Daily Variation of Body Temperature: An Analysis of Influencing Physiological Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Characteristics of Circadian Rhythms
2.2. Daily Rhythmicity of Body Temperature
- -
- The mean level, like the mean of any set of data.
- -
- The amplitude refers to the different values (range) of the body temperature excursion in a cycle.
- -
- The time of the daily peak is defined as the acrophase.
- -
- Robustness describes the way in which the rhythm takes on a regularity [2].
3. Some Factors Affecting Body Temperature and Its Circadian Rhythm
3.1. Circadian Rhythm of Temperature following Exercise
3.2. Use of Non-Invasive Instruments for Temperature Measurement
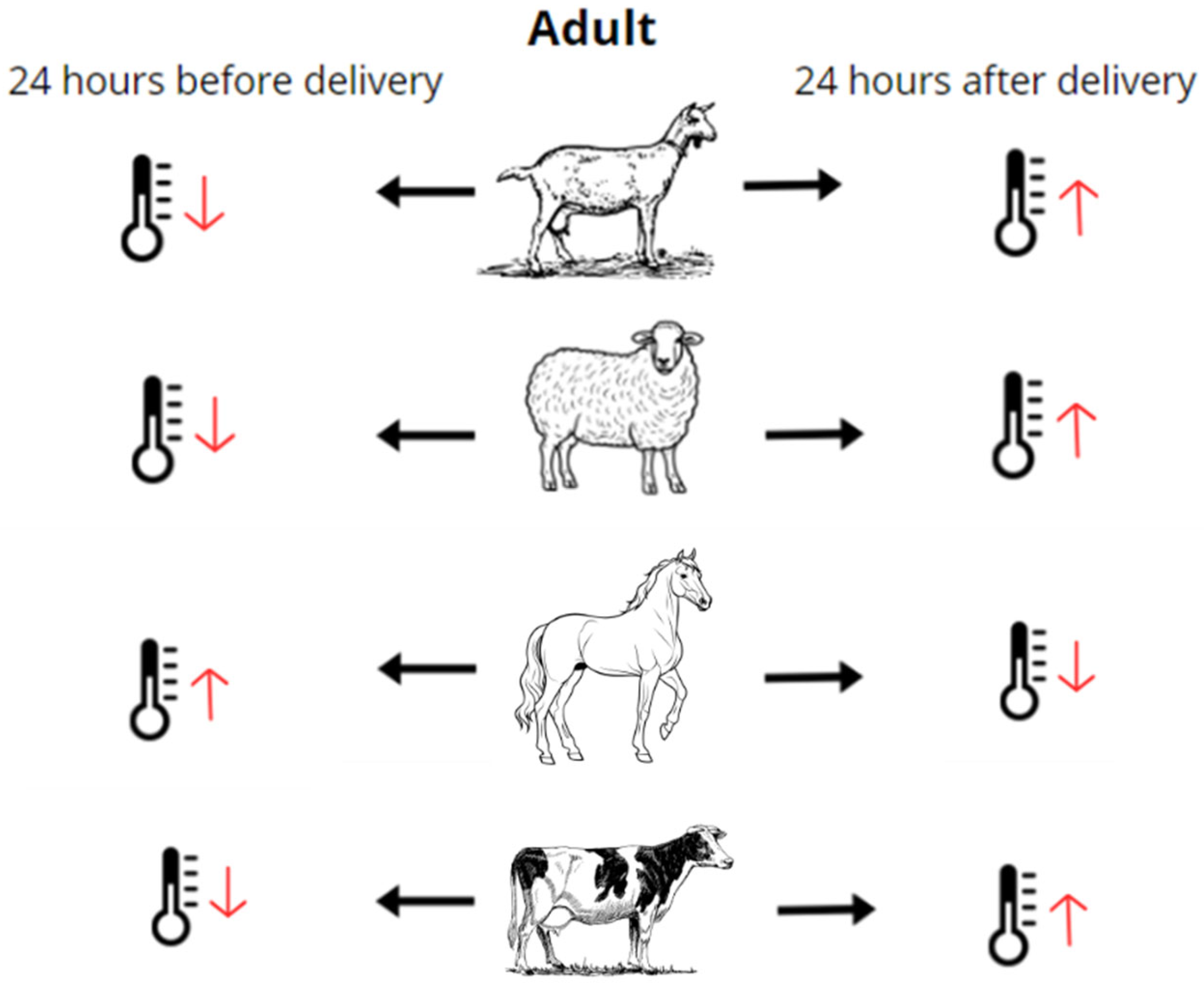
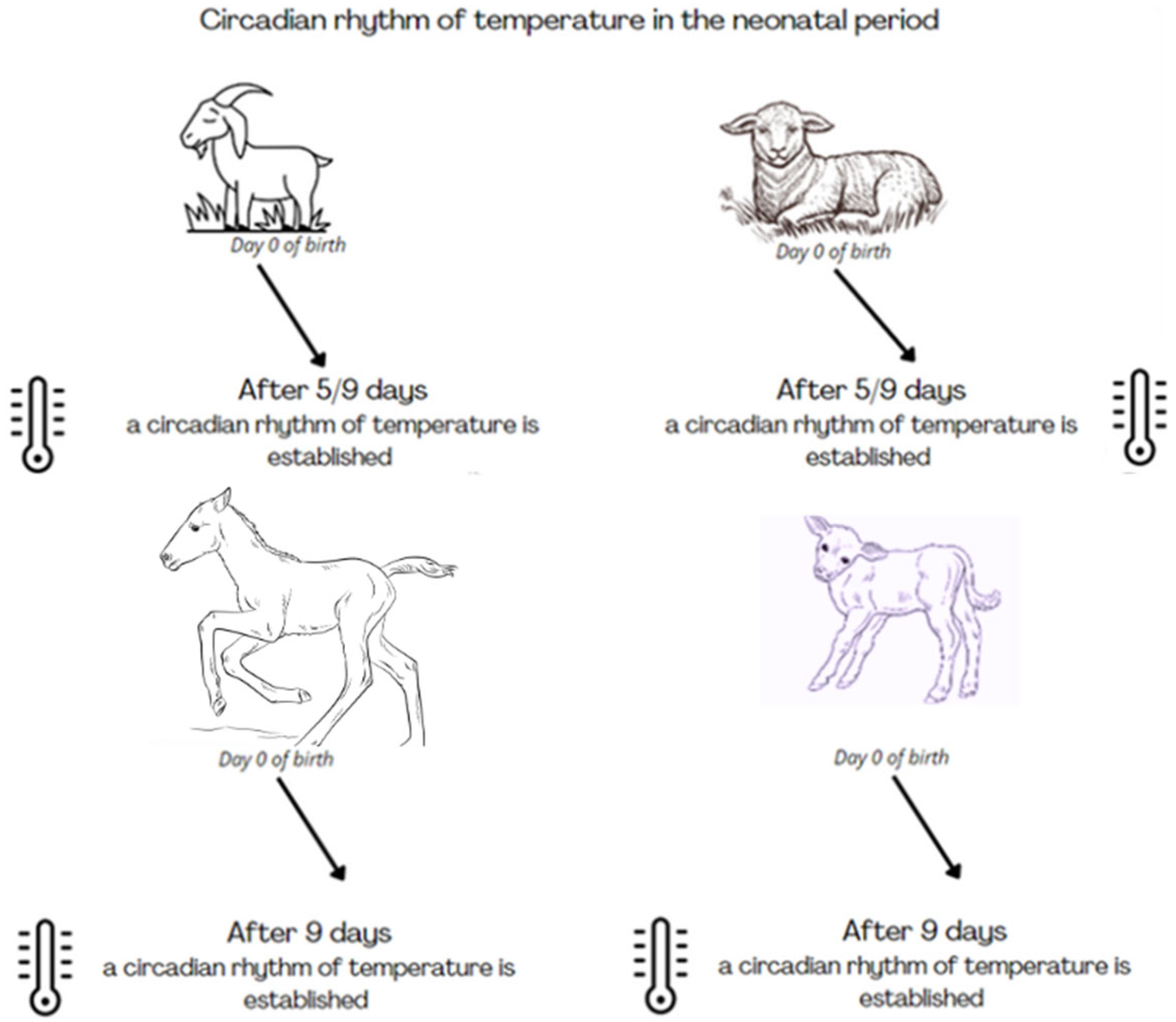
3.3. Daily Variation of Body Temperature during the Gestation and Neonatal Period
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Piccione, G.; Gianesella, M.; Morgante, M.; Refinetti, R. Daily Rhythmicity of Core and Surface Temperatures of Sheep Kept under Thermoneutrality or in the Cold. Res. Vet. Sci. 2013, 95, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R.; Piccione, G. Intra- and Inter-Individual Variability in the Circadian Rhythm of Body Temperature of Rats, Squirrels, Dogs, and Horses. J. Therm. Biol. 2005, 30, 139–146. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Arfuso, F.; Giudice, E.; Gianesella, M.; Fazio, F.; Panzera, M.; Piccione, G. Infrared Methodologies for the Assessment of Skin Temperature Daily Rhythm in Two Domestic Mammalian Species. J. Therm. Biol. 2020, 92, 102677. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Caola, G.; Refinetti, R. Circadian Rhythms of Body Temperature and Liver Function in Fed and Food-Deprived Goats. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 134, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Fazio, F.; Giudice, E.; Refinetti, R. Body Size and the Daily Rhythm of Body Temperature in Dogs. J. Therm. Biol. 2009, 34, 171–175. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Liu, T. Study on Body Temperature Detection of Pig Based on Infrared Technology: A Review. Artific. Intellig. Agricult. 2019, 1, 14–26. [Google Scholar] [CrossRef]
- Le Gal, P.-Y.; Andrieu, N.; Bruelle, G.; Dugué, P.; Monteil, C.; Moulin, C.-H.; Penot, E.; Ryschawy, J. Modelling Mixed Crop-Livestock Farms for Supporting Farmers’ Strategic Reflections: The CLIFS Approach. Comput. Electron. Agric. 2022, 192, 106570. [Google Scholar] [CrossRef]
- Stewart, M.; Stafford, K.J.; Dowling, S.K.; Schaefer, A.L.; Webster, J.R. Eye Temperature and Heart Rate Variability of Calves Disbudded with or without Local Anaesthetic. Physiol. Behav. 2008, 93, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Cui, J.; Yuan, H.; Cheng, M. Application and Research Progress of Infrared Thermography in Temperature Measurement of Livestock and Poultry Animals: A Review. Comput. Electron. Agric. 2023, 205, 107586. [Google Scholar] [CrossRef]
- Piccione, G.; Giudice, E.; Fazio, F.; Mortola, J.P. The Daily Rhythm of Body Temperature, Heart and Respiratory Rate in Newborn Dogs. J. Comp. Physiol. B 2010, 180, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Gebremedhin, K.G. Thermal Biology of Domestic Animals. Annu. Rev. Anim. Biosci. 2015, 3, 513–532. [Google Scholar] [CrossRef]
- Piccione, G.; Refinetti, R. Thermal Chronobiology of Domestic Animals. Front. Biosci. 2003, 8, 258–264. [Google Scholar]
- Murphy, B.A.; Elliott, J.A.; Sessions, D.R.; Vick, M.M.; Kennedy, E.L.; Fitzgerald, B.P. Rapid Phase Ad-justment of Melatonin and Core Body Temperature Rhythms Following a 6-h Advance of the Light/Dark Cycle in the Horse. J. Circadian Rhythm. 2007, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Barrell, G.K.; Thrun, L.A.; Brown, M.E.; Viguié, C.; Karsch, F.J. Importance of Photoperiodic Signal Quality to Entrainment of the Circannual Reproductive Rhythm of the Ewe. Biol. Reprod. 2000, 63, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, K.; Zhang, K.; Li, Y.; Gu, H.; Liu, H.; Yang, Z.; Cai, D. The Circadian Physiology: Implications in Livestock Health. Int. J. Mol. Sci. 2021, 22, 2111. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S.; Hong, H.-K.; Ko, C.H.; McDearmon, E.L. The Genetics of Mammalian Circadian Order and Disorder: Implications for Physiology and Disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Hamra, J.G.; Kamerling, S.G.; Wolfsheimer, K.J.; Bagwell, C.A. Diurnal Variation in Plasma Ir-Beta-Endorphin Levels and Experimental Pain Thresholds in the Horse. Life Sci. 1993, 53, 121–129. [Google Scholar] [CrossRef]
- Stull, C.L.; Rodiek, A.V. Physiological Responses of Horses to 24 Hours of Transportation Using a Commercial van during Summer Conditions. J. Anim. Sci. 2000, 78, 1458–1466. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G.; Refinetti, R. Feeble Weekly Rhythmicity in Hematological, Cardiovascular, and Thermal Parameters in the Horse. Chronobiol. Int. 2004, 21, 571–589. [Google Scholar] [CrossRef]
- Smith, J.E.; Barnes, A.L.; Maloney, S.K. A Nonsurgical Method Allowing Continuous Core Temperature Monitoring in Mares for Extended Periods, Including during Endurance Exercise. Equine Vet. J. 2006, 38, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Arfuso, F.; Fazio, F.; Giudice, E.; Panzera, M.; Piccione, G. Rhythmic Function of Body Temperature, Breathing and Heart Rates in Newborn Goats and Sheep during the First Hours of Life. J. Vet. Behav. 2017, 18, 29–36. [Google Scholar] [CrossRef]
- Mendel, V.E.; Raghavan, G.V. A STUDY OF DIURNAL TEMPERATURE PATTERNS IN SHEEP. J. Physiol. 1964, 174, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.L.; Fewell, J.E. Arousal Response from Sleep to Tracheal Obstruction in Lambs during Postnatal Maturation. Pediatr. Res. 1994, 36, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Araki, C.T.; Nakamura, R.M.; Kam, L.W.G.; Clarke, N.L. Diurnal Temperature Patterns of Early Lactating Cows with Milking Parlor Cooling. J. Dairy Sci. 1985, 68, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Lefcourt, A.M.; Huntington, J.B.; Akers, R.M.; Wood, D.L.; Bitman, J. Circadian and Ultradian Rhythms of Body Temperature and Peripheral Concentrations of Insulin and Nitrogen in Lactating Dairy Cows. Domest. Anim. Endocrinol. 1999, 16, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Ammer, S.; Lambertz, C.; Gauly, M. Is Reticular Temperature a Useful Indicator of Heat Stress in Dairy Cattle? J. Dairy Sci. 2016, 99, 10067–10076. [Google Scholar] [CrossRef] [PubMed]
- Arfuso, F.; Iliev, A.; Fazio, F.; Rizzo, M.; Saoca, C.; Piccione, G. Variations of Serum Mitochondrial Uncoupling Protein 1 (UCP1) Levels and Rectal Temperature in Capra Hircus. Archiv. Anat. Physiol. 2017, 2, 007–010. [Google Scholar] [CrossRef]
- Piccione, G.; Bertolucci, C.; Costa, A.; Di Mauro, S.; Caola, G. Daily Rhythm of Body and Auricle Temperature in Goats Kept at Two Different Ambient Temperatures. Biol. Rhythm Res. 2005, 36, 309–314. [Google Scholar] [CrossRef]
- Piccione, G.; Giannetto, C.; Fazio, F.; Giudice, E. Accuracy of Auricular Temperature Determination as Body Temperature Index and Its Daily Rhythmicity in Healthy Dog. Biol. Rhythm Res. 2011, 42, 437–443. [Google Scholar] [CrossRef]
- Wrenn, T.R.; Bitman, J.; Sykes, J.F. Body Temperature Variations in Dairy Cattle during the Estrous Cycle and Pregnancy. J. Dairy Sci. 1958, 41, 1071–1076. [Google Scholar] [CrossRef]
- Kendall, P.E.; Tucker, C.B.; Dalley, D.E.; Clark, D.A.; Webster, J.R. Milking Frequency Affects the Circadian Body Temperature Rhythm in Dairy Cows. Livest. Sci. 2008, 117, 130–138. [Google Scholar] [CrossRef]
- Mayilsamy, S.; Sakthivel, J.; Ayyasamy, M.; Pushpadass, H.A.; Muniyandi, S.; Ramesha, K. Influence of Circadian Rhythm, Breed, Stage of Lactation, Milk Yield and Parity on Body and Udder Skin Surface Temperature of Lactating Cows Monitored by Infrared Thermography. J. Appl. Anim. Res. 2023, 51, 406–413. [Google Scholar] [CrossRef]
- Fazio, F.; Arfuso, F.; Rizzo, M.; Giannetto, C.; Giudice, E.; Zanghì, E.; Piccione, G. Livestock Handling and Road Transport Influence Some Oxidative Stress Parameters in Ewes. J. Vet. Behav. 2018, 26, 5–10. [Google Scholar] [CrossRef]
- Padalino, B.; Raidal, S.L. Effects of Transport Conditions on Behavioural and Physiological Responses of Horses. Animals 2020, 10, 160. [Google Scholar] [CrossRef]
- Meléndez, D.M.; Marti, S.; Haley, D.B.; Schwinghamer, T.D.; Schwartzkopf-Genswein, K.S. Effects of conditioning, source, and rest on indicators of stress in beef cattle transported by road. PLoS ONE 2021, 16, e0244854. [Google Scholar] [CrossRef] [PubMed]
- Macartney, J.E.; Bateman, K.G.; Ribble, C.S. Health Performance of Feeder Calves Sold at Conventional Auctions versus Special Auctions of Vaccinated or Conditioned Calves in Ontario. J. Am. Vet. Med. Assoc. 2003, 223, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Proctor, H.S.; Carder, G. Nasal Temperatures in Dairy Cows Are Influenced by Positive Emotional State. Physiol. Behav. 2015, 138, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Pedernera-Romano, C.; Ruiz de la Torre, J.L.; Badiella, L.; Manteca, X. Effect of Perphenazine Enanthate on Open-Field Test Behaviour and Stress-Induced Hyperthermia in Domestic Sheep. Pharmacol. Biochem. Behav. 2010, 94, 329–332. [Google Scholar] [CrossRef]
- Rocha, L.M.; Devillers, N.; Maldague, X.; Kabemba, F.Z.; Fleuret, J.; Guay, F.; Faucitano, L. Validation of Anatomical Sites for the Measurement of Infrared Body Surface Temperature Variation in Response to Handling and Transport. Animals 2019, 9, 425. [Google Scholar] [CrossRef]
- Verdegaal, E.-L.J.M.M.; Howarth, G.S.; McWhorter, T.J.; Delesalle, C.J.G. Is Continuous Monitoring of Skin Surface Temperature a Reliable Proxy to Assess the Thermoregulatory Response in Endurance Horses During Field Exercise? Front. Vet. Sci. 2022, 9, 894146. [Google Scholar] [CrossRef]
- Esteves Trindade, P.H.; de Camargo Ferraz, G.; Pereira Lima, M.L.; Negrão, J.A.; Paranhos da Costa, M.J.R. Eye Surface Temperature as a Potential Indicator of Physical Fitness in Ranch Horses. J. Equine Vet. Sci. 2019, 75, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Arfuso, F.; Alberghina, D.; Giudice, E.; Gianesella, M.; Piccione, G. Monitoring Changes in Body Surface Temperature Associated with Treadmill Exercise in Dogs by Use of Infrared Methodology. J. Therm. Biol. 2017, 69, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Scoppetta, F.; Tartaglia, M.; Renzone, G.; Avellini, L.; Gaiti, A.; Scaloni, A.; Chiaradia, E. Plasma Protein Changes in Horse after Prolonged Physical Exercise: A Proteomic Study. J. Proteom. 2012, 75, 4494–4504. [Google Scholar] [CrossRef] [PubMed]
- Seabra, J.C.; Dittrich, J.R.; Vale, M.M.; Janiszewski, J.R.; Hollanda, R.S. de Eye temperature change in response to race training in thoroughbred horses at the Jockey Club. Arch. Vet. Sci. 2019, 24, 50–59. [Google Scholar]
- Ott, E.A. Influence of Temperature Stress on the Energy and Protein Metabolism and Requirements of the Working Horse. Livest. Prod. Sci. 2005, 92, 123–130. [Google Scholar] [CrossRef]
- Hodgson, D.R.; Davis, R.E.; McConaghy, F.F. Thermoregulation in the Horse in Response to Exercise. Br. Vet. J. 1994, 150, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C. Blood Pressure Control--Special Role of the Kidneys and Body Fluids. Science 1991, 252, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Assenza, A.; Bergero, D.; Congiu, F.; Tosto, F.; Giannetto, C.; Piccione, G. Evaluation of Serum Electrolytes and Blood Lactate Concentration During Repeated Maximal Exercise in Horse. J. Equine Vet. Sci. 2014, 34, 1175–1180. [Google Scholar] [CrossRef]
- Rizzo, M.; Arfuso, F.; Giudice, E.; Abbate, F.; Longo, F.; Piccione, G. Core and Surface Temperature Modification During Road Transport and Physical Exercise in Horse After Acupuncture Needle Stimulation. J. Equine Vet. Sci. 2017, 55, 84–89. [Google Scholar] [CrossRef]
- Piccione, G.; Giannetto, C.; Assenza, A.; Fazio, F.; Caola, G. Serum Electrolyte and Protein Modification during Different Workload in Jumper Horse. Comp. Clin. Pathol. 2007, 16, 103–107. [Google Scholar] [CrossRef]
- Giannetto, C.; Di Pietro, S.; Falcone, A.; Pennisi, M.; Giudice, E.; Piccione, G.; Acri, G. Thermographic Ocular Temperature Correlated with Rectal Temperature in Cats. J. Therm. Biol. 2021, 102, 103104. [Google Scholar] [CrossRef]
- Mccafferty, D.J. The Value of Infrared Thermography for Research on Mammals: Previous Applications and Future Directions. Mammal Rev. 2007, 37, 207–223. [Google Scholar] [CrossRef]
- Aragona, F.; Di Pietro, S.; Arfuso, F.; Fazio, F.; Piccione, G.; Giudice, E.; Giannetto, C. Correlation between Ocular and Rectal Temperature with Intra Ocular Pressure in Horse during Exercise. Animals 2022, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Arfuso, F.; Acri, G.; Piccione, G.; Sansotta, C.; Fazio, F.; Giudice, E.; Giannetto, C. Eye Surface Infrared Thermography Usefulness as a Noninvasive Method of Measuring Stress Response in Sheep during Shearing: Correlations with Serum Cortisol and Rectal Temperature Values. Physiol. Behav. 2022, 250, 113781. [Google Scholar] [CrossRef] [PubMed]
- Soroko, M.; Howell, K. Infrared Thermography: Current Applications in Equine Medicine. J. Equine Vet. Sci. 2018, 60, 90–96.e2. [Google Scholar] [CrossRef]
- Prochno, H.C.; Barussi, F.M.; Bastos, F.Z.; Weber, S.H.; Bechara, G.H.; Rehan, I.F.; Michelotto, P.V. Infrared Thermography Applied to Monitoring Musculoskeletal Adaptation to Training in Thoroughbred Race Horses. J. Equine Vet. Sci. 2020, 87, 102935. [Google Scholar] [CrossRef] [PubMed]
- Bertolucci, C.; Giannetto, C.; Fazio, F.; Piccione, G. Seasonal Variations in Daily Rhythms of Activity in Athletic Horses. Animal 2008, 2, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Grasso, F.; Fazio, F.; Giudice, E. The Effect of Physical Exercise on the Daily Rhythm of Platelet Aggregation and Body Temperature in Horses. Vet. J. 2008, 176, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Yoshida, M.; Samura, K.; Matsumoto, H.; Ikemoto, F.; Tagawa, M. Ranges of Diurnal Variation and the Pattern of Body Temperature, Blood Pressure and Heart Rate in Laboratory Beagle Dogs. Exp. Anim. 2002, 51, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Zanghi, B.M. Eye and Ear Temperature Using Infrared Thermography Are Related to Rectal Temperature in Dogs at Rest or With Exercise. Front. Vet. Sci. 2016, 3, 111. [Google Scholar] [CrossRef] [PubMed]
- Silva-Gómez, S.E.; Rodríguez-Galván, G.; Hernández-Zepeda, J.S.; Zaragoza-Martínez, L.; Palestina-González, M.I. Donkey for transport and load. Actas Iberoam. Conserv. Anim. 2017, 10, 83–87. [Google Scholar]
- Ake, A.S.; Ayo, J.O.; Aluwong, T.; Mohammed, A. Effects of Packing (Load Carrying) on Body Temperatures and Their Circadian Rhythms in Donkeys (Equus asinus) during the Hot-Dry Season. J. Therm. Biol. 2023, 113, 103497. [Google Scholar] [CrossRef] [PubMed]
- Zakari, F.O.; Ayo, J.O.; Rekwot, P.I.; Kawu, M.U.; Minka, N.S. Daily Rhythms of Rectal and Body Surface Temperatures in Donkeys during the Cold-Dry (Harmattan) and Hot-Dry Seasons in a Tropical Savannah. Int. J. Biometeorol. 2018, 62, 2231–2243. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.D.; Eijsvogels, T.M.H.; Daanen, H.A.M. Exercise under Heat Stress: Thermoregulation, Hydration, Performance Implications, and Mitigation Strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef] [PubMed]
- Hilmer, S.; Algar, D.; Neck, D.; Schleucher, E. Remote Sensing of Physiological Data: Impact of Long Term Captivity on Body Temperature Variation of the Feral Cat (Felis catus) in Australia, Recorded via Thermochron iButtons. J. Therm. Biol. 2010, 35, 205–210. [Google Scholar] [CrossRef]
- Taweel, H.Z.; Tas, B.M.; Smit, H.J.; Tamminga, S.; Elgersma, A. A Note on Eating Behaviour of Dairy Cows at Different Stocking Systems—Diurnal Rhythm and Effects of Ambient Temperature. Appl. Anim. Behav. Sci. 2006, 98, 315–322. [Google Scholar] [CrossRef]
- Piccione, G.; Rizzo, M.; Casella, S.; Marafioti, S.; Fazio, F. Application of the iButton® for Measurement of the Rumen Temperature Circadian Rhythms in Lambs. Biol. Rhythm Res. 2014, 45, 375–381. [Google Scholar] [CrossRef]
- Nikkhah, A.; Furedi, C.J.; Kennedy, A.D.; Crow, G.H.; Plaizier, J.C. Effects of Feed Delivery Time on Feed Intake, Milk Production, and Blood Metabolites of Dairy Cows. J. Dairy Sci. 2008, 91, 4249–4260. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.A.; María, G.A.; Estévez-Moreno, L.X.; Miranda-De La Lama, G.C. Daily Rhythms of Body Temperature around Lambing in Sheep Measured Non-Invasively. Biol. Rhythm Res. 2020, 51, 988–993. [Google Scholar] [CrossRef]
- Sakatani, M.; Sugano, T.; Higo, A.; Naotsuka, K.; Hojo, T.; Gessei, S.; Uehara, H.; Takenouchi, N. Vaginal Temperature Measurement by a Wireless Sensor for Predicting the Onset of Calving in Japanese Black Cows. Theriogenology 2018, 111, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kendall, P.E.; Webster, J.R. Season and Physiological Status Affects the Circadian Body Temperature Rhythm of Dairy Cows. Livest. Sci. 2009, 125, 155–160. [Google Scholar] [CrossRef]
- Suarez-Trujillo, A.; Hoang, N.; Robinson, L.; McCabe, C.J.; Conklin, D.; Minor, R.C.; Townsend, J.; Plaut, K.; George, U.Z.; Boerman, J.; et al. Effect of Circadian System Disruption on the Concentration and Daily Oscillations of Cortisol, Progesterone, Melatonin, Serotonin, Growth Hormone, and Core Body Temperature in Periparturient Dairy Cattle. J. Dairy Sci. 2022, 105, 2651–2668. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E.; Bruce Currie, W. Partitioning of Nutrients During Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef] [PubMed]
- Kalyesubula, M.; Casey, T.M.; Reicher, N.; Sabastian, C.; Wein, Y.; Bar Shira, E.; Hoang, N.; George, U.Z.; Shamay, A.; Plaut, K.; et al. Physiological State and Photoperiod Exposures Differentially Influence Circadian Rhythms of Body Temperature and Prolactin and Relate to Changes in Mammary PER1 Expression in Late Pregnant and Early Lactation Dairy Goats. Small Rumin. Res. 2021, 200, 106394. [Google Scholar] [CrossRef]
- Abecia, J.-A.; Forcada, F.; Vázquez, M.-I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Pérez-Pe, R.; Casao, A. Role of Melatonin on Embryo Viability in Sheep. Reprod. Fertil. Dev. 2019, 31, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Caola, G.; Refinetti, R. The Circadian Rhythm of Body Temperature of the Horse. Biol. Rhythm Res. 2002, 33, 113–119. [Google Scholar] [CrossRef]


| Species | Place of Collection | Mean Temperature | Oscillation Range | Acrophase | References |
|---|---|---|---|---|---|
| Equus caballus | rectum | 38.0 | 0.4 | 15:00 | [18] |
| rectum | 37.4 | 0.3 | 20:00 | [19] | |
| rectum | 38.2 | 0.4 | 23:00 | [20] | |
| rectum | 38.4 | 0.7 | 14:00 | [2] | |
| rectum | 37.4 | 1.0 | 12:00 | [21] | |
| rectum | 37.4 | 0.3 | 19:00 | [22] | |
| Ovis aries | carotidy artery | 38.7 | 1.0 | 18:00 | [23] |
| intraperitoneal | 39.3 | 0.3 | 14:00 | [24] | |
| Ovis aries (lambs) | rumen | 39.5 | 0.4 | 19:00 | [1] |
| Bos taurus | vagina | 38.6 | 0.5 | 18:00 | [25] |
| udder | 38.9 | 0.5 | 22:00 | [26] | |
| rectum | 38.3 | 1.4 | 14:00 | [5] | |
| retina | 38.1 | 0.4 | 10:00 | [27] | |
| Capra hircus | rectum | 38.8 | 0.2 | 14:00 | [13] |
| Capra hircus | rectum | 38.4 | 0.4 | 19:00 | [28] |
| Capra hircus | rectum | 38.4 | 0.5 | 19:00 | [22] |
| Canis familiaris | rectum | 39.1 | 0.5 | 11:00 | [13] |
| rectum | 38.7 | 0.7 | 11:00 | [29] | |
| rectum | 39.0 | 0.8 | 11:00 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrigo, F.; Arfuso, F.; Faggio, C.; Piccione, G. Daily Variation of Body Temperature: An Analysis of Influencing Physiological Conditions. Appl. Sci. 2024, 14, 5413. https://doi.org/10.3390/app14135413
Arrigo F, Arfuso F, Faggio C, Piccione G. Daily Variation of Body Temperature: An Analysis of Influencing Physiological Conditions. Applied Sciences. 2024; 14(13):5413. https://doi.org/10.3390/app14135413
Chicago/Turabian StyleArrigo, Federica, Francesca Arfuso, Caterina Faggio, and Giuseppe Piccione. 2024. "Daily Variation of Body Temperature: An Analysis of Influencing Physiological Conditions" Applied Sciences 14, no. 13: 5413. https://doi.org/10.3390/app14135413
APA StyleArrigo, F., Arfuso, F., Faggio, C., & Piccione, G. (2024). Daily Variation of Body Temperature: An Analysis of Influencing Physiological Conditions. Applied Sciences, 14(13), 5413. https://doi.org/10.3390/app14135413








