Using an Uptake Enhancer to Mitigate Nitrogen Leaching While Enhancing Uptake Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Pre-Planting Soil Sampling and Analysis
2.3. Treatment
2.4. Experimental Procedure
2.5. Sampling and Analyses
2.5.1. Sampling and Analyses of Soil
2.5.2. Sampling and Analyses of Plant Samples
2.6. Statistical Analysis of Data
2.7. Ethical Approval
3. Results
3.1. Effect of Uptake Enhancer Application on N Distribution
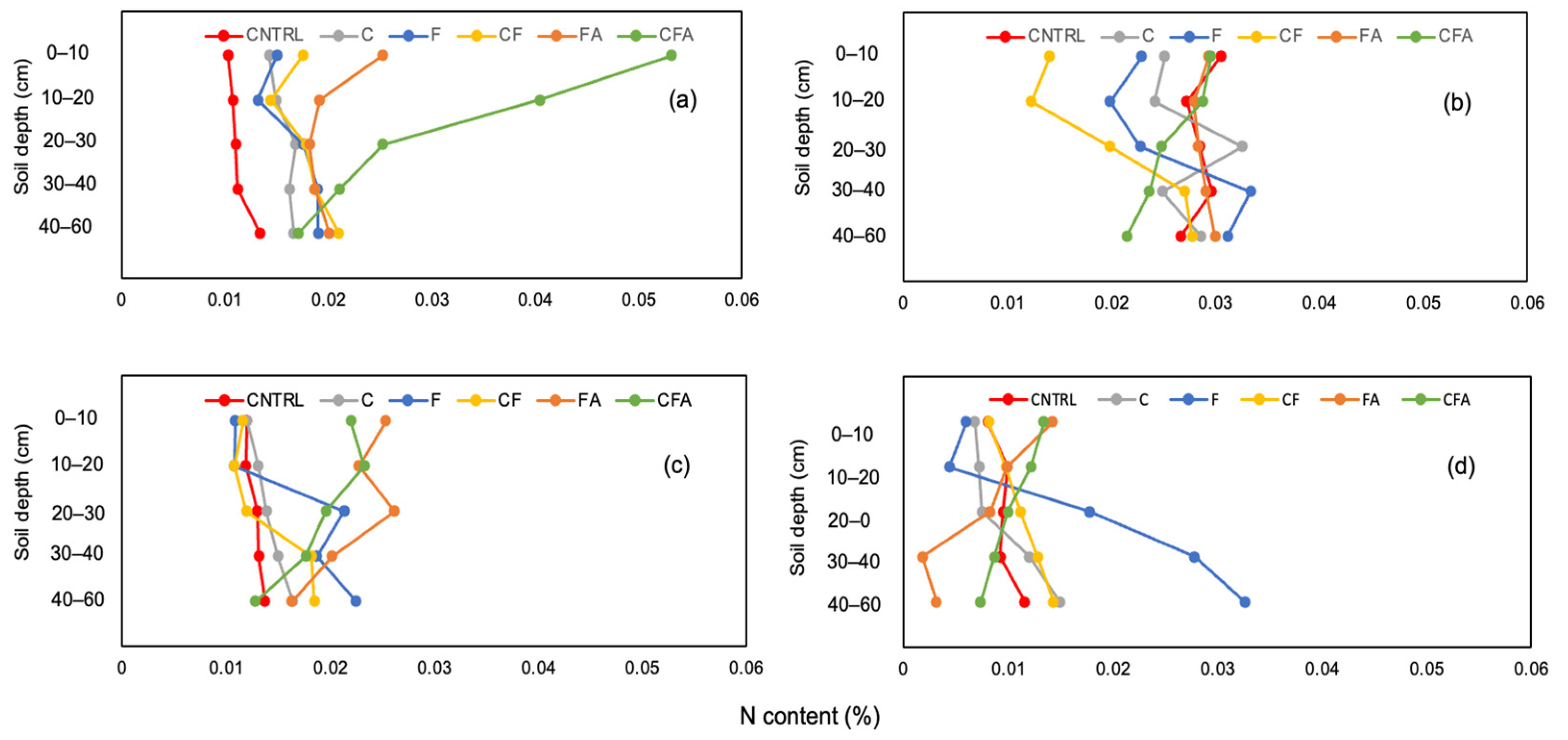
3.1.1. Effect of Uptake Enhancer Application on N Distribution through a Soil Profile
3.1.2. Effect of Uptake Enhancer Application on N Distribution Patterns
3.2. Effect of Uptake Enhancer Application on Vegetative Growth of Oats
3.2.1. Morphological Parameters
Plant Height
Above-Ground FM and DM
Root Measurements
Leaf Area
3.2.2. Physiological Parameters and Yield Component Measurements
Number of Seeds per Plant
Total Yield
Plant Analysis for N
4. Discussion
4.1. Effect of Uptake Enhancer on N Distribution through a Soil Profile
4.2. Effect of Uptake Enhancer Application on Vegetative Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, B.L.; Zheng, Z.; Pageau, D.; Vera, C.; Fregeau-Reid, J.; Xue, A.; Yan, W. Nitrogen and phosphorus uptake, yield and agronomic traits of oat cultivars as affected by fertilizer N rates under diverse environments. Nutr. Cycl. Agroecosyst. 2017, 108, 245–265. [Google Scholar] [CrossRef]
- Anas, S.M.; Alam, M.; Umair, M. Performance of one-way composite reinforced concrete slabs under explosive-induced blast loading. IOP Conf. Ser. Earth Environ Sci. 2020, 614, 012094. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 2011. [Google Scholar]
- Graham, P.H.; Vance, C.P. Nitrogen fixation in perspective: An overview of research and extension needs. Field Crop Res. 2000, 65, 93–106. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil 2004, 274, 1–36. [Google Scholar] [CrossRef]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2005. [Google Scholar]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.A.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2019, 105, 1141–1157. [Google Scholar] [CrossRef]
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Delhaize, E.; Frommer, W.B.; Guerinot, M.L.; Harrison, M.J.; Herrera-Estrella, L.; Horie, T.; Kochian, L.V.; Munns, R.; Nishizawa, N.K.; et al. Using membrane transporters to improve crops for sustainable food production. Nature 2013, 497, 60–66. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Glass, A.D.M.; Siddiqi, M.Y.; Kirk, G.J.D. Comparative kinetic analysis of ammonium and nitrate acquisition by tropical lowland rice: Implications for rice cultivation and yield potential. New Phytol. 2000, 145, 471–476. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. How plant root exudates shape the nitrogen cycle. Trends. Plant Sci. 2017, 22, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, J. The nitrogen cascade. Biosci. Biotechnol. Biochem. 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Erisman, J.W.; Bleeker, A.; Galloway, J.; Sutton, M.S. Reduced nitrogen in ecology and the environment. Environ. Pollut. 2007, 150, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A.; Bleeker, A.; Howard, C.M.; Erisman, J.W.; Abrol, Y.P.; Bekunda, M.; Datta, A.; Davidson, E.; De Vries, W.; Oenema, O.; et al. Our Nutrient World: The Challenge to Produce more Food and Energy with Less Pollution; Centre for Ecology and Hydrology: Edinburgh, UK, 2013. [Google Scholar]
- Ostendorf, M. What Is Going on with Fertilizer Prices? 2021. Available online: https://www.agriculture.com/news/crops/skyrocketing-fertilizer-market-has-farmers-analysts-and-companies-weighing-in (accessed on 4 September 2022).
- Kousar, P.; Ali, L.; Raza, A.; Maqbool, A.; Maqbool, S.; Rasheed, S.; Irum, N. Effect of different levels of nitrogen on the economic yield of wheat (Triticum aestivum L.) variety Aas-11. Int. J. Agron. Agric. Res. 2015, 6, 7–11. [Google Scholar]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Hazen, J.L. Adjuvants—Terminology, classification, and chemistry. Weed Technol. 2000, 14, 773–784. [Google Scholar] [CrossRef]
- Van Huyssteen, C.W. Relating the South African Soil Taxonomy to the World Reference Base for Soil Resources; Sun Media Publishers: Bloemfontein, South Africa, 2020. [Google Scholar]
- FSSA Fertilizer Handbook, 6th ed.; FSSA: Pretoria, South Africa, 2007.
- Meier, U. Growth Stages of Mono and Dicotyledonous Plants; Blackwell: Oxford, UK, 1997. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 961–1010. [Google Scholar]
- Bohm, W. Methods of Studying Root Systems; Springer: Berlin, Germany, 1979; pp. 95–138. [Google Scholar]
- Liu, Y.J.; Tong, Y.P.; Zhu, Y.G.; Ding, H.; Smith, F.A. Leaf chlorophyll readings as an indicator for spinach yield and nutritional quality with different nitrogen fertilizer applications. J. Plant Nutr. 2004, 29, 1207–1217. [Google Scholar] [CrossRef]
- Fixen, D.J.; Grove, J.H. Nitrate-nitrogen distribution in soil profiles under various cropping systems. Soil Sci. Soc. Am. J. 1990, 54, 1139–1144. [Google Scholar]
- Baratella, V.; Renzaglia, M.; Trinchera, A. Effect of surfactant as adjuvant for irrigation/fertigation in vegetables production: Preliminary results on lettuce. Acta Hortic. 2014, 1123, 157–164. [Google Scholar] [CrossRef]
- Smith, J.A.; Weber, J.B.; Reed, J.D.; Blum, U. Characterization of the effects of adjuvant and herbicide formulation on herbicide absorption and translocation. Weed Sci. 2007, 55, 59–68. [Google Scholar]
- Pinton, R.; Varanini, Z.; Nannipieri, P. The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Midha, L.K.; Duhan, B.S.; Arya, S. Performance of promising entries of oat (Avena sativa L.) under different nitrogen levels. Forage Res. 2015, 41, 122–125. [Google Scholar]
- Singh, R.; Singh, S.; Singh, R. Chlorophyll content and leaf area of oats as influenced by nitrogen and phosphorus fertilization. J. Indian Soc. Soil Sci. 2002, 50, 503–505. [Google Scholar]
- Wei, W.; Liu, S.; Cui, D.; Ding, X. Interaction between nitrogen fertilizer and biochar fertilization on crop yield and soil chemical quality in a temperate region. J. Agric. Sci. 2015, 159, 106–115. [Google Scholar] [CrossRef]
- Gu, X.; Yang, X.; Jiang, L.; Huang, S.; Zhou, H.; Zhu, J.; Chen, Y.; Li, Y.; Liu, Y. Nitrogen Foliage Application at Anthesis Improves Grain Yield and Quality of Wheat in a Genotype-Dependent Manner. Agronomy 2023, 13, 3077. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Singh, R.; Singh, P. Role of adjuvants in crop production: A review. J. Pharmacogn. Phytochem. 2020, 9, 1–8. [Google Scholar]
| Treatment | Crop or No Crop | Fertilizer [ANO 21% (L ha−1)] | Uptake Enhancer (1 mL L−1 Fertilizer) |
|---|---|---|---|
| CNTRL | No crop | ||
| C | Crop | ||
| F | No crop | 50 | |
| CF | Crop | 50 | |
| FA | No crop | 50 | 1 |
| CFA | Crop | 50 | 1 |
| Sampling Time 1 (4 Weeks) | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | |||||||
| Depth (cm) | CNTRL | C | F | CF | FA | CFA | p-Value |
| 0–10 | 0.010 a (±0.0012) | 0.014 a (±0.0014) | 0.015 a (±0.0035) | 0.018 ab (±0.0034) | 0.025 b (±0.0046) | 0.053 c (±0.0051) | <0.001 |
| 10–20 | 0.011 a (±0.0009) | 0.015 a (±0.0027) | 0.013 a (±0.0030) | 0.014 a (±0.0025) | 0.019 a (±0.0061) | 0.040 b (±0.0056) | <0.001 |
| 20–30 | 0.011 a (±0.0014) | 0.017 ab (±0.0035) | 0.018 ab (±0.0012) | 0.018 ab (±0.0034) | 0.018 ab (±0.0083) | 0.025 b (±0.0018) | 0.005 |
| 30–40 | 0.011 a (±0.0086) | 0.016 a (±0.0025) | 0.019 b (±0.0019) | 0.019 b (±0.0015) | 0.019 b (±0.0036) | 0.021 b (±0.0041) | 0.001 |
| 40–50 | 0.013 a (±0.0015) | 0.017 ab (±0.0031) | 0.019 ab (±0.0031) | 0.02 b (±0.0031) | 0.020 ab (±0.0044) | 0.017 ab (±0.0035) | 0.042 |
| Sampling Time 2 (8 Weeks) | |||||||
| Treatments | |||||||
| Depth (cm) | CNTRL | C | F | CF | FA | CFA | p-Value |
| 0–10 | 0.031 b (±0.0037) | 0.025 b (±0.0036) | 0.023 b (±0.0024) | 0.014 a (±0.0021) | 0.029 b (±0.0043) | 0.030 b (±0.0014) | <0.001 |
| 10–20 | 0.027 c (±0.0049) | 0.024 bc (±0.0040) | 0.020 b (±0.0013) | 0.012 a (±0.0018) | 0.028 c (±0.0032) | 0.029 c (±0.0019) | <0.001 |
| 20–30 | 0.029 ab (±0.0056) | 0.033 b (±0.0042) | 0.023 ab (±0.0061) | 0.020 a (±0.0026) | 0.028 ab (±0.0045) | 0.025 ab (±0.0017) | 0.009 |
| 30–40 | 0.030 a (±0.0055) | 0.025 a (±0.0054) | 0.033 a (±0.0067) | 0.027 a (±0.0033) | 0.029 a (±0.0059) | 0.024 a (±0.0012) | 0.132 |
| 40–50 | 0.027 a (±0.0084) | 0.029 a (±0.0025) | 0.031 a (±0.0052) | 0.028 a (±0.0038) | 0.030 a (±0.0043) | 0.022 a (±0.0013) | 0.129 |
| Sampling Time 3 (12 Weeks) | |||||||
| Treatments | |||||||
| Depth (cm) | CNTRL | C | F | CF | FA | CFA | p-Value |
| 0–10 | 0.012 a (±0.0005) | 0.012 a (±0.0006) | 0.011 a (±0.0005) | 0.012 a (±0.0010) | 0.025 b (±0.0039) | 0.022 b (±0.0011) | <0.001 |
| 10–20 | 0.012 a (±0.0007) | 0.013 a (±0.0007) | 0.011 a (±0.0005) | 0.011 a (±0.0015) | 0.023 b (±0.0054) | 0.023 b (±0.0012) | <0.001 |
| 20–30 | 0.013 a (±0.0013) | 0.014 a (±0.0004) | 0.021 ab (±0.0013) | 0.012 a (±0.0009) | 0.026 b (±0.0110) | 0.020 ab (±0.0013) | 0.002 |
| 30–40 | 0.013 a (±0.0017) | 0.015 ab (±0.0010) | 0.019 ab (±0.0044) | 0.018 ab (±0.0013) | 0.020 b (±0.0037) | 0.018 ab (±0.0023) | 0.018 |
| 40–50 | 0.014 a (±0.0031) | 0.016 a (±0.0012) | 0.022 b (±0.0024) | 0.018 ab (±0.0016) | 0.016 a (±0.0044) | 0.013 a (±0.0016) | <0.001 |
| Sampling Time 4 (16 Weeks) | |||||||
| Treatments | |||||||
| Depth (cm) | CNTRL | C | F | CF | FA | CFA | p-Value |
| 0–10 | 0.008 a (±0.0081) | 0.007 a (±0.0018) | 0.006 a (±0.0032) | 0.008 a (±0.0014) | 0.014 b (±0.0025) | 0.013 b (±0.0013) | <0.001 |
| 10–20 | 0.010 bc (±0.0008) | 0.007 ab (±0.0021) | 0.004 a (±0.0009) | 0.010 bc (±0.0020) | 0.010 bc (±0.0015) | 0.012 c (±0.0016) | <0.001 |
| 20–30 | 0.010 a (±0.0053) | 0.007 a (±0.0018) | 0.018 b (±0.0017) | 0.011 a (±0.0006) | 0.008 a (±0.0019) | 0.010 a (±0.0012) | <0.001 |
| 30–40 | 0.009 ab (±0.0063) | 0.012 b (±0.0024) | 0.028 c (±0.0040) | 0.013 b (±0.0014) | 0.002 a (±0.0008) | 0.009 ab (±0.0013) | <0.001 |
| 40–50 | 0.012 ab (±0.0060) | 0.015 b (±00026) | 0.033 c (±0.0072) | 0.014 b (±0.0017) | 0.003 a (±0.0027) | 0.007 ab (±0.0009) | <0.001 |
| Sampling Time | 4 Weeks | 8 Weeks | 12 Weeks | 16 Weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | C | CF | CFA | C | CF | CFA | C | CF | CFA | C | CF | CFA |
| Plant height (cm) | 39.9 a (±5.7) | 38.2 a (±6.6) | 40.1 a (±4.9) | 48.3 a (±19.4) | 54.4 b (±25.9) | 55.4 b (±7.9) | 72.4 a (±11.1) | 75.9 a (±8.4) | 75.3 a (±5.9) | 79.0 a (±7.8) | 80.4 a (±6.8) | 83.0 a (±5.1) |
| Above-ground FM (g) | 0.85 a (±0.52) | 0.58 a (±0.48) | 0.78 a (±0.12) | 19.1 b (±3.6) | 18.1 ab (±3.6) | 17.5 a (±1.45) | 14.2 a (±1.5) | 16.8 a (±4.1) | 18.1 a (±3.5) | 14.5 a (±1.5) | 16.3 a (±1.6) | 14.9 a (±1.5) |
| Above-ground DM (g) | 0.39 a (±0.18) | 0.22 a (±0.17) | 0.27 a (±0.04) | 6.09 b (±1.01) | 5.62 ab (±0.93) | 5.54 a (±0.84) | 5.65 a (±2.15) | 6.94 a (±1.41) | 6.96 a (±1.21) | 5.13 a (±0.35) | 7.19 a (±0.59) | 6.92 a (±1.12) |
| Root volume (ml) | 0.09 b (±0.01) | 0.04 a (±0.01) | 0.08 b (±0.01) | 0.20 a (±0.14) | 0.18 a (±0.09) | 0.25 a (±0.11) | 1.60 a (±0.58) | 1.98 ab (±1.11) | 3.34 b (±1.31) | 2.25 a (±0.58) | 3.05 ab (±1.11) | 4.53 b (±1.31) |
| Root FM (g) | 1.94 a (±0.79) | 1.27 a (±0.72) | 1.81 a (±0.45) | 7.51 a (±2.35) | 7.86 a (±2.09) | 8.09 a (±2.67) | 2.55 a (±0.45) | 5.95 a (±2.25) | 9.94 b (±4.17) | 3.33 a (±0.74) | 4.04 b (±1.13) | 3.45 ab (±0.41) |
| Root DM (g) | 1.07 b (±0.59) | 0.55 a (±0.21) | 1.06 b (±0.19) | 2.85 a (±0.63) | 3.16 a (±0.93) | 3.45 a (±1.47) | 1.12 a (±0.33) | 2.39 ab (±0.86) | 2.72 b (±0.97) | 1.71 ab (±0.36) | 2.07 b (±0.37) | 1.61 a (±0.11) |
| Leaf area (cm2) | 152 a (±87) | 97 a (±14) | 176 a (±91) | 241 a (±80) | 224 a (±60) | 222 a (±27) | 67 a (±11) | 80 a (±26) | 101 a (±22) | 63 a (±14) | 75 a (±21) | 83 a (±14) |
| Sampling Time | 4 Weeks | 8 Weeks | 12 Weeks | 16 Weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | C | CF | CFA | C | CF | CFA | C | CF | CFA | C | CF | CFA |
| Chlorophyll content (CU) | 15.8 a (±8.3) | 18.0 a (±7.2) | 17.6 a (±10.8) | 10.3 a (±6.4) | 16.2 b (±8.8) | 14.1 ab (±7.1) | 3.4 a (±2.8) | 5.1 a (±3.1) | 5.6 a (±2.5) | 1.3 a (±0.6) | 1.4 a (±0.5) | 1.3 a (±0.6) |
| Panicle FM (g) | _ | _ | _ | _ | _ | _ | 3.82 a (±1.04) | 5.66 a (±0.93) | 5.73 a (±0.96) | 5.52 a (±1.44) | 7.32 b (±1.67) | 6.48 ab (±1.85) |
| Panicle DM (g) | _ | _ | _ | _ | _ | _ | 1.34 a (±0.76) | 2.43 a (±0.83) | 2.42 a (±1.24) | 3.01 a (±1.68) | 4.19 b (±1.23) | 4.24 b (±1.78) |
| Nr of seeds per plant | _ | _ | _ | _ | _ | _ | 54 a (±8) | 75 a (±16) | 73 a (±12) | 68 a (±19) | 92 ab (±22) | 100 b (±24) |
| Total yield—seed mass (g) | _ | _ | _ | _ | _ | _ | 1.34 a (±1.23) | 2.54 a (±1.67) | 2.43 a (±1.87) | 3.19 a (±1.33) | 4.43 b (±2.11) | 4.51 b (±1.98) |
| N content in plants (%) | 1.63 a (±0.18) | 1.69 a (±0.13) | 1.95 a (±0.51) | 0.59 a (±0.06) | 0.65 a (±0.12) | 0.68 a (±0.11) | 0.27 a (±0.04) | 0.26 a (±0.06) | 0.33 a (±0.06) | 0.32 a (±0.04) | 0.30 a (±0.03) | 0.33 a (±0.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somi, Z.; Kotzé, E.; Van der Watt, E. Using an Uptake Enhancer to Mitigate Nitrogen Leaching While Enhancing Uptake Efficiency. Appl. Sci. 2024, 14, 5271. https://doi.org/10.3390/app14125271
Somi Z, Kotzé E, Van der Watt E. Using an Uptake Enhancer to Mitigate Nitrogen Leaching While Enhancing Uptake Efficiency. Applied Sciences. 2024; 14(12):5271. https://doi.org/10.3390/app14125271
Chicago/Turabian StyleSomi, Zoyolo, Elmarie Kotzé, and Elmarie Van der Watt. 2024. "Using an Uptake Enhancer to Mitigate Nitrogen Leaching While Enhancing Uptake Efficiency" Applied Sciences 14, no. 12: 5271. https://doi.org/10.3390/app14125271
APA StyleSomi, Z., Kotzé, E., & Van der Watt, E. (2024). Using an Uptake Enhancer to Mitigate Nitrogen Leaching While Enhancing Uptake Efficiency. Applied Sciences, 14(12), 5271. https://doi.org/10.3390/app14125271






