Eco-Friendly Preservation of Pharaonic Wooden Artifacts using Natural Green Products
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Archaeological Objects
2.2. Isolation and Identification of Deteriorating Fungi
2.3. Ageing and Biodeterioration of Experimental Aged Wooden Blocks
2.4. Green Eco-Friendly Natural Products
2.5. Antifungal Effects of the Essential Oils and Plant Extracts on the Highly Deterioration-Causing Fungal Isolates
2.6. Active Constituents of the Most Efficient Essential Oils
2.7. Effect of the Most Efficient Essential Oils on the Enzymatic Activities of the Highly Frequent Deterioration-Causing Fungal Species
2.8. Essential Oils Efficiency on the Preservation of the Experimental Aged Wood Samples against the Deterioration-causing Fungal Species
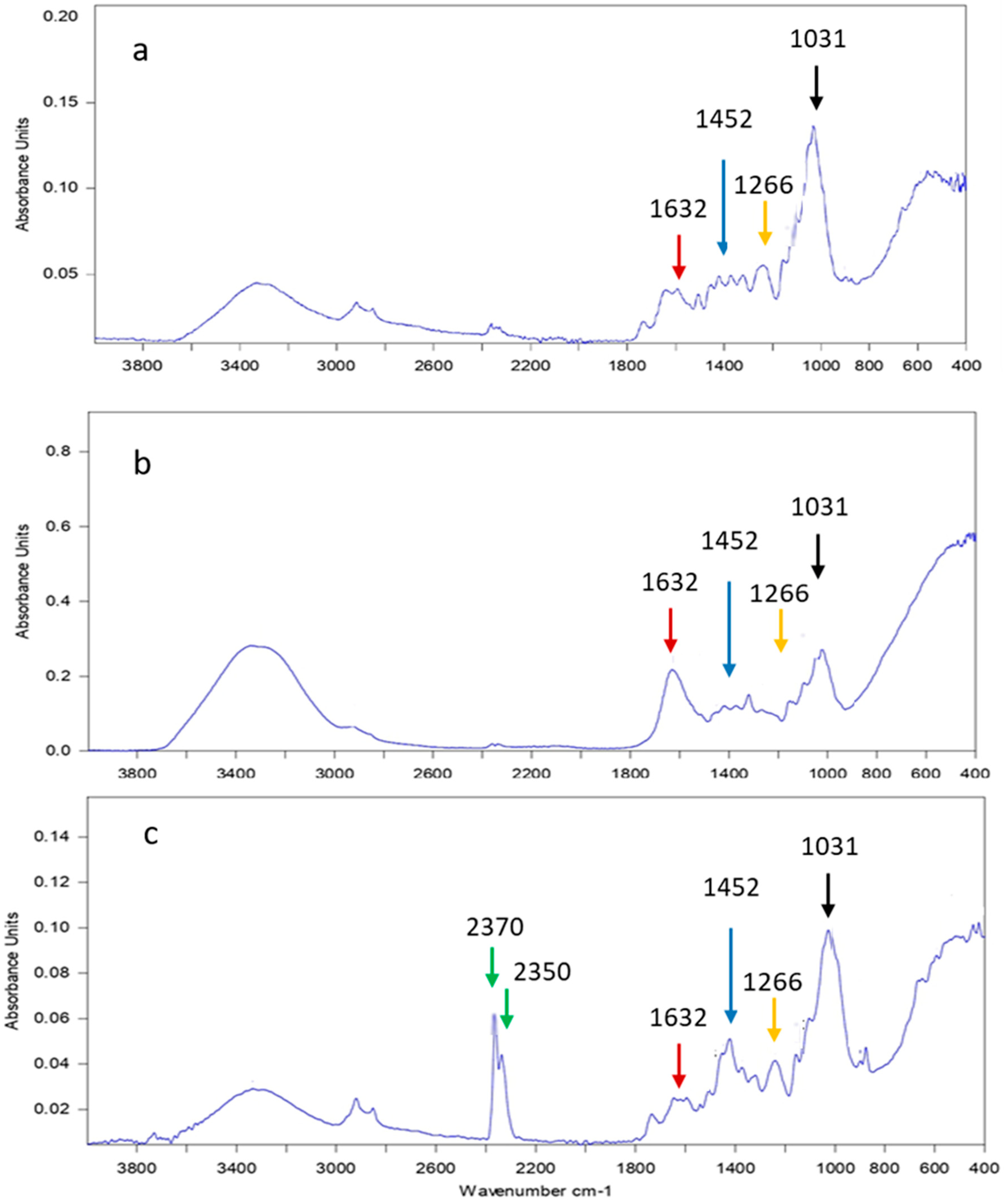
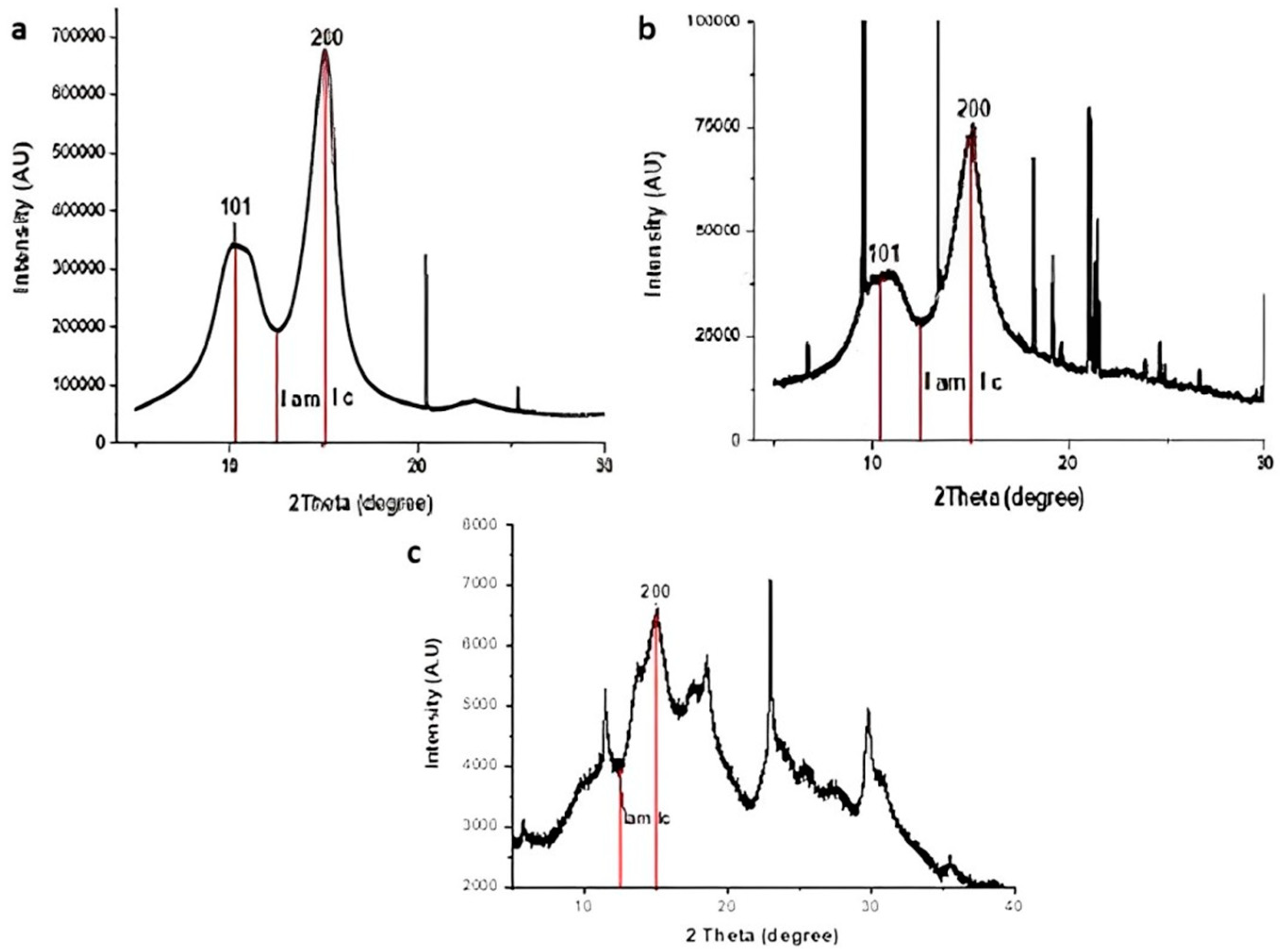
2.9. Effect of the Most Efficient Essential Oil on the Characterization of the Experimental Aged Wood Samples
2.10. Statistical Analysis
3. Results
3.1. Identification of Fungi Isolated from the Ten Archaeological Wooden Objects
3.2. Identification of the Highly Frequent Fungal Species
3.3. Experimental Aged Wooden Blocks Biodeterioration
3.4. Effect of the Plant Extracts on the Highly Frequent Fungal Species
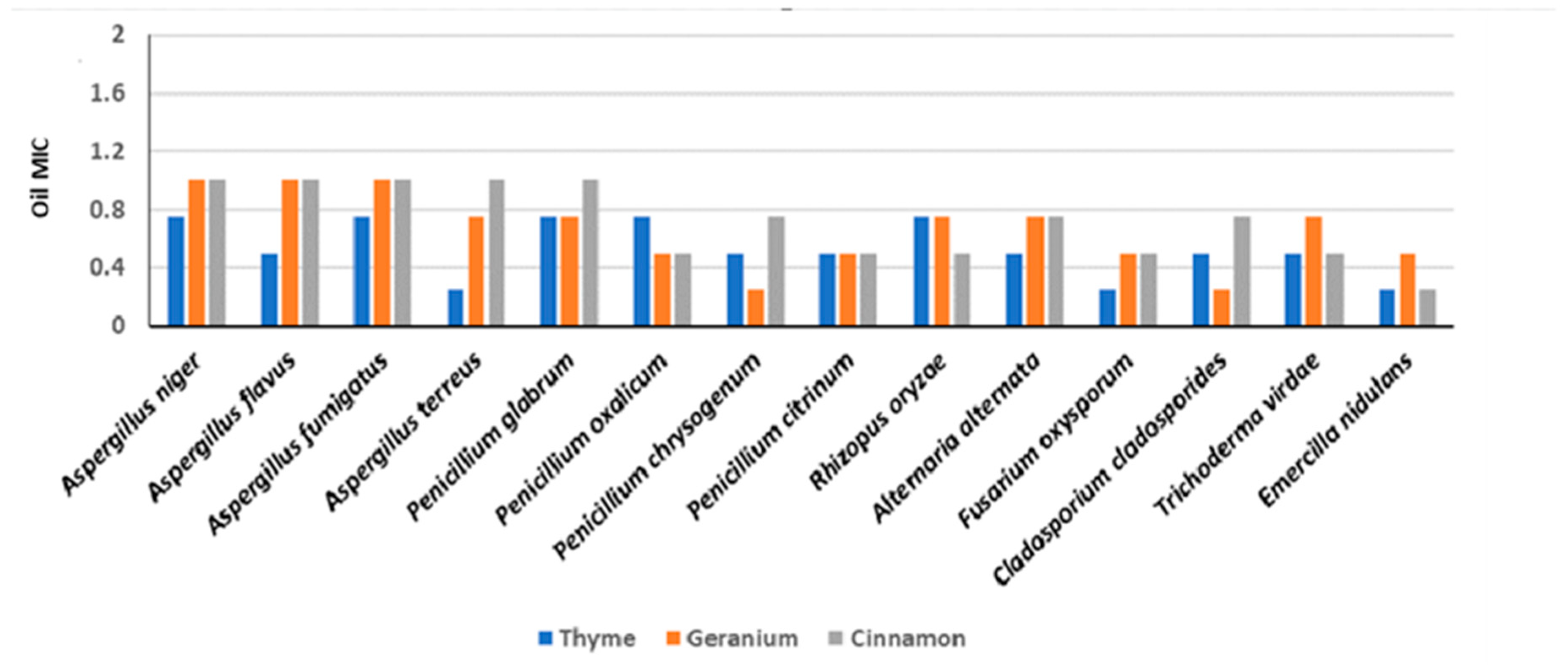
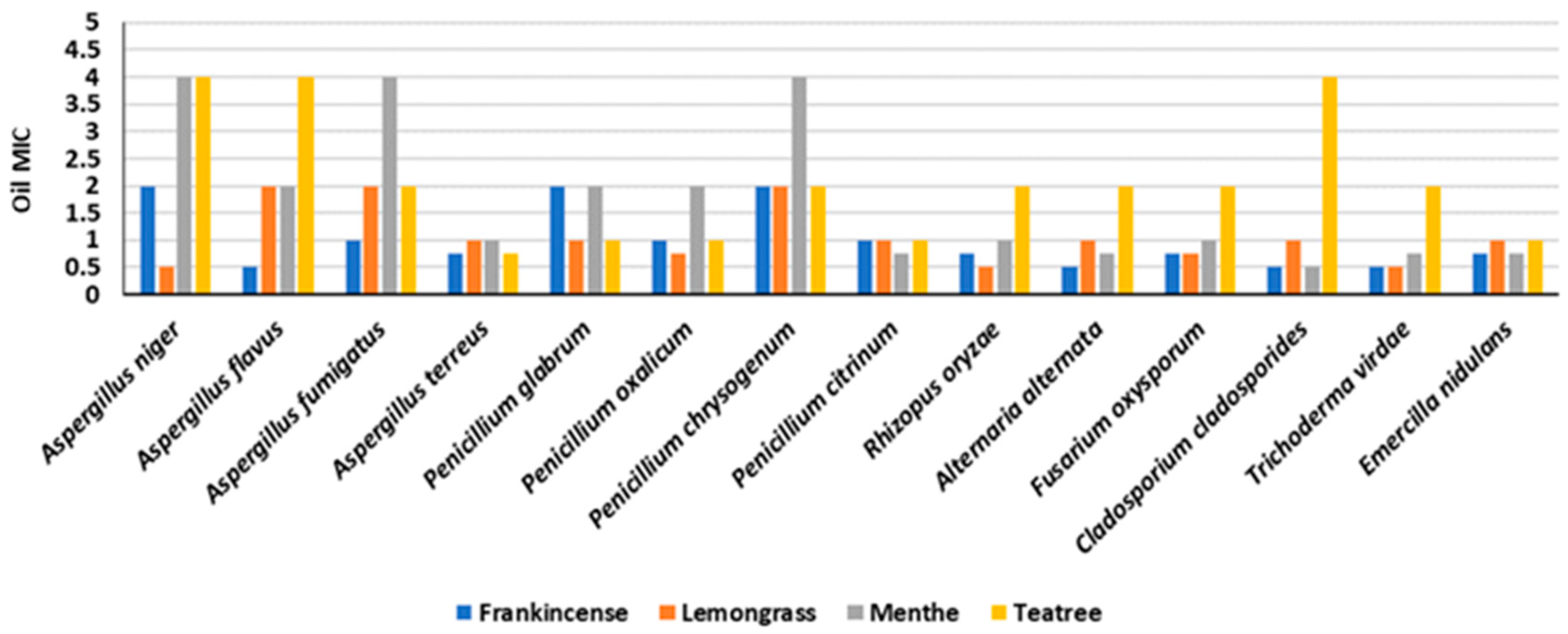
3.5. Effect of Essential Oils (MICs) on Fungal Isolates and Chemical Composition
3.6. Preservation Effects of Essential Oils on Experimental Wooden Blocks
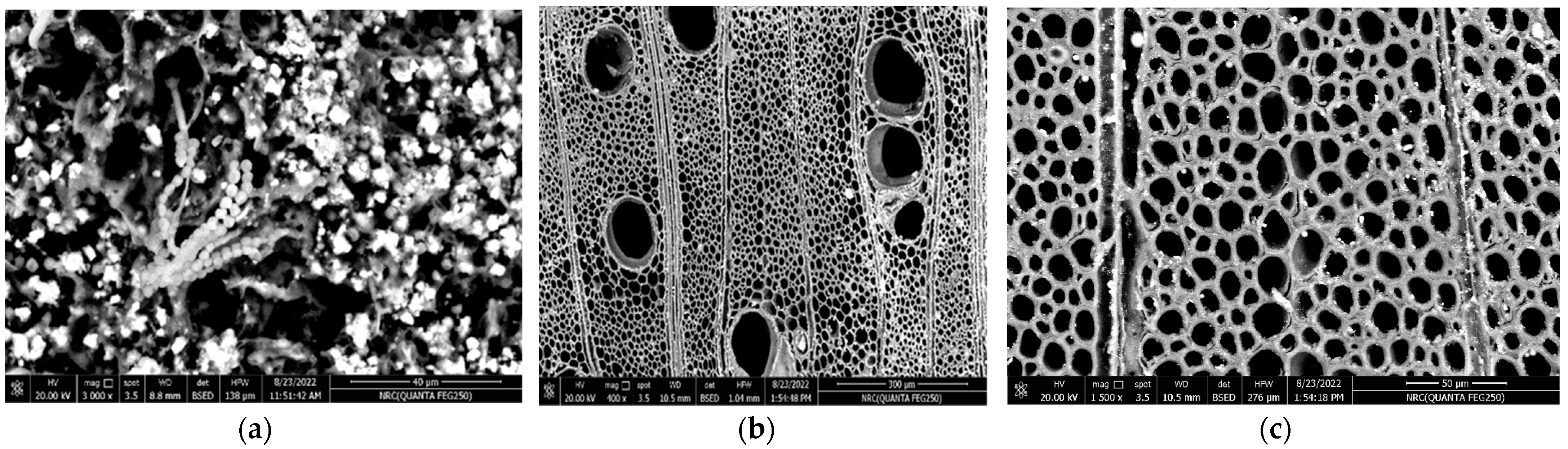
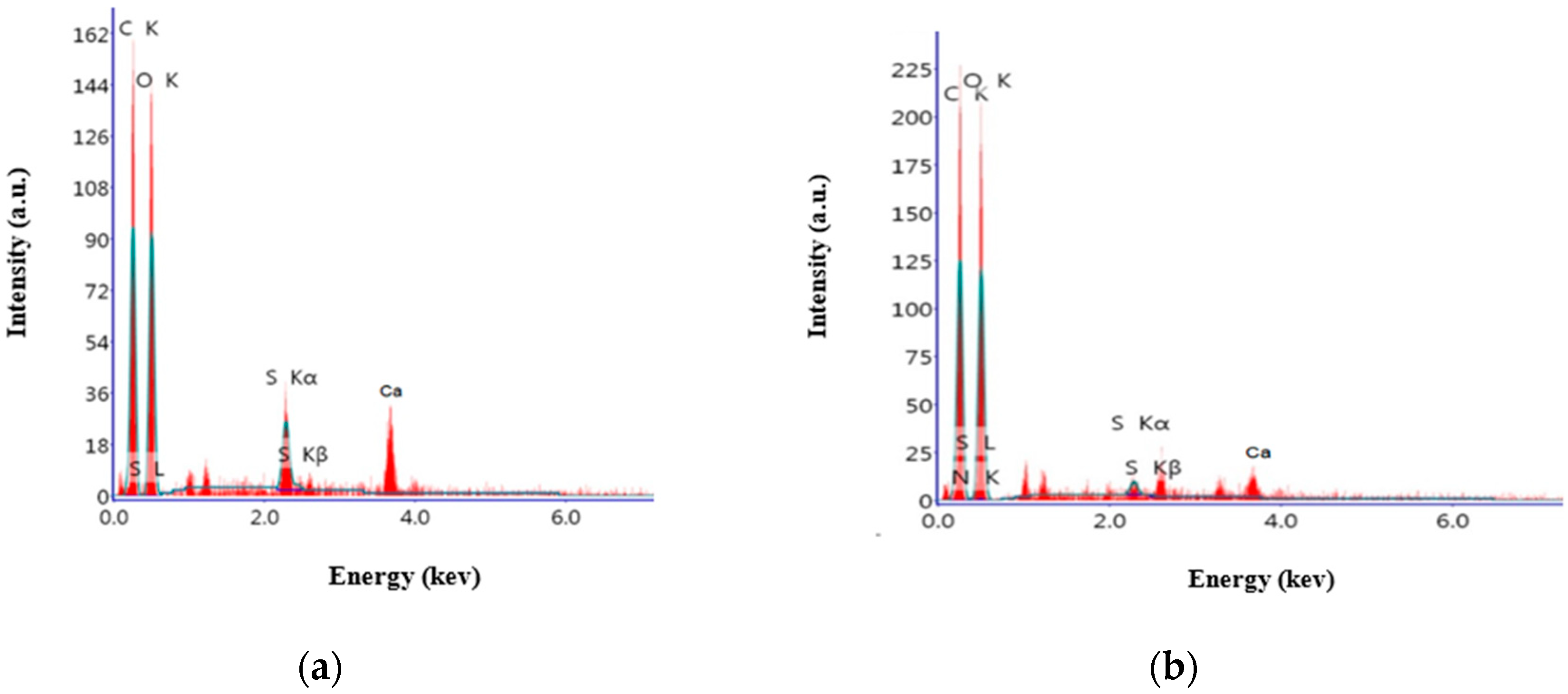
3.7. Analyses of A. nilotica Experimental Wooden Cubes Treated with Thyme Oil as the Most Effective EO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blondel, F.; Huebner, S.R.; Pearson, C.; Stoffel, M. Mummy labels: A witness to the use and processing of wood in Roman Egypt. Int. J. Wood Cult. 2023, 3, 192–223. [Google Scholar] [CrossRef]
- Savković, Ž.; Stupar, M.; Unković, N.; Knežević, A.; Vukojević, J.; Ljaljević Grbić, M. Fungal deterioration of cultural heritage objects. In Biodegradation Technology of Organic and Inorganic Pollutants; Mendes, K.F., de Sousa, R.N., Mielke, K.C., Eds.; IntechOpen: London, UK, 2021; pp. 267–288. [Google Scholar] [CrossRef]
- Flyen, A.C.; Thuestad, A.E. A review of fungal decay in historic wooden structures in polar regions. Conserv. Manag. Archaeol. Sites 2023, 24, 3–35. [Google Scholar] [CrossRef]
- Li, T.; Cui, L.; Song, X.; Cui, X.; Wei, Y.; Tang, L.; Mu, Y.; Xu, Z. Wood decay fungi: An analysis of worldwide research. J. Soils Sediments 2022, 22, 1688–1702. [Google Scholar] [CrossRef]
- Blanchette, R.A. A review of microbial deterioration found in archaeological wood from different environments. Int. Biodeterior. Biodegrad. 2000, 46, 189–204. [Google Scholar] [CrossRef]
- Gadd, G.; Fomina, M.; Pinzari, F. Fungal biodeterioration and preservation of cultural heritage, artwork, and historical artifacts: Extremophily and adaptation. Microbiol. Mol. Biol. Rev. 2024, 88, e00200–e00222. [Google Scholar] [CrossRef] [PubMed]
- Atwa, D.M.; Ibrahim, S.; Stani, C.; Birarda, G.; Ali, N.; Abdullah, E.; Vaccari, L.; Grenni, P.; Visca, A.; Badr, Y.; et al. Biodeterioration assessment of a unique old pharaonic kingdom wooden statue using advanced diagnostic techniques. Appl. Sci. 2022, 12, 7020. [Google Scholar] [CrossRef]
- Geweely, N.S. A novel comparative review between chemical, natural essential oils and physical (ozone) conservation of archaeological objects against microbial deterioration. Geomicrobiol. J. 2022, 39, 531–540. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Zikeli, F.; Humar, M.; Biscontri, M.; Bergamasco, S.; Romagnoli, M. Essential oils from Thymus spp. as natural biocide against common brown-and white-rot fungi in degradation of wood products: Antifungal activity evaluation by in vitro and FTIR analysis. Eur. J. Wood Wood Prod. 2023, 81, 747–763. [Google Scholar] [CrossRef]
- Geweely, N.S. Purification and characterization of acido-thermophilic xylanase from Aspergillus terreus. Aust. J. Basic Appl. Sci. 2011, 5, 214–219. [Google Scholar]
- Broda, M. Natural compounds for wood protection against fungi—A review. Molecules 2020, 25, 3538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Bhandari, B.; Wang, M. Basic sensory properties of essential oils from aromatic plants and their applications: A critical review. Crit. Rev. Food Sci. Nutr. 2023, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, F.; Bartolini, M.; Plissonnier, M.L.; Esposito, A.; Galotta, G.; Ricci, S.; Davidde Petriaggi, B.; Pedone, C.; Di Giovanni, A.; Piazza, S.; et al. Essential oils as alternative biocides for the preservation of waterlogged archaeological wood. Microorganisms 2020, 8, 2015. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.; Enwemiwe, V.; Ejeromedoghene, O.; Adebayo, A.; Ogunyemi, O.; Fu, F. Production and functionalities of specialized metabolites from different organic sources. Metabolites 2022, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Skóra, J.; Gutarowska, B.; Pielech-Przybylska, K.; Stępień, Ł.; Pietrzak, K.; Piotrowska, M.; Pietrowski, P. Assessment of microbiological contamination in the work environments of museums, archives and libraries. Aerobiologia 2015, 31, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M. Antifungal agents in wood protection—A review. Molecules 2020, 27, 6392. [Google Scholar] [CrossRef] [PubMed]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Kew, UK, 1971; 237p. [Google Scholar]
- Gilman, J.C. A Manual of Soil Fungi, Indian Edition Published by Arrangement with the Original American Publishers; The Iowa State University Press: Ames, IA, USA, 1974; pp. 217–251. [Google Scholar]
- Raper, K.B.; Funnel, D.I. The Genus Aspergillus; Robert E. Ktieger Publ. Co.:: New York, NY, USA, 1977; 686p. [Google Scholar]
- Pitt, J.I. The Genus Pencillium and Its Teleomorph States Eupencillim and Talaromyces; Academic Press: New York, NY, USA, 1979; pp. 50–55. [Google Scholar]
- Samson, R.A.; Reenen-Koekstra, E.S. Introduction to Food–Borne Fungi, 3rd ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 1988; 299p. [Google Scholar]
- Moubasher, A.H. Soil fungi in Qatar and other Arab countries. Econ. Bot. 1996, 50, 242. [Google Scholar] [CrossRef]
- Kern, M.E.; Blevins, K.S. Laboratory procedures for fungal culture and isolation. In Medical Mycology: A Self-Instructional Text, 2nd ed.; FA Davis Company: Philadelphia, PA, USA, 1997; pp. 27–72. [Google Scholar]
- Klich, M.A. Identification of Common Aspergillus Species; Centraal Bureau Voor Schim: Utrecht, The Netherlands, 2002; 316p. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; Academic Press: London, UK, 2007; Volume 1, 672p. [Google Scholar]
- Siddiquee, S. Practical Handbook of the Biology and Molecular Diversity of Trichoderma Species from Tropical Regions; Springer International Publishing: Cham, Swizerland, 2017; Volume 431. [Google Scholar]
- Kwiatkowska, M.; Ważny, R.; Turnau, K.; Wójcik, A. Fungi as deterioration agents of historic glass plate negatives of Brandys family collection. Int. Biodeterior. Biodegrad. 2016, 115, 133–140. [Google Scholar] [CrossRef]
- Geweely, N.S.; Abu Taleb, A.; Ibrahim, S.; Grenni, P.; Caneva, G.; Galotta, G.; Abdalla, M.; Atwa, D.M.; Plaisier, J.R.; Antonelli, F. New data on relevant ancient Egyptian wooden artifacts: Identification of wooden species and study of the state of conservation with multidisciplinary analyses. Archaeometry 2023, 65, 165–183. [Google Scholar] [CrossRef]
- Sambrook, J.F.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Veneranda, M.; Blanco-Zubiaguirre, L.; Roselli, G.; Di Girolami, G.; Castro, K.; Madariaga, J.M. Evaluating the exploitability of several essential oils constituents as a novel biological treatment against cultural heritage biocolonization. Microchem. J. 2018, 138, 1–6. [Google Scholar] [CrossRef]
- Bahmani, M.; Schmidt, O. Plant essential oils for environment-friendly protection of wood objects against fungi. Maderas. Ciencia Tecnología 2018, 20, 325–332. [Google Scholar] [CrossRef]
- Mohareb, A.S.; Badawy, M.E.; Abdelgaleil, S.A. Antifungal activity of essential oils isolated from Egyptian plants against wood decay fungi. J. Wood Sci. 2013, 59, 499–505. [Google Scholar] [CrossRef]
- Anda, R.R.; Koch, G.; Richter, H.-G.; Talavera, F.J.F.; Guzmán, J.A.S.; Satyanarayana, K.G. Formation of heartwood, chemical composition of extractives and natural durability of plantation-grown teak wood from Mexico. Holzforschung 2019, 73, 547–557. [Google Scholar] [CrossRef]
- Varshney, V.K.; Pandey, A.; Onial, P.K.; Dayal, R. Antifungal activity of phytochemicals from Eucalyptus hybrid leaves against some plant pathogenic and wood decay fungi. Arch. Phytopathol. Plant Prot. 2012, 45, 2347–2354. [Google Scholar] [CrossRef]
- Salem, M.Z.; Zidan, Y.E.; El Hadidi, N.M.; Mansour, M.M.; Elgat, W.A.A. Evaluation of usage three natural extracts applied to three commercial wood species against five common molds. Int. Biodeterior. Biodegrad. 2016, 110, 206–226. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ricci, A.; Serio, A.; Chaves-López, C.; Mazzarrino, G.; D’Amato, S.; Lo Sterzo, C.; Paparella, A. Characterization of essential oils obtained from Abruzzo autochthonous plants: Antioxidant and antimicrobial activities assessment for food application. Foods 2018, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.; Salem, M.Z. Evaluation of wood treated with some natural extracts and Paraloid B-72 against the fungus Trichoderma harzianum: Wood elemental composition, in-vitro and application evidence. Int. Biodeterior. Biodegrad. 2015, 100, 62–69. [Google Scholar] [CrossRef]
- Reidel, R.V.B.; Cioni, P.L.; Pistelli, L. Volatiles from different plant parts of Punica granatum grown in Tuscany (Italy). Sci. Hortic. 2018, 231, 49–55. [Google Scholar] [CrossRef]
- Ominyi, M.C.; Ogbonna, J.C.; Nwoba, E.G.; Nwagu, K.E.; Ukachi, R. Isolation and screening of α-amylase and glucoamylase producing fungi and their application in bioethanol production. Int. J. Sci. Nat. 2013, 4, 44–50. [Google Scholar]
- Ram, L.; Kaur, K.; Sharma, S. Screening isolation and characterization of cellulase producing microorganisms from soil. Int. J. Pharm. Sci. Invent. 2014, 3, 12–18. [Google Scholar]
- Marathe, S.K.; Vashistht, M.A.; Prashanth, A.; Parveen, N.; Chakraborty, S.; Nair, S.S. Isolation, partial purification, biochemical characterization and detergent compatibility of alkaline protease produced by Bacillus subtilis, Alcaligenes faecalis and Pseudomonas aeruginosa obtained from sea water samples. J. Genet. Eng. Biotechnol. 2018, 16, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Humar, H.; Pohleven, F. Influence of a nitrogen supplement on the growth of wood decay fungi and decay of wood. Int. Biodeterior. Biodegradation 2005, 56, 34–39. [Google Scholar] [CrossRef]
- Balzano, V.; Cavaliere, E.; Fanetti, M.; Gardonio, S.; Gavioli, L. The role of substrate on thermal evolution of Ag/TiO2 nanogranular thin films. Nanomaterials 2021, 11, 2253. [Google Scholar] [CrossRef] [PubMed]
- Colao, F.; Fantoni, R.; Fiorani, L.; Palucci, A.; Gomoiu, I. Compact scanning lidar fluorosensor for investigations of biodegradation on ancient painted surfaces. J. Optoelectron. Adv. Mater. 2005, 7, 3197–3208. [Google Scholar]
- Fazio, A.T.; Papinutti, L.; Gómez, B.A.; Parera, S.D.; Romero, A.R.; Siracusano, G.; Maier, M.S. Fungal deterioration of a Jesuit South American polychrome wood sculpture. Int. Biodeterior. Biodegrad. 2010, 64, 694–701. [Google Scholar] [CrossRef]
- Ahmad, A.; Elserogy, A.; Al-Muheisen, Z.; Villeneuve, F.; El-Oqlah, A. The conservation of a wooden nabataean coffin box from Jordan-application of non-destructive ultrasonic technique. Wood Res. 2018, 63, 1–14. [Google Scholar]
- Abdallah, M.; Kamal, H.M.; Abdrabou, A. Investigation, preservation and restoration processes of an ancient Egyptian wooden offering table. Int. J. Conserv. Sci. 2016, 7, 1047–1064. [Google Scholar]
- Helmi, F.M.; Ali, N.M.; Ismael, S.M. Nanomaterials for the inhibition of microbial growth on ancient Egyptian funeral masks. Mediterr. Archaeol. Archaeom. 2015, 15, 87–95. [Google Scholar] [CrossRef]
- Osman, M.E.; El-Shaphy, A.A.; Meligy, D.A.; Ayid, M.M. Survey for fungal decaying archaeological wood and their enzymatic activity. Int. J. Conserv. Sci. 2014, 5, 295–308. [Google Scholar]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Mellon, J.E.; Cotty, P.J.; Dowd, M.K. Aspergillus flavus hydrolases: Their roles in pathogenesis and substrate utilization. Appl. Microbiol. Biotechnol. 2007, 77, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.M.; Giudicessi, S.L.; Vitale, R.G. Is the fungus Aspergillus a threat to cultural heritage? J. Cult. Herit. 2021, 51, 107–124. [Google Scholar] [CrossRef]
- Cappitelli, F.; Vicini, S.; Piaggio, P.; Abbruscato, P.; Princi, E.; Casadevall, A.; Nosanchuk, J.D.; Zanardini, E. Investigation of fungal deterioration of synthetic paint binders using vibrational spectroscopic techniques. Macromol. Biosci. 2005, 5, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Sumthong, P.; Damveld, R.A.; Choi, Y.H.; Arentshorst, M.; Ram, A.F.; Van Den Hondel, C.A.; Verpoorte, R. Activity of quinones from teak (Tectona grandis) on fungal cell wall stress. Planta Medica 2006, 72, 943–944. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.; Leithoff, H.; Meier, D.; Puls, J.; Richter, H.G.; Faix, O. Heartwood extractives and natural durability of plantation-grown teakwood (Tectona grandis L.). A case study. Holz RohWerkst 2003, 61, 473–474. [Google Scholar] [CrossRef]
- Chang Su, Y.; Ho, C.L.; Wang, I.C.; Chang, S.T. Antifungal activities and chemical compositions of essential oils from leaves of four eucalypts. Taiwan J. Sci. 2006, 21, 49–61. [Google Scholar]
- El Bergadi, F.; Laachari, F.; Sadiki, M.; Megzari, A.; El-Abed, S.; Iraqui, H.M.; Ibnsouda, K.S. Antifungal effect of Moroccan Lawsonia inermis leaf extracts on the growth of filamentous fungi isolated from historical wood. Int. J. Curr. Res. 2014, 7, 14237–14240. [Google Scholar]
- Radaelli, M.; Silva, B.P.D.; Weidlich, L.; Hoehne, L.; Flach, A.; Costa, L.A.M.A.D.; Ethur, E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef]
- Amany, A.E.; Geweely, N.S. Comparative studies on the biochemistry of Penicillium albicans (alkalosensitive) and Verticillium lateritium, (facultative alkalophile) with special reference to the role of sodium ion in alkalophily. Eur. J. Soil Biol. 1999, 35, 1–7. [Google Scholar] [CrossRef]
- Baghdady, K.Z.; Tolba, S.T.; Houssien, S.S. Biogenic deterioration of Egyptian limestone monuments: Treatment and conservation. J. Cult. Herit. 2019, 38, 118–125. [Google Scholar] [CrossRef]
- Noshyutta, W.; Osman, E.; Mansour, M. An investigation of the biological fungicidal activity of some essential used as preservatives for a 19th century Egyptian Coptic cellulosic manuscript. Int. J. Conserv. Sci. 2016, 7, 41–56. [Google Scholar]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.; Poucet, T.; Li, Y. The potential of plant proteins as antifungal agents for agricultural applications. Synth. Syst. Biotechnol. 2022, 7, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Tahlan, V. Antimicrobial Activity of Essential Oil Emulsions and Possible Synergistic Effect on Food Borne Pathogens. Master’s Thesis, Wayne State University, Detroit, MI, USA, 2014. Available online: http://digitalcommons.wayne.edu/oa_theses (accessed on 10 February 2023).
- Wang, H.; Yang, Z.; Ying, G.; Yang, M.; Nian, Y.; Wei, F.; Kong, W. Antifungal evaluation of plant essential oils and their major components against toxigenic fungi. Ind. Crops Prod. 2018, 120, 180–186. [Google Scholar] [CrossRef]
- Axinte, L.; Cuzman, O.A.; Feci, E.; Palanti, S.; Tiano, P. Cinnamaldehyde, a potential active agent for the conservation of wood and stone religious artefacts. Eur. J. Sci. Theol. 2011, 7, 25–34. [Google Scholar]
- Chittenden, C.; Singh, T. Antifungal activity of essential oils against wood degrading fungi and their applications as wood preservatives. Int. Wood Prod. J. 2011, 2, 44–48. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, P.F.; Chang, S.T. Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresour. Technol. 2005, 96, 813–818. [Google Scholar] [CrossRef]
- Li, C.C.; Yu, H.F.; Chang, C.H.; Liu, Y.T.; Yao, H.T. Effects of lemongrass oil and citral on hepatic drug-metabolizing enzymes, oxidative stress, and acetaminophen toxicity in rats. J. Food Drug Anal. 2018, 26, 432–438. [Google Scholar] [CrossRef]
- Ishkeh, S.R.; Asghari, M.; Shirzad, H.; Alirezalu, A.; Ghasemi, G. Lemon verbena (Lippia citrodora) essential oil effects on antioxidant capacity and phytochemical content of raspberry (Rubus ulmifolius subsp. sanctus). Sci. Hortic. 2019, 248, 297–304. [Google Scholar] [CrossRef]
- Felšöciová, S.; Vukovic, N.; Jeżowski, P.; Kačániová, M. Antifungal activity of selected volatile essential oils against Penicillium sp. Open Life Sci. 2020, 15, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Pánek, M.; Reinprecht, L.; Hulla, M. Ten essential oils for beech wood protection—Efficacy against wood-destroying fungi and moulds, and effect on wood discoloration. BioResources 2014, 9, 5588–5603. [Google Scholar] [CrossRef]
- Schroder, T.; Gaskin, S.; Ross, K.; Whiley, H. Antifungal activity of essential oils against fungi isolated from air. Int. J. Occup. Environ. Health 2017, 23, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Stupar, M.; Grbić, M.L.; Džamić, A.; Unković, N.; Ristić, M.; Jelikić, A.; Vukojević, J. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. S. Afr. J. Bot. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- Farooq, A.; Choudhary, I.; Tahara, S.; Başer, K.H.C.; Demirci, F. Detoxification of terpinolene by plant pathogenic fungus Botrytis cinerea. Z. Für Naturforschung C 2002, 57, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.A.; Ismail, K.M.I.; Amany, H.A.; El-Lill, A.; Geweely, N.S. Biochemical studies of purified extracellular xylanases from Aspergillus versicolor. Int. J. Bot. 2008, 4, 41–48. [Google Scholar] [CrossRef]
- Geweely, N.S. Purification and characterization of intracellular urease enzyme isolated from Rhizopus oryzae. Biotechnology 2006, 5, 358–364. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ceylan, O.; Targan, S.; Zeljković, S.Ć. Chemical composition and biological activities of the essential oils of two endemic Nepeta species. Ind. Crops Prod. 2018, 125, 5–8. [Google Scholar] [CrossRef]
- Xu, F.; Gu, D.; Wang, M.; Zhu, L.; Chu, T.; Cui, Y.; Tian, J.; Wang, Y.; Yang, Y. Screening of the potential α-amylase inhibitor in essential oil from Cedrus deodara cones. Ind. Crops Prod. 2017, 103, 251–256. [Google Scholar] [CrossRef]
- Abolellil, A.; Geweely, N.S. An enrichment of xylanolytic organism with high pH optima. Biotechnology 2005, 4, 49–55. [Google Scholar] [CrossRef]
- Geweely, N.S.; Nawar, L.S. Production, optimization, purification and properties of uricase isolated from some fungal flora in Saudi Arabian soil. Aust. J. Basic Appl. Sci. 2011, 5, 220–230. [Google Scholar]
- Yang, J.; Gu, D.; Wang, M.; Kou, D.; Guo, H.; Tian, J.; Yang, Y. In silico-assisted identification of α-amylase inhibitor from the needle oil of Pinus tabulaeformis Carr. Ind. Crops Prod. 2019, 111, 360–363. [Google Scholar] [CrossRef]
- Ekinci, M.S.; Dalfesoğlu, İ.A.K.; Ozkose, E. Effects of essential oils supplementation on survival rate and lignocellulolytic enzyme activities of rumen fungi isolated from cattle. KSU J. Nat. Sci. 2017, 20, 235–241. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Chavez-Lopez, C.; Viuda-Martos, M.; Serio, A.; Delgado-Ospina, A.; Perez-Alvarez, J.A.; Ospina, N.; la Tora, S.; Palmieri, S.; Paparella, A. Sub-lethal concentrations of Colombian Austroeupatorium inulifolium (HBK) essential oil and its effect on fungal growth and the production of enzymes. Ind. Crops Prod. 2016, 87, 315–323. [Google Scholar] [CrossRef]
- Wilson, C.L.; Solar, J.M.; El Ghaouth, A.; Wisniewski, M.E. Rapid evaluation of plant extracts and essential oils for antifungal activity against Botrytis cinerea. Plant Dis. 1997, 81, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.C.; Young, G.D.; Oberg, C.J. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. J. Essent. Oil Res. 2000, 12, 639–649. [Google Scholar] [CrossRef]
- Voda, K.; Boh, B.; Vrtačnik, M.; Pohleven, F. Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot Trametes versicolor and the brown-rot Coniophora puteana. Int. Biodeterior. Biodegrad. 2003, 51, 51–59. [Google Scholar] [CrossRef]
- Sparacello, S.; Gallo, G.; Faddetta, T.; Megna, B.; Nicotra, G.; Bruno, B.; Palla, F. Thymus vulgaris essential oil and hydro-alcoholic solutions to counteract wooden artwork microbial colonization. Appl. Sci. 2021, 11, 8704. [Google Scholar] [CrossRef]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential oils as natural biocides in conservation of cultural heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef]
- Palla, F.; Caruana, E.; Di Carlo, E.; Rotolo, V. Plant essential oils in controlling fungal colonization on wooden substrate. Borziana 2021, 2, 5–14. [Google Scholar] [CrossRef]
- El-Shahawy, A.; Elsawi, N.M.; Baker, W.; Khorshid, F.; Geweely, N.S. Spectral analysis, molecular orbital calculations and antimicrobial activity of PMF-G fraction extracted from PM-701. Int. J. Pharma Bio Sci. 2010, 1, 1–19. [Google Scholar]
- Yan, J.; Niu, Y.; Wu, C.; Shi, Z.; Zhao, P.; Naik, N.; Yuan, B. Antifungal effect of seven essential oils on bamboo. Adv. Compos. Hybrid Mater. 2021, 4, 552–561. [Google Scholar] [CrossRef]
- Geweely, N.S. Non-toxic fumigation and alternative control techniques against fungal colonization for preserving archaeological oil painting. Int. J. Bot. 2006, 2, 353–362. [Google Scholar] [CrossRef]
- Nuopponen, M.; Vuorinen, T.; Viitaniemi, P.; Jamsa, S. Effects of heat treatment on the behaviour of extractives in softwood studied by FTIR spectroscopic methods. Wood Sci. Technol. 2003, 37, 109–115. [Google Scholar] [CrossRef]
- Aluong, E.M.A.; Redington, M. Fourier transform infrared analyses of Bog and Modern Oak wood (Quercus petraea) extractives. Wood Sci. Technol. 2004, 38, 181–190. [Google Scholar] [CrossRef]
- Nuopponen, M. FTIR and UV Raman Spectroscopic Studies on Thermal Modification of Scots Pine Wood and Its Extractable Compounds. Ph.D. Thesis, Helsinki University of Technology, Helsinki, Finland, 2005; pp. 1–29. [Google Scholar]
- Geweely, N.S. New frontiers review on some recent conservation techniques of organic and inorganic archaeological artefacts against microbial deterioration. Front. Microbiol. 2023, 14, 1146582. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Elgat, W.A.; El-Hefny, M.; Salem, M.Z.; Taha, A.S.; Al Farraj, D.A.; Abdel-Salam, E.M. New approach for using of Mentha longifolia L. and Citrus reticulata L. essential oils as wood-biofungicides: GC-MS, SEM, and MNDO quantum chemical studies. Materials 2021, 14, 1361. [Google Scholar] [CrossRef]
- Martinez-Pacheco, M.M.; Reynoso, W.E.; León, J.C.; González, D.R.; García, A.F.; Rojas, A.M.; Becerra, C.V. Pinewood protection against sapstain using citrus essential oils. Revista Árvore 2022, 46, 1–12. [Google Scholar] [CrossRef]

| Object No. | Object Name | Period/Dynasty | Dimensions | Provenance | ID |
|---|---|---|---|---|---|
| 1 | Wooden statue of a seated man | Old Kingdom (2686–2181 BC) | Length 127 cm Width (shoulders) 47 cm Base (length) 58 cm | Saqqara excavations site (Memphite region) | SR2/15969 |
| 2 | Anthropoid wooden coffin with cartonnage mummy of Nespahettawi, son of Neskhonsupahred (male) | New Kingdom (1550–1069 BC) | Length 179 cm Width (shoulders) 55 cm Width (foot) 32 cm | Deir el-Bahari, (Upper Egypt, Luxor) | SR3/643 |
| 3 | Wooden box of Padimen’s son | New Kingdom (1550–1069 BC) | Length 43.5 cm Width 39 cm Depth 37.5 cm | Deir el-Bahari (Upper Egypt, Luxor) | CG5028 |
| 4 | Inner coffin of Khonsuemrenep (lid) | 3rd intermediate period Dynasty 21 | Length 195 cm Width 42 cm Depth 32 cm | Deir el-Bahari (Upper Egypt, Luxor) | SR7/23476(b).2 |
| 5 | Upper part of a wooden statue of a woman (Wife of Kaaper) | Old Kingdom (2686–2181 BC) | Length 61 cm Width 25 cm Depth cm | Saqqara excavations site (Memphite region) | SR 2/14958 |
| 6 | Wooden block statue of Kanefer with figure of Ptah | New Kingdom (1550–1069 BC) | Length 18.7 cm Width 13 cm Depth 8.5 cm | Saqqara excavations site (Memphite region) | SR 5/13750 |
| 7 | Wooden Shabti box | New Kingdom (1550–1069 BC) | Height 27 cm Depth 40 cm | Deir el-Bahari (Upper Egypt, Luxor) | SR 4/9099 |
| 8 | Wooden statue of an Ibis perching | Late period Dynasty 26 | Length 32.5 cm Width 12 cm Depth 6.5 cm | Tuna El-Gabal Al Minya Governorate Middle Egypt | SR 3/6135 |
| 9 | Wooden box engraved with lions, gazelles, and calves | New Kingdom (1550–1069 BC) | Height 16 cm Diameter 8 cm | Fayoum Region Sidmant al Jabal | SR 3/1885.1 |
| 10 | Painted wooden anthropoid coffin of Taa (lid) | Late period Dynasty 26 | Length 170 cm Width 48 cm Depth 30 cm | Deir el-Bahari (Upper Egypt, Luxor) | SR 4/11307 |
| No. | Common Name | Latin Name | Family | Used Part |
|---|---|---|---|---|
| 1 | Cinnamon | Cinnamomum cassia | Lauraceae | Bark |
| 2 | Eucalyptus | Eucalyptus globulus | Myrtaceae | Leaves |
| 3 | Frankincense | Boswellia carterii | Burseraceae | Seeds |
| 4 | Geranium | Pelargonium graveolens | Geraniaceae | Leaves |
| 5 | Lavender | Lavandula latifolia | Lamiaceae | Leaves |
| 6 | Lemongrass | Cymbopogon citratus | Poaceae | Leaves |
| 7 | Menthe | Mentha piperita | Lamiaceae | Leaves |
| 8 | Rosemary | Rosmarinus officinalis | Lamiaceae | Leaves |
| 9 | Tea tree | Melaleuca alternifolia | Myrtaceae | Leaves |
| 10 | Thyme | Thymus vulgaris | Lamiaceae | Leaves |
| No. | Common Name | Latin Name | Family | Used Part |
|---|---|---|---|---|
| 1 | Basil | Ocimum basilicum | Lamiaceae | Leaves |
| 2 | Eucalyptus | Eucalyptus camaldulensis | Myrtaceae | Leaves |
| 3 | Henna | Lawsonia inermis | Lythraceae | Leaves |
| 4 | Melia | Melia azedarach | Meliaceae | Leaves |
| 5 | Teak | Tectona grandis | Verbenaceae | Leaves |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geweely, N.S.; Abu Taleb, A.M.; Grenni, P.; Caneva, G.; Atwa, D.M.; Plaisier, J.R.; Ibrahim, S. Eco-Friendly Preservation of Pharaonic Wooden Artifacts using Natural Green Products. Appl. Sci. 2024, 14, 5023. https://doi.org/10.3390/app14125023
Geweely NS, Abu Taleb AM, Grenni P, Caneva G, Atwa DM, Plaisier JR, Ibrahim S. Eco-Friendly Preservation of Pharaonic Wooden Artifacts using Natural Green Products. Applied Sciences. 2024; 14(12):5023. https://doi.org/10.3390/app14125023
Chicago/Turabian StyleGeweely, Neveen S., Amira M. Abu Taleb, Paola Grenni, Giulia Caneva, Dina M. Atwa, Jasper R. Plaisier, and Shimaa Ibrahim. 2024. "Eco-Friendly Preservation of Pharaonic Wooden Artifacts using Natural Green Products" Applied Sciences 14, no. 12: 5023. https://doi.org/10.3390/app14125023






