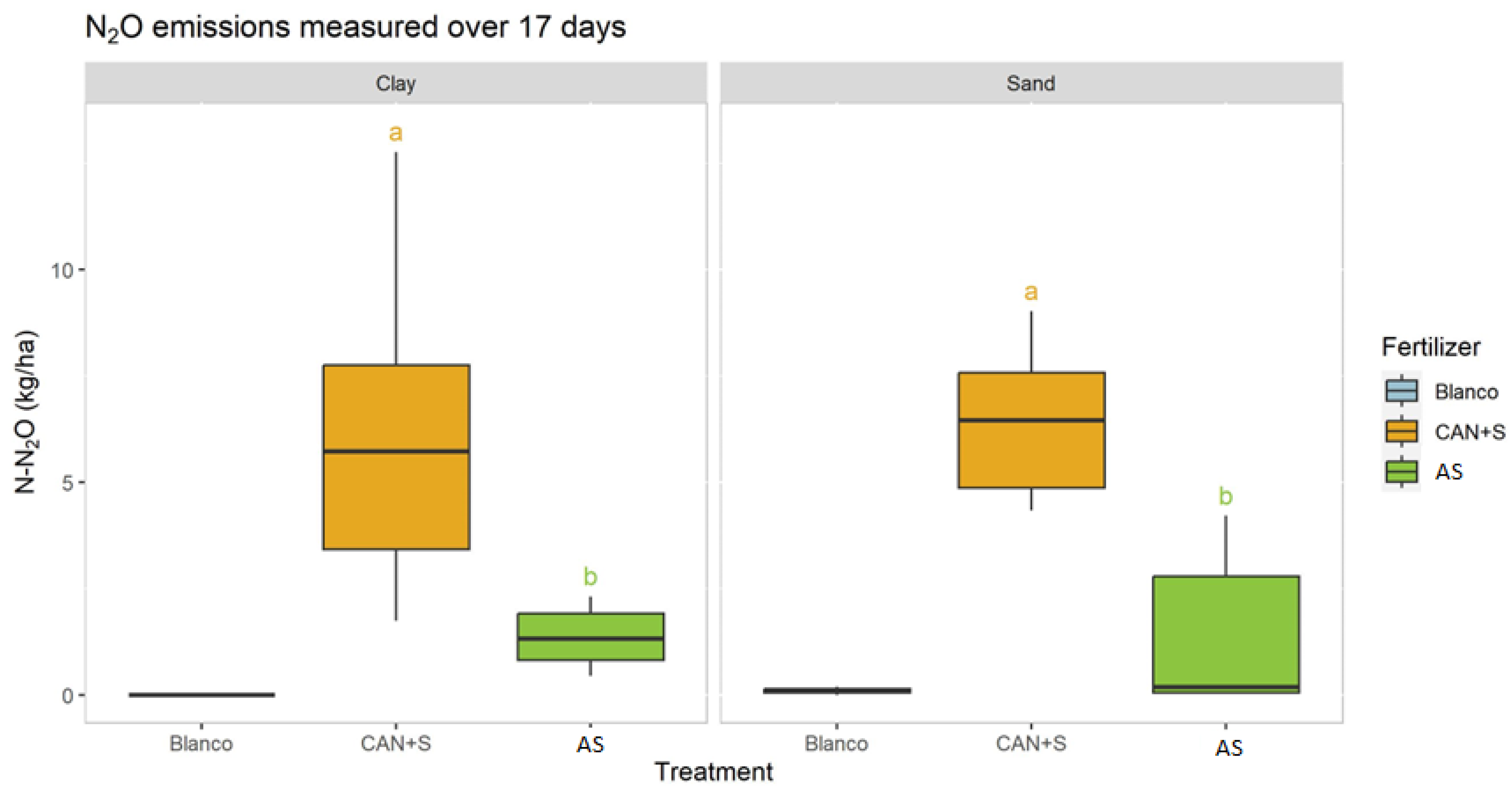
- NH3 and GHG emissions, in this research: methane (CH4) and nitrous oxide (N2O) are influenced by soil type, soil pH, and the fertilizers.
- NH3 emissions of liquid ammonium sulfate obtained from processed manure are lower than for the original livestock manure, while the NH3 emissions from the remaining treated products (the solid fraction of the digestate and the stripped liquid digestate) are similar or slightly higher than the original livestock manure, resulting in the lower net emission of the processed products compared to the original manure.
2. Materials and Methods
2.1. Introduction
2.2. Greenhouse Experiment
2.3. Field Demonstration 2021 and 2022
Statistics
2.4. Sheltered Flux Chamber Experiment
3. Results
3.1. Greenhouse Experiment, Yield and N Uptake
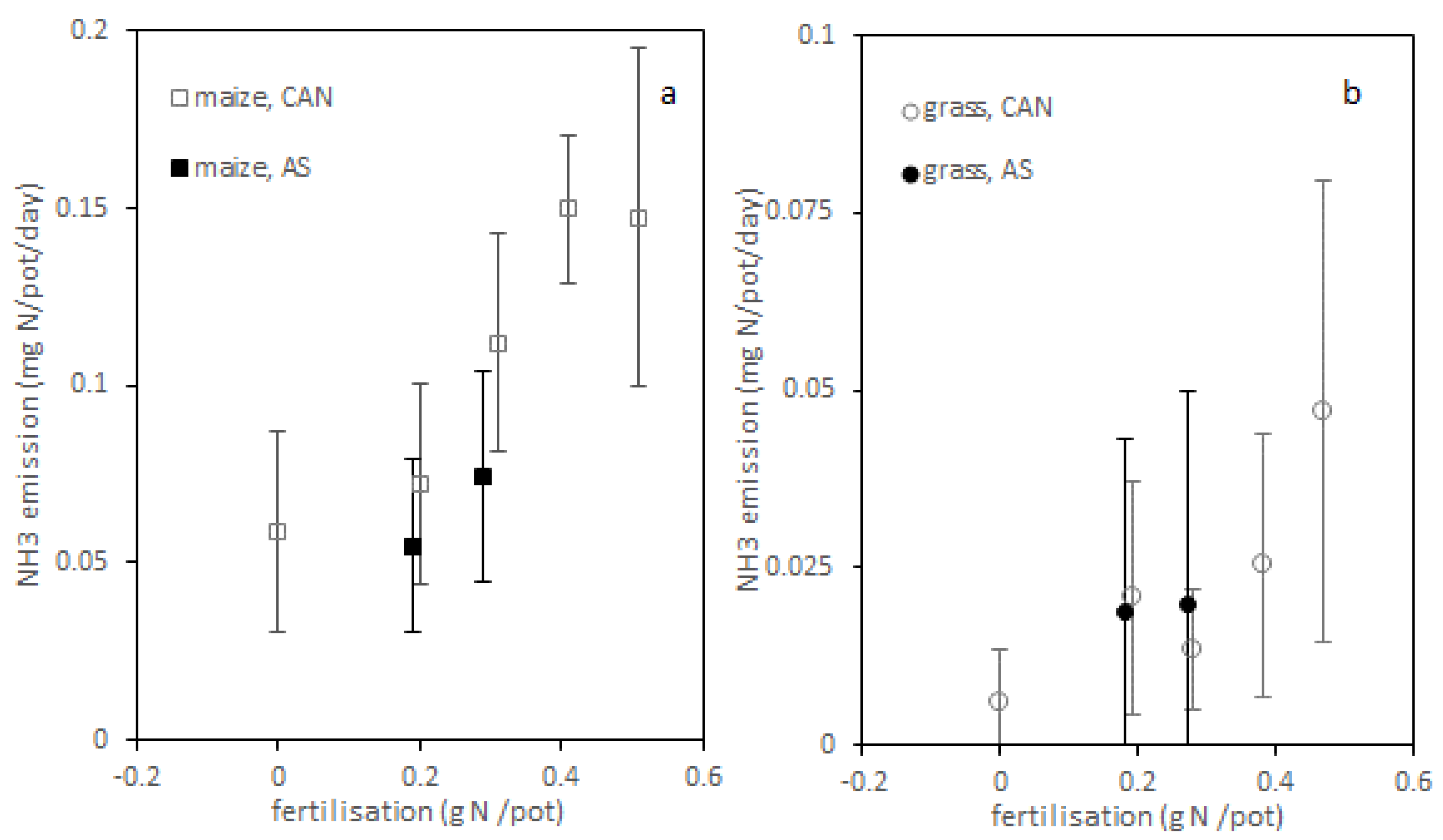
3.2. Greenhouse Experiment, Gaseous Emissions
3.3. Field Demonstration in 2021 and 2022
3.4. Sheltered Flux Chamber Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Crop | Soil | XY Coordinates | Soil Type | Layer (cm) | Nmin | pH | OM | <2 µm | <50 µm | CaCO3 | K | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grass 2021 | Sand | 51.99175, 5.64489 | Umbric Gleysol | 0–10 | 39 | 5.7 | 4.4 | 4 | 15 | <0.2 | 102 | 2.8 |
| Clay | 51.96380, 5.62833 | eutric cambisol | 0–10 | 47 | 6.3 | 8.8 | 43 | 31 | 1.1 | 61 | 0.6 | |
| Grass 2022 | Sand | 51.99241, 5.64582 | Umbric Gleysol | 0–10 | 56 | 6.0 | 1.8 | 8 | 17 | <0.2 | 17 | 0.9 |
| Clay | 51.96422, 5.62797 | eutric cambisol | 0–10 | 112 | 6.6 | 8.1 | 39 | 30 | 1.2 | 26 | 0.4 | |
| Maize 2021 | Sandy | 51.99262, 5.65325 | Umbric Gleysol | 0–25 | - | 5.8 | 2.9 | 3 | 13 | 0.2 | 122 | 2.3 |
| Clay | 51.95542, 5.63467 | Calcaric cambisol | 0–25 | - | 7.3 | 1.7 | 33 | 46 | 2.1 | 70 | 1.7 |
| AS1 2021 | AS2 2021 | CAN + S | Gypsum | AS3 2022 | ||
|---|---|---|---|---|---|---|
| Unit | Grass | Maize | Grass and Maize | Grass and Maize | Grass | |
| Dry matter | % | 27.9 | 29.07 | 98.82 | 99.51 | 37 |
| N tot | % | 5.81 | 6.09 | 22.8 | 0.025 | 7.75 |
| NH4-N% | % | 5.5 | 5.17 | 10.82 | <0.01 | 7.25 |
| S tot | % | 6.78 | 6.92 | 7.1 | 24.2 | 8.17 |
| S water soluble | % | 6.77 | 6.81 | 3.3 | 2.36 | n.d |
| Bulk density | g L−1 | 1137 | 1176 | 1174 | 1435 | n.d. |
| pH | - | 4.3 | 4.2 | - | - | 3.4 |
| TOC | % | 0.12 | 0.07 | 0.9 | 1.61 | <0.18 |
| Treatment | Product Applied | Total Applied | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop | Soil | AS | CAN* | CaSO4 | N | SO3 | P2O5 | K2O | MgO | Na2O | Cu | Zn | |
| Grass 2021 | Sand | CAN-S | - | 500 | 450 | 114 | 361 | 15 | 85 | 50 | 30 | 2 | 0 |
| AS | 1910 | - | - | 111 | 325 | ||||||||
| Clay | CAN-S | - | 450 | 410 | 103 | 328 | 75 | 80 | 55 | 0 | 0 | 0 | |
| AS | 1740 | - | - | 101 | 296 | ||||||||
| Grass 2022 | Sand | blank | 0 | 0 | 0 | 0 | 0 | 15/25 | 180/100 | 25/25 | 10 | 2 | 0 |
| CAN-S | - | 483/387 | 419/336 | 116/93 | 326/262 | ||||||||
| AS | 1600/1282 | - | - | 116/93 | 326/262 | ||||||||
| Clay | blank | 0 | 0 | 0 | 0 | 0 | 95/25 | 110/50 | 75/75 | 0 | 0 | 0 | |
| CAN-S | - | 441/366 | 383/318 | 106/88 | 298/247 | ||||||||
| AS | 1462/1213 | - | - | 106/88 | 298/247 | ||||||||
| Maize 2021 | Sand | CAN-S | - | 600 | 530 | 137 | 427 | 0 | 50 | 0 | 0 | 0.5 | 1 |
| AS | 2250 | - | 50 | 137 | 420 | ||||||||
| Clay | CAN-S | - | 690 | 600 | 157 | 486 | 10 | 55 | 0 | 0 | 0 | 0.5 | |
| AS | 2570 | - | 55 | 157 | 478 | ||||||||
| Soil | Layer | pH | OM | <2 μm | <50 μm | CaCO3 | K | P | WHC | NO3 | NH4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| clay | 0–25 | 7.3 | 1.7 | 33 | 46 | 2.1 | 70 | 1.7 | 425 | 5.6 | 3.5 |
| sandy | 0–25 | 5.4 | 3.1 | 2 | 12 | <0.2 | 70 | 2 | 245 | 17.7 | 0.6 |
| Treatment | Product Applied | Total Applied | |||||
|---|---|---|---|---|---|---|---|
| Crop | Soil | Code | AS | CAN | CaSO4 | N | SO3 |
| maize | clay | 0 | 0 | 0 | 0 | 0 | 0 |
| CAN 50 | 0 | 1 | 1 | 0.26 | 0.47 | ||
| CAN 75 | 0 | 1.5 | 1.5 | 0.39 | 0.70 | ||
| CAN 100 | 0 | 2 | 2 | 0.52 | 0.93 | ||
| CAN 125 | 0 | 2.5 | 2.5 | 0.65 | 1.16 | ||
| AS 50 | 4.43 | 0 | 4.43 | 0.25 | 2.06 | ||
| AS 75 | 6.64 | 0 | 6.64 | 0.37 | 3.09 | ||
| sand | 0 | 0 | 0 | 0 | 0 | 0 | |
| CAN 50 | 0 | 0.78 | 0.78 | 0.20 | 0.36 | ||
| CAN 75 | 0 | 1.18 | 1.18 | 0.31 | 0.55 | ||
| CAN 100 | 0 | 1.57 | 1.57 | 0.41 | 0.73 | ||
| CAN 125 | 0 | 1.966 | 1.966 | 0.51 | 0.91 | ||
| AS 50 | 3.49 | 0 | 3.49 | 0.19 | 1.62 | ||
| AS 75 | 5.23 | 0 | 5.23 | 0.29 | 2.43 | ||
| grass | clay | 0 | 0 | 0 | 1.77 | 0 | 0.82 |
| CAN 50 | 0 | 0.726 | 1.77 | 0.19 | 0.82 | ||
| CAN 75 | 0 | 1.09 | 1.77 | 0.28 | 0.82 | ||
| CAN 100 | 0 | 1.45 | 1.77 | 0.38 | 0.82 | ||
| CAN 125 | 0 | 1.81 | 1.77 | 0.47 | 0.82 | ||
| AS 50 | 3.22 | 0 | 0.6 | 0.18 | 0.28 | ||
| AS 75 | 4.83 | 0 | 0 | 0.27 | 0 | ||
| sand | 0 | 0 | 0 | 1.77 | 0.00 | 0.82 | |
| CAN 50 | 0 | 0.726 | 1.77 | 0.19 | 0.82 | ||
| CAN 75 | 0 | 1.09 | 1.77 | 0.28 | 0.82 | ||
| CAN 100 | 0 | 1.45 | 1.77 | 0.38 | 0.82 | ||
| CAN 125 | 0 | 1.81 | 1.77 | 0.47 | 0.82 | ||
| AS 50 | 3.22 | 0 | 0.6 | 0.18 | 0.28 | ||
| AS 75 | 4.83 | 0 | 0 | 0.27 | 0 | ||
| Unit | AS 2023 | CS | |
|---|---|---|---|
| Dry matter | % | 37.4 | 9.4 |
| N tot | % | 4.598 | 0.347 |
| NH4-N% | % | 4.49 | 0.16 |
| S tot | % | 5.25 | 0.054 |
| Bulk density | g L−1 | 1100 | 989 |
| pH | 5.0 | 7.5 |
| Soil Extractable N (kg of N ha−1) | |||||
|---|---|---|---|---|---|
| Soil | Treatment | 0–30 | 30–60 | 60–90 | Sum |
| Maize 2021 | |||||
| Sand | CAN + S | 17 | 26 | 23 | 66 |
| AS | 17 | 25 | 21 | 63 | |
| Clay | CAN + S | 18 | 15 | 9 | 42 |
| AS | 19 | 16 | 10 | 45 | |
| Grass 2021 * | |||||
| Grass 2022 | |||||
| Sand | CAN + S | 26 | 28 | 8 | 62 |
| AS | 34 | 32 | 10 | 76 | |
| Control | 8 | 14 | 4 | 26 | |
| Clay | CAN + S | 30 | 32 | 11 | 73 |
| AS | 35 | 39 | 12 | 86 | |
| Control | 26 | 24 | 8 | 58 | |
| n.s. | |||||
| Soil Extractable S (kg of S ha−1) | |||||
| 0–30 | 30–60 | 60–90 | Sum | ||
| Sand | CAN + S | 340 | 167 | 17 | 524 |
| AS | 129 | 110 | 23 | 262 | |
| Control | 29 | 28 | 14 | 71 | |
| Clay | CAN + S | 247 | 102 | 27 | 377 |
| AS | 160 | 97 | 33 | 290 | |
| Control | 22 | 22 | 17 | 61 | |
| n.s. | |||||
| Sand | Clay | |||
|---|---|---|---|---|
| Grass | Maize | Grass | Maize | |
| CAN 50 | 0.01% | 0.00% | 0.00% | 0.03% |
| CAN 75 | 0.02% | 0.00% | 0.00% | 0.00% |
| CAN 100 | 0.03% | 0.00% | 0.01% | 0.01% |
| CAN 125 | 0.00% | 0.00% | 0.02% | 0.02% |
| AS 50 | 0.04% | 0.00% | 0.03% | 0.00% |
| AS 75 | 0.00% | 0.00% | 0.00% | 0.02% |
| Dm | N | P | K | Ca | Mg | Na | S | ||
|---|---|---|---|---|---|---|---|---|---|
| Maize 2021 | |||||||||
| clay | CAN + S | 19.2 ± 0.3 | 157 ± 23 | 27 ± 3 | 161 ± 14 | 40 ± 1 | 23 ± 2 | 1.0 ± 0.1 | 15 ± 1 |
| AS | 18.3 ± 0.8 | 178 ± 9 | 28 ± 3 | 154 ± 12 | 38 ± 2 | 22 ± 1 | 0.9 ± 0.1 | 15 ± 0.5 | |
| sand | CAN + S | 22.1 ± 1 | 235 ± 14 | 33 ± 2 | 288 ± 12 | 38 ± 2 | 23 ± 1 | 1.0 ± 0.1 | 18 ± 1 |
| AS | 23 ± 1.1 | 252 ± 13 | 38 ± 2 | 256 ± 12 | 37 ± 2 | 24 ± 1 | 1.0 ± 0.1 | 19 ± 1 | |
| Grass 2021 | |||||||||
| clay | CAN + S | 6.6 ± 0.3 | 118 ± 5 | 21 ± 0.3 | 178 ± 14 | 26 ± 1 | 8 ± 0.3 | 9 ± 2 | 19 ± 1 |
| AS | 6.7 ± 0.6 | 131 ± 5 | 21 ± 1 | 178 ± 10 | 28 ± 3 | 9 ± 1 | 10 ± 3 | 21 ± 2 | |
| sand | CAN + S | 7.7 ± 0.8 | 142 ± 15 | 24 ± 2 | 145 ± 27 | 29 ± 5 | 13 ± 2 | 17 ± 1 | 22 ± 2 |
| AS | 6.8 ± 0.6 | 132 ± 13 | 22 ± 2 | 141 ± 16 | 23 ± 4 | 11 ± 1 | 18 ± 1 | 21 ± 2 | |
| Grass 2022 | |||||||||
| clay | Blank | 2.9 | 46.1 | 8.3 | 55.3 | 12.5 | 3.8 | 1.7 | 5.1 |
| CAN + S | 6.3 ± 0.4 | 181 ± 11 | 19 ± 1 | 156 ± 11 | 33 ± 3 | 11 ± 1 | 13 ± 1 | 23 ± 2 | |
| AS | 5.9 ± 0.6 | 171 ± 16 | 18 ± 2 | 146 ± 13 | 30 ± 3 | 10 ± 1 | 11 ± 3 | 28 ± 3 | |
| sand | Blank | 3.2 | 41 | 7.3 | 48 | 15.7 | 4.8 | 4 | 6.2 |
| CAN + S | 7.0 ± 0.1 | 182 ± 12 | 23 ± 1 | 96 ± 9 | 44 ± 2 | 16 ± 1 | 31 ± 7 | 27 ± 2 | |
| AS | 6.9 ± 0.2 | 191 ± 18 | 24.2 ± 0.3 | 96 ± 14 | 37 ± 1 | 15 ± 1 | 31 ± 6 | 30 ± 2 | |

References
- Huygens, D.; Orveillon, G.; Lugato, E.; Tavazzi, S.; Comero, S.; Jones, A.; Gawlik, B.; Saveyn, H. Technical Proposals for the Safe Use of Processed Manure above the Threshold Established for Nitrate Vulnerable Zones by the Nitrates Directive (91/676/EEC). JRC121636 Publications Office; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Brienza, C.; Snauwaert, E.; De Dobbelaere, A.; De Mey, J.; Vaneeckhaute, C.; Michels, E.; Schoumans, O.; Adani, F.; Meers, E. Production and performance of bio-based mineral fertilizers from agricultural waste using ammonia (stripping-) scrubbing technology. Waste Manag. 2019, 89, 265–274. [Google Scholar] [CrossRef] [PubMed]
- van Puffelen, J.L.; Brienza, C.; Regelink, I.C.; Sigurnjak, I.; Adani, F.; Meers, E.; Schoumans, O.F. Performance of a full-scale processing cascade that separates agricultural digestate and its nutrients for agronomic reuse. Sep. Purif. Technol. 2022, 297, 121501. [Google Scholar] [CrossRef]
- Meers, E.; Michels, E.; Rietra, R.; Velthof, G. Biorefinery of Inorganics: Recovering Mineral Nutrients from Biomass and Organic Waste; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Schils, R.; Schröder, J.; Velthof, G. Fertilizer Replacement Value: Linking Organic Residues to Mineral Fertilizers. In Biorefinery Inorganics Recovering Mineral Nutrients from Biomass and Organic Waste; Meers, E., Velthof, G., Michels, E., Rietra, R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 189–214. [Google Scholar] [CrossRef]
- Westerik, D.; Hoffland, E.; Hijbeek, R. Nitrogen fertilizer replacement values of organic amendments: Determination and prediction. Nutr. Cycl. Agroecosyst. 2023. [CrossRef]
- van der Weerden, T.; Noble, A.; Beltran, I.; Hutchings, N.; Thorman, R.; de Klein, C.; Amon, B. Influence of key factors on ammonia and nitrous oxide emission factors for excreta deposited by livestock and land-applied manure. Sci. Total Environ. 2023, 889, 164066. [Google Scholar] [CrossRef] [PubMed]
- Kros, H.; Cals, T.; Gies, E.; Groenendijk, P.; Lesschen, J.P.; Voogd, J.C.; Hermans, T.; Velthof, G. Region oriented and integrated approach to reduce emissions of nutrients and greenhouse gases from agriculture in The Netherlands. Sci. Total Environ. 2024, 909, 168501. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.; Hinton, N.; Cloy, J.; Topp, C.; Rees, R.; Williams, J.; Misselbrook, T.; Chadwick, D. How do emission rates and emission factors for nitrous oxide and ammonia vary with manure type and time of application in a Scottish farmland? Geoderma 2016, 264, 81–93. [Google Scholar] [CrossRef]
- Reuland, G.; Sigurnjak, I.; Dekker, H.; Michels, E.; Meers, E. The potential of digestate and the liquid fraction of digestate as chemical fertiliser substitutes under the RENURE criteria. Agronomy 2021, 11, 1374. [Google Scholar] [CrossRef]
- Sommer, S.; Génermont, S.; Cellier, P.; Hutchings, N.; Olesen, J.; Morvan, T. Processes controlling ammonia emission from livestock slurry in the field. Eur. J. Agron. 2003, 19, 465–486. [Google Scholar] [CrossRef]
- Müller, B.; Hartung, J.; von Cossel, M.; Lewandowski, I.; Müller, T.; Bauerle, A. On-farm use of recycled liquid ammonium sulphate in Southwest Germany using a participatory approach. Nutr. Cycl. Agroecosyst. 2023. [CrossRef]
- EC. Commission Directive (Draft) Amending Council Directive 91/676/EEC as Regards the Use of Certain Fertilising Materials from Livestock Manure. Ares (2024)2885619 19/04/2024. Available online: https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/14242-Commission-Directive-amending-Annex-III-of-the-Nitrates-Directive_en (accessed on 25 April 2024).
- Reijneveld, J.A.; van Oostrum, M.J.; Brolsma, K.M.; Fletcher, D.; Oenema, O. Empower innovations in routine soil testing. Agronomy 2022, 12, 191. [Google Scholar] [CrossRef]
- Anonymous. Fertilization Recommendation for Grassland, and Forage Crops. Commissie Bemesting Grasland en Voedergewassen. 2012. Available online: https://edepot.wur.nl/413891 (accessed on 25 April 2024). (In Dutch).
- Novozamsky, I.; Houba, V.; Van Eck, R.; Van Vark, W. A novel digestion technique for multi-element plant analysis. Commun. Soil Sci. Plant Anal. 1983, 14, 239–248. [Google Scholar] [CrossRef]
- Lubbers, I.M.; Brussaard, L.; Otten, W.; Van Groenigen, J.W. Earthworm-induced N mineralization in fertilized grassland increases both N2O emission and crop-N uptake. Eur. J. Soil Sci. 2011, 62, 152–161. [Google Scholar] [CrossRef]
- Van der Stelt, B.; Temminghoff, E.; Van Vliet, P.; Van Riemsdijk, W. Volatilization of ammonia from manure as affected by manure additives, temperature and mixing. Bioresour. Technol. 2007, 98, 3449–3455. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS-14256-1; ISO (International Organization for Standardization), Soil Quality—Determination of Nitrate, Nitrite and Ammonium in Field-Moist Soils by Extraction with Potassium Chloride Solution. ISO (International Organization for Standardization): Geneva, Switzerland, 2003.
- Houba, V.; Temminghoff, E.; Gaikhorst, G.; Van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- De Ruijter, F.; Huijsmans, J.; Rutgers, B. Ammonia volatilization from crop residues and frozen green manure crops. Atmos. Environ. 2010, 44, 3362–3368. [Google Scholar] [CrossRef]
- EEA. EMEP/EEA Air Pollutant Emission Inventory Guidebook 2023; European Environment Agency: Copenhagen, Denmark, 2013; Available online: https://www.eea.europa.eu/publications/emep-eea-guidebook-2023/part-b-sectoral-guidance-chapters/3-agriculture/3-d-agricultural-soils-2023/view (accessed on 23 May 2024).
- Huijsmans, J.; Schröder, J.; Mosquera, J.; Vermeulen, G.; Ten Berge, H.; Neeteson, J. Ammonia emissions from cattle slurries applied to grassland: Should application techniques be reconsidered? Soil Use Manag. 2016, 32, 109–116. [Google Scholar] [CrossRef]
- Huijsmans, J.; Vermeulen, G.; Hol, J.; Goedhart, P. A model for estimating seasonal trends of ammonia emission from cattle manure applied to grassland in The Netherlands. Atmos. Environ. 2018, 173, 231–238. [Google Scholar] [CrossRef]
- van Bruggen, C.; Bannink, A.; Groenestein, C.; Huijsmans, J.; Luesink, H.H.; Oude Voshaar, S.V.; van der Sluis, S.M.; Velthof, G.L.; Vonk, J. Emissies naar Lucht uit de Landbouw uit de Landbouw in 2015. Berekeningen met het Model NEMA; WOt-Technical Report 98; Wettelijke Onderzoekstaken Natuur & Milieu: Wageningen, The Netherlands, 2015. (In Dutch) [Google Scholar] [CrossRef]
- Schlossberg, M.J.; McGraw, B.A.; Hivner, K.R. Comparing closed chamber measures of ammonia volatilization from Kentucky bluegrass fertilized by granular urea. J. Environ. Hortic. 2018, 36, 85–91. [Google Scholar] [CrossRef]
- Alexander, J.R.; Spackman, J.A.; Wilson, M.L.; Fernández, F.G.; Venterea, R.T. Capture efficiency of four chamber designs for measuring ammonia emissions. Agrosyst. Geosci. Environ. 2021, 4, e20199. [Google Scholar] [CrossRef]
- De Klein, C.A.M.; Van Logtestijn, R.S.P. Denitrification in grassland soils in The Netherlands in relation to irrigation, N-application rate, soil water content and soil temperature. Soil Biol. Biochem. 1996, 28, 231–237. [Google Scholar] [CrossRef]
- van Bruggen, C.; Bannink, A.; Bleeker, A.; Bussink, D.W.; Dooren, H.J.C.v.; Groenestein, C.M.; Huijsmans, J.F.M.; Kros, J.; Lagerwerf, L.A.; Oltmer, K.; et al. Emissies naar Lucht uit de Landbouw Berekend met NEMA voor 1990–2021; WOt-Report 242; Wettelijke Onderzoekstaken Natuur & Milieu: Wageningen, The Netherlands, 2023. (In Dutch) [Google Scholar] [CrossRef]
- Suttle, N.F. Mineral Nutrition of Livestock; CAB International: Wallingford, UK, 2022. [Google Scholar]
- National Research Council. Mineral Tolerance of Animals. Second Revised Edition, 2005; National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Drewnoski, M.; Pogge, D.; Hansen, S. High-sulfur in beef cattle diets: A review. J. Anim. Sci. 2014, 92, 3763–3780. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; López, S.; Montanholi, Y.; Smith, B.; Haas, L.; Miller, S.; France, J. A meta-analysis of the effects of dietary copper, molybdenum, and sulfur on plasma and liver copper, weight gain, and feed conversion in growing-finishing cattle. J. Anim. Sci. 2013, 91, 5714–5723. [Google Scholar] [CrossRef] [PubMed]
- McLaren, R. Effects of fertilizers on the sulphur content of herbage. Grass Forage Sci. 1976, 31, 99–103. [Google Scholar] [CrossRef]
- Keer, J.; McLaren, R.; Swift, R. The sulphur status of intensive grassland sites in southern Scotland. Grass Forage Sci. 1986, 41, 183–190. [Google Scholar] [CrossRef]
- Dijksterhuis, G.H.; Oenema, O. Studies on the effectiveness of various sulfur fertilizers under controlled conditions. Fertil. Res. 1990, 22, 147–159. [Google Scholar] [CrossRef]
- Kulczycki, G.; Sacała, E.; Koszelnik-Leszek, A.; Milo, Ł. Perennial Ryegrass (Lolium perenne L.) Response to Different Forms of Sulfur Fertilizers. Agriculture 2023, 13, 1773. [Google Scholar] [CrossRef]
- Reijneveld, J.; Abbink, G.; Termorshuizen, A.; Oenema, O. Relationships between soil fertility, herbage quality and manure composition on grassland-based dairy farms. Eur. J. Agron. 2014, 56, 9–18. [Google Scholar] [CrossRef]
- Lamers, L.P.; Tomassen, H.B.; Roelofs, J.G. Sulfate-induced eutrophication and phytotoxicity in freshwater wetlands. Environ. Sci. Technol. 1998, 32, 199–205. [Google Scholar] [CrossRef]
- EN 15936:2022; Soil, Waste, Treated Biowaste and Sludge—Determination of Total Organic Carbon (TOC) by Dry Combustion. CEN (European Committee for Standardization): Brussels, Belgium, 2022. Available online: https://www.nen.nl/en/nen-en-15936-2022-en-293461 (accessed on 23 May 2024).
- ISO 11885, 2009; ISO (International Organization for Standardization), Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO (International Organization for Standardization): Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36250.html (accessed on 23 May 2024).
- ISO 17294-2, 2016; ISO (International Organization for Standardization), Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/82245.html (accessed on 23 May 2024).
- VDLUFA. Methodenbuch Band II.2 Sekundärrohstoffdünger, Kultursubstrate und Bodenhilfsstoffe (Grundwerk 2000); VDLUFA: Speyer, Germany, 2000. [Google Scholar]
- DIN 38406-5, 1983; DIN (Deutsches Institut für Normung), German Standard Methods for the Examination of Water, Waste Water and Sludge—Cations (group E)—Determination of Ammonia-Nitrogen (E5). DIN: Berlin, Germany, 1983. Available online: https://www.din.de/de/mitwirken/normenausschuesse/naw/wdc-beuth:din21:1061756 (accessed on 23 May 2024).
- VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs- und ForschungsAnstalten e. V). Methodenbuch Band I Böden (Grundwerk 1991); VDLUFA: Speyer, Germany, 1991. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rietra, R.; van Dijk, K.; Schoumans, O. Environmental Effects of Using Ammonium Sulfate from Animal Manure Scrubbing Technology as Fertilizer. Appl. Sci. 2024, 14, 4998. https://doi.org/10.3390/app14124998
Rietra R, van Dijk K, Schoumans O. Environmental Effects of Using Ammonium Sulfate from Animal Manure Scrubbing Technology as Fertilizer. Applied Sciences. 2024; 14(12):4998. https://doi.org/10.3390/app14124998
Chicago/Turabian StyleRietra, René, Kimo van Dijk, and Oscar Schoumans. 2024. "Environmental Effects of Using Ammonium Sulfate from Animal Manure Scrubbing Technology as Fertilizer" Applied Sciences 14, no. 12: 4998. https://doi.org/10.3390/app14124998





