Oxygen Self-Diffusion in Fluorite High Entropy Oxides
Abstract
:1. Introduction
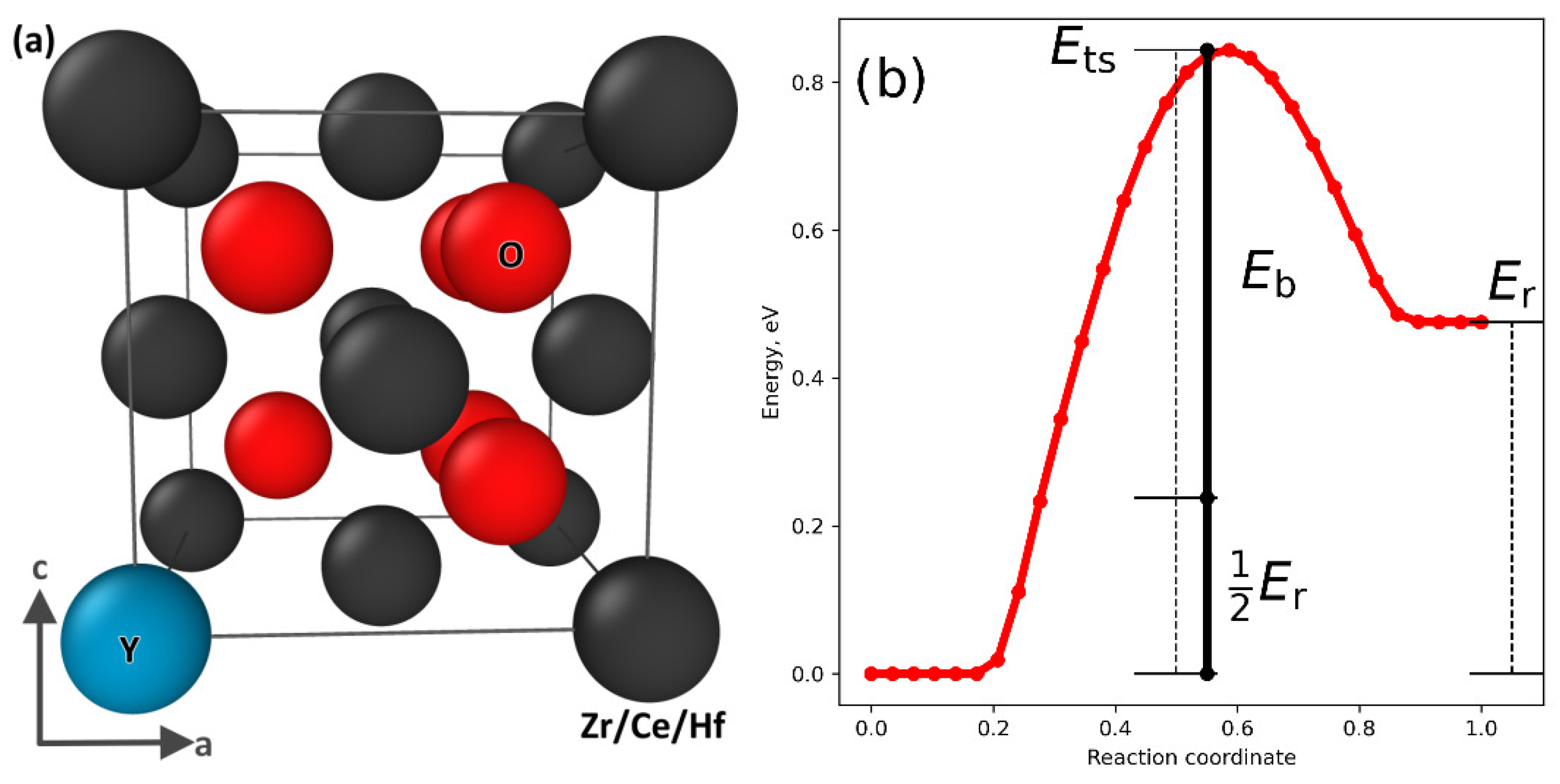
2. Methodology
- An 8 × 8 × 8 supercell of the fluorite structure is created with a random assignment of cations according to a specified stoichiometry. Oxygen ion vacancies are introduced onto the oxygen ion sublattice to provide charge balance.
- An equilibration series of simulations which consist of the following:
- Annealing is at a set temperature of 1500 K to equilibrate the distribution of oxygen vacancies.
- A 2 ns simulation at constant (zero) pressure a set temperature of Tset is used to establish the equilibrium cell parameters at temperature.
- The cell is then set to the average value of the lattice parameters, and a further 50 ps of simulation time is run to allow the vacancy distribution to adapt to the altered cell volume.
- The statistics run is calculated in a constant volume, a constant energy ensemble (i.e., without the thermostat) for a simulation time of 10 ns or the time required for the mean squared displacement of the oxygen ions to reach 10 Å2. The latter condition allows for efficient early termination of compositions with clear values of diffusivity. The simulation time and displacement of the ions R(t) is recorded in this last step and used to calculate the average temperature T (~Tset) and mean squared displacement () of the oxygen ions.
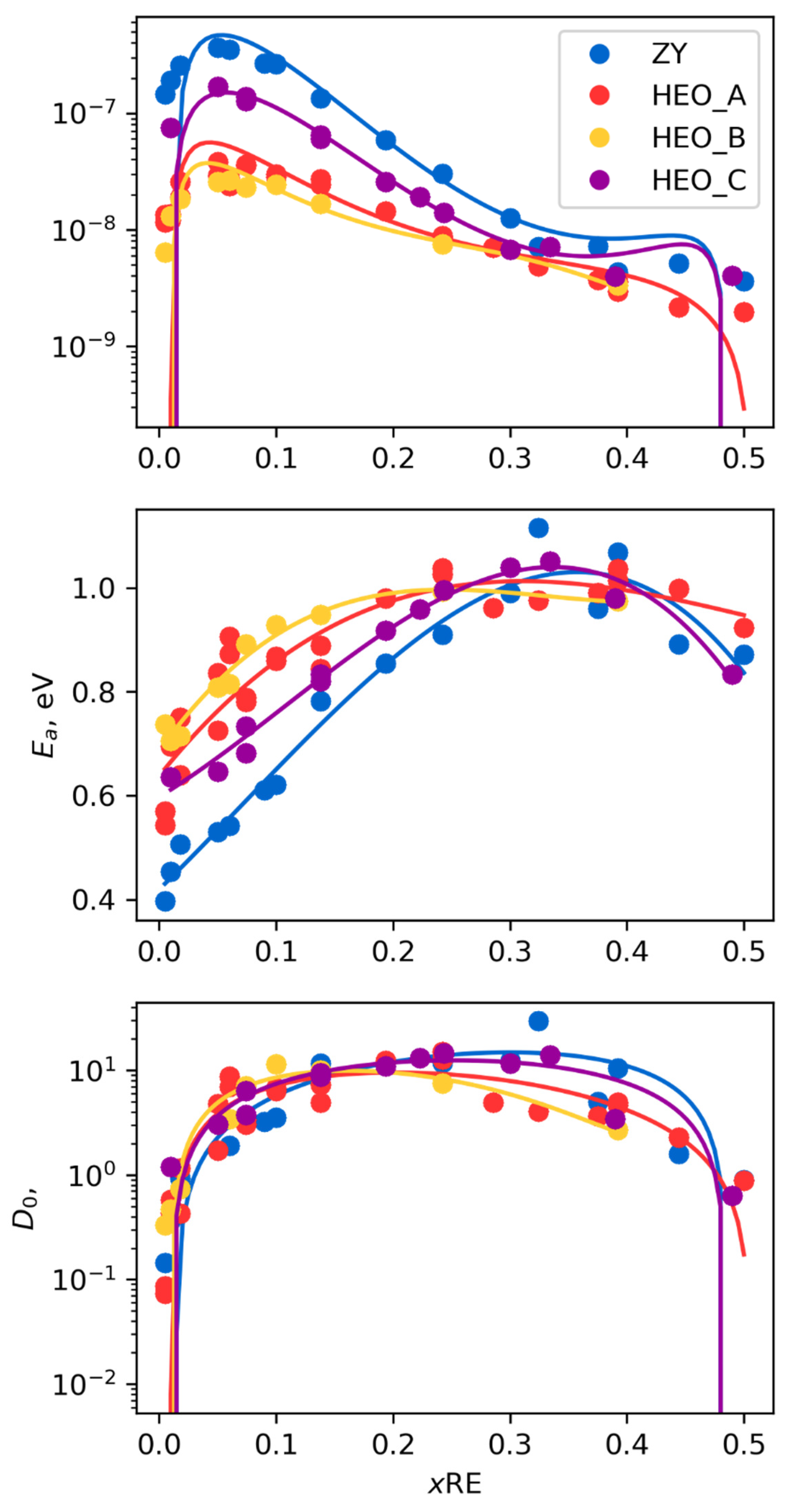
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Tarascon, J.M. Key challenges in future Li-battery research. Philos. Trans. R. Soc. Lond. A 2010, 368, 3227–3241. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Tarancón, A.; Burriel, M.; Santiso, J.; Skinner, S.J.; Kilner, J.A. Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2010, 20, 3799–3813. [Google Scholar] [CrossRef]
- Kushima, A.; Yildiz, B. Oxygen ion diffusivity in strained yttria stabilized zirconia: Where is the fastest strain? J. Mater. Chem. 2010, 20, 4809–4819. [Google Scholar] [CrossRef]
- Souza, R.A.D.; Ramadan, A.; Hörner, S. Modifying the barriers for oxygen-vacancy migration in fluorite-structured CeO2 lectrolytes through strain: A computer simulation study. Energy Environ. Sci. 2012, 5, 5445–5453. [Google Scholar] [CrossRef]
- Rushton, M.J.D.; Chroneos, A. Impact of uniaxial strain and doping on oxygen diffusion in CeO2. Sci. Rep. 2014, 4, 6068. [Google Scholar] [CrossRef]
- Chiabrera, F.; Garbayo, I.; López-Conesa, L.; Martín, G.; Ruiz-Caridad, A.; Walls, M.; Ruiz-González, L.; Kordatos, A.; Núñez, M.; Morata, A.; et al. Engineering transport in manganites by tuning local nonstoichiometry in grain boundaries. Adv. Mater. 2019, 31, 1805360. [Google Scholar] [CrossRef]
- Baiutti, F.; Chiabrera, F.; Acosta, M.; Diercks, D.; Parfitt, D.; Santiso, J.; Wang, X.; Cavallaro, A.; Morata, A.; Wang, H.; et al. A high-entropy manganite in an ordered nanocomposite for long-term application in solid oxide cells. Nat. Commun. 2021, 12, 2660. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Gali, A.; George, E.P. Tensile properties of high- and medium-entropy alloys. Intermetallics 2013, 39, 74–78. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef]
- Bonnet, E.; Grenier, J.C.; Bassat, J.M.; Jacob, A.; Delatouche, B.; Bourdais, S. On the ionic conductivity of some zirconia-derived high-entropy oxides. J. Eur. Ceram. Soc. 2021, 41, 4505–4515. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-entropy oxides: Fundamental aspects and electrochemical properties. Adv. Mater. 2019, 31, 1806236. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Chen, K.P.; Pei, X.T.; Tang, L.; Cheng, H.R.; Li, Z.M.; Li, C.W.; Zhang, X.W.; An, L.A. A five-component entropy-stabilized fluorite oxide. J. Eur. Ceram. Soc. 2018, 38, 4161–4164. [Google Scholar] [CrossRef]
- Chellali, M.R.; Sarkar, A.; Nandam, S.H.; Bhattacharya, S.S.; Breitung, B.; Hahn, H.; Velasco, L. On the homogeneity of high entropy oxides: An investigation at the atomic scale. Scipta Mater. 2019, 166, 58–63. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.Y.; Huang, C.Y.; Nieto, A.; Chen, R.K.; Luo, J. From high-entropy ceramics to compositionally-complex ceramics: A case study of fluorite oxides. J. Eur. Ceram. Soc. 2020, 40, 2120–2129. [Google Scholar] [CrossRef]
- Dabrowa, J.; Szymczak, M.; Zajusz, M.; Mikula, A.; Mozdzierz, M.; Berent, K.; Wytrwal-Sarna, M.; Bernasik, A.; Stygar, M.; Swierczek, K. Stabilizing fluorite structure in ceria-based high-entropy oxides: Influence of Mo addition on crystal structure and transport properties. J. Eur. Ceram. Soc. 2020, 40, 5870–5881. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Dell’Agli, G. A simple and effective predictor to design novel fluorite-structured High Entropy Oxides (HEOs). Acta. Mater. 2021, 202, 181–189. [Google Scholar] [CrossRef]
- Chen, K.P.; Ma, J.X.; Tan, C.A.X.; Li, C.W.; An, L.A. An anion-deficient high-entropy fluorite oxide with very low density. Ceram. Inter. 2021, 47, 21207–21211. [Google Scholar] [CrossRef]
- Su, L.; Chen, X.; Xu, L.; Eldred, T.; Smith, J.; DellaRova, C.; Wang, H.J.; Gao, W.P. Visualizing the formation of high-entropy fluorite oxides from an amorphous precursor at atomic resolution. ACS Nano 2022, 16, 21397–21496. [Google Scholar] [CrossRef]
- Nundy, S.; Tatar, D.; Kojcinovic, J.; Ullah, H.; Chosh, A.; Mallick, T.K.; Meinusch, R.; Smarsly, B.M.; Tahir, A.A.; Djerdj, I. Bandgap engineering in novel fluorite-type rare earth high-entropy oxides (RE-HEOs) with computational and experimental validation for photocatalytic water splitting applications. Adv. Sustain. Syst. 2022, 6, 2200067. [Google Scholar] [CrossRef]
- Kotsonis, G.N.; Almishal, S.S.I.; Vieira, F.M.D.; Crespi, V.H.; Dabo, I.; Rost, C.M.; Maria, J.P. High-entropy oxides: Harnessing crystalline disorder for emergent functionality. J. Am. Ceram. Soc. 2023, 106, 5587–5611. [Google Scholar] [CrossRef]
- Ma, B.; Wen, Z.Q.; Qin, J.D.; Wu, Z.Y.; Liu, J.X.; Lv, Y.M.; Yu, J.J.; Zhao, Y.H. Synthesis and microstructure of (Ce0.2Zr0.2La0.2Sm0.2Nd0.2)O2-δ high-entropy oxides characterized by fluorite structure. Ceram. Inter. 2024, 50, 1981–1989. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum Scales. Comp. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Buckingham, R.A.; Lennard-Jones, J.E. The classical equation of state of gaseous helium, neon and argon. Proc. R. Soc. London A 1938, 168, 264–283. [Google Scholar]
- Grimes, R.W.; Busker, G.; McCoy, M.A.; Chroneos, A.; Kilner, J.A.; Chen, S.P. The Effect of Ion Size on Solution Mechanism and Defect Cluster Geometry. Ber. Bunsenges. Phys. Chem. 1997, 101, 1204. [Google Scholar] [CrossRef]
- Rupasov, D.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W.; Istomin, S.Y.; Antipov, E.V. Oxygen diffusion in Sr0.75Y0.25CoO2.625: A molecular dynamics study. Phys. Rev. B 2009, 79, 172102. [Google Scholar] [CrossRef]
- Ebmeyer, W.; Dholabhai, P.P. High-throughput prediction of oxygen vacancy defect migration near misfit dislocations in SrTiO3/BaZrO3 heterostructures. Mater. Adv. 2024, 5, 315–328. [Google Scholar]
- Gale, J.D. GULP: Capabilities and prospects. Z. Für Krist.-Cryst. Mater. 2005, 220, 552–554. [Google Scholar] [CrossRef]
- Gale, J.D. GULP: A Computer Program for the Symmetry-Adapted Simulation of Solids. J. Chem. Soc. Faraday Trans. 1997, 93, 629–637. [Google Scholar] [CrossRef]
- Kushima, A.; Parfitt, D.; Chroneos, A.; Yildiz, B.; Kilner, J.A.; Grimes, R.W. Interstitialcy diffusion of oxygen in tetragonal La2CoO4+δ. Phys. Chem. Chem. Phys. 2011, 13, 2242–2249. [Google Scholar] [CrossRef]
- He, X.; Zhu, X.; Epstein, A.; Mo, Y. Statistical variances of diffusional properties from ab initio molecular dynamics simulations. npj Comput. Mater. 2018, 4, 18. [Google Scholar] [CrossRef]
- Varotsos, P.; Alexopoulos, K. Thermodynamics of Point Defects and their Relation with the Bulk Properties; North-Holland: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Cooper, M.W.D.; Grimes, R.W.; Fitzpatrick, M.E.; Chroneos, A. Modeling oxygen self-diffusion in UO2 under pressure. Solid State Ionics 2015, 282, 26–30. [Google Scholar] [CrossRef]
- Chroneos, A. Connecting point defect parameters with bulk properties to describe diffusion in solids. Appl. Phys. Rev. 2016, 3, 041304. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chroneos, A. Oxygen Self-Diffusion in Fluorite High Entropy Oxides. Appl. Sci. 2024, 14, 5309. https://doi.org/10.3390/app14125309
Chroneos A. Oxygen Self-Diffusion in Fluorite High Entropy Oxides. Applied Sciences. 2024; 14(12):5309. https://doi.org/10.3390/app14125309
Chicago/Turabian StyleChroneos, Alexander. 2024. "Oxygen Self-Diffusion in Fluorite High Entropy Oxides" Applied Sciences 14, no. 12: 5309. https://doi.org/10.3390/app14125309



