Design and Evaluation of Face Mask Filtration: Mechanisms, Formulas, and Fluid Dynamics Simulations
Abstract
:1. Introduction
2. Modelling
2.1. Brownian Diffusion
2.2. Inertial Impaction
2.3. Interception
2.4. Total Filtration Efficiency
2.5. Mathematical Analysis Based on Laminar Flow Model
2.6. Quadrant-Based Average Velocity
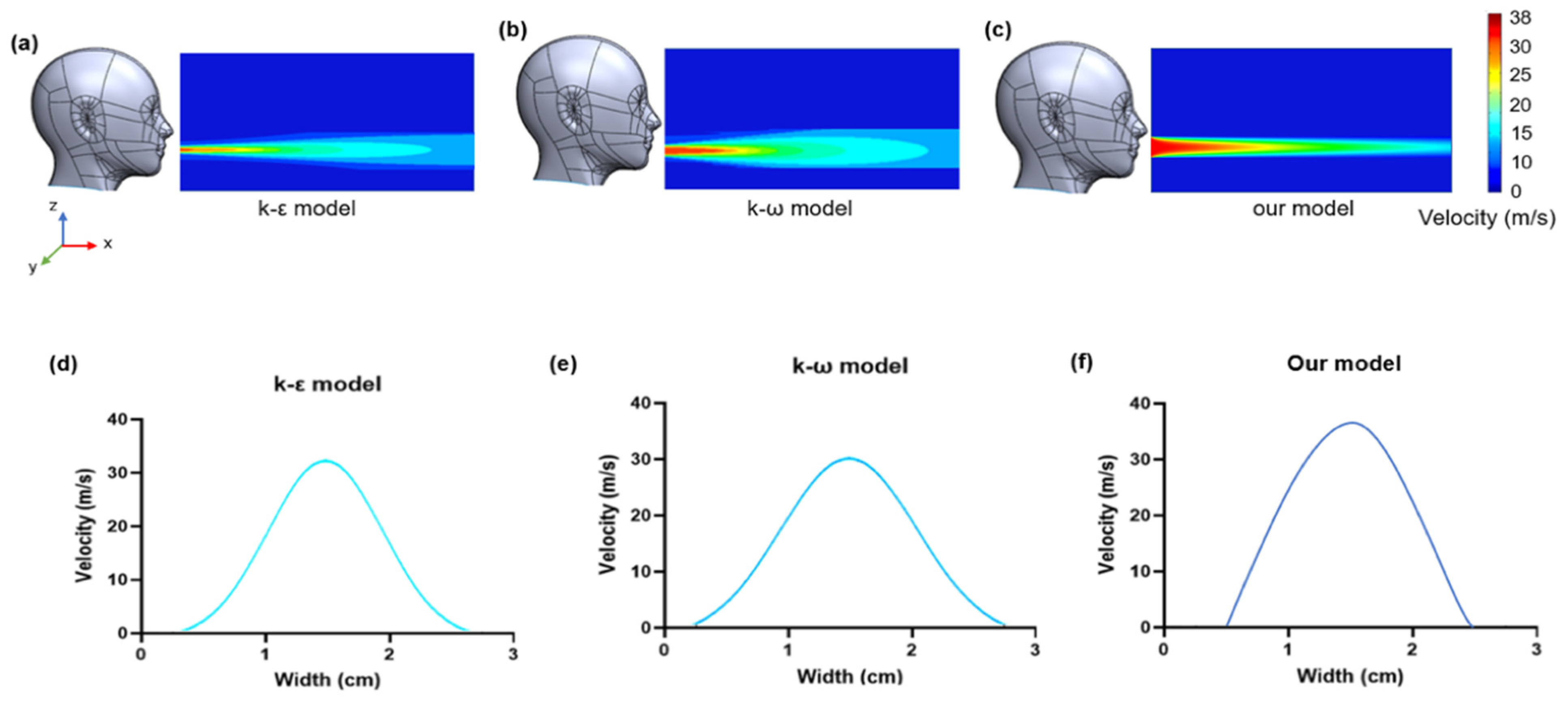
2.7. Turbulent Flow Model
3. Results and Discussions
3.1. CAD Designs
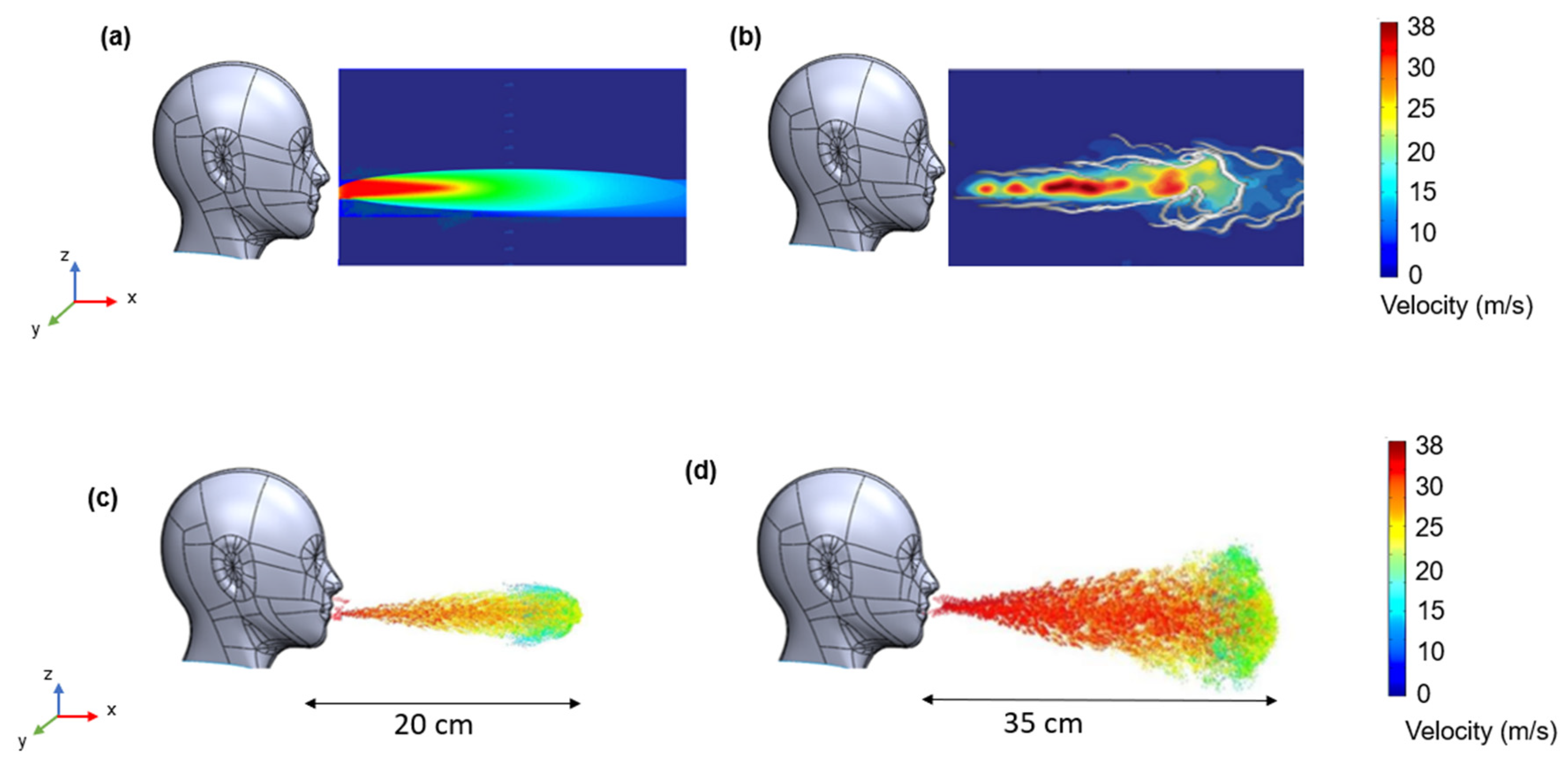
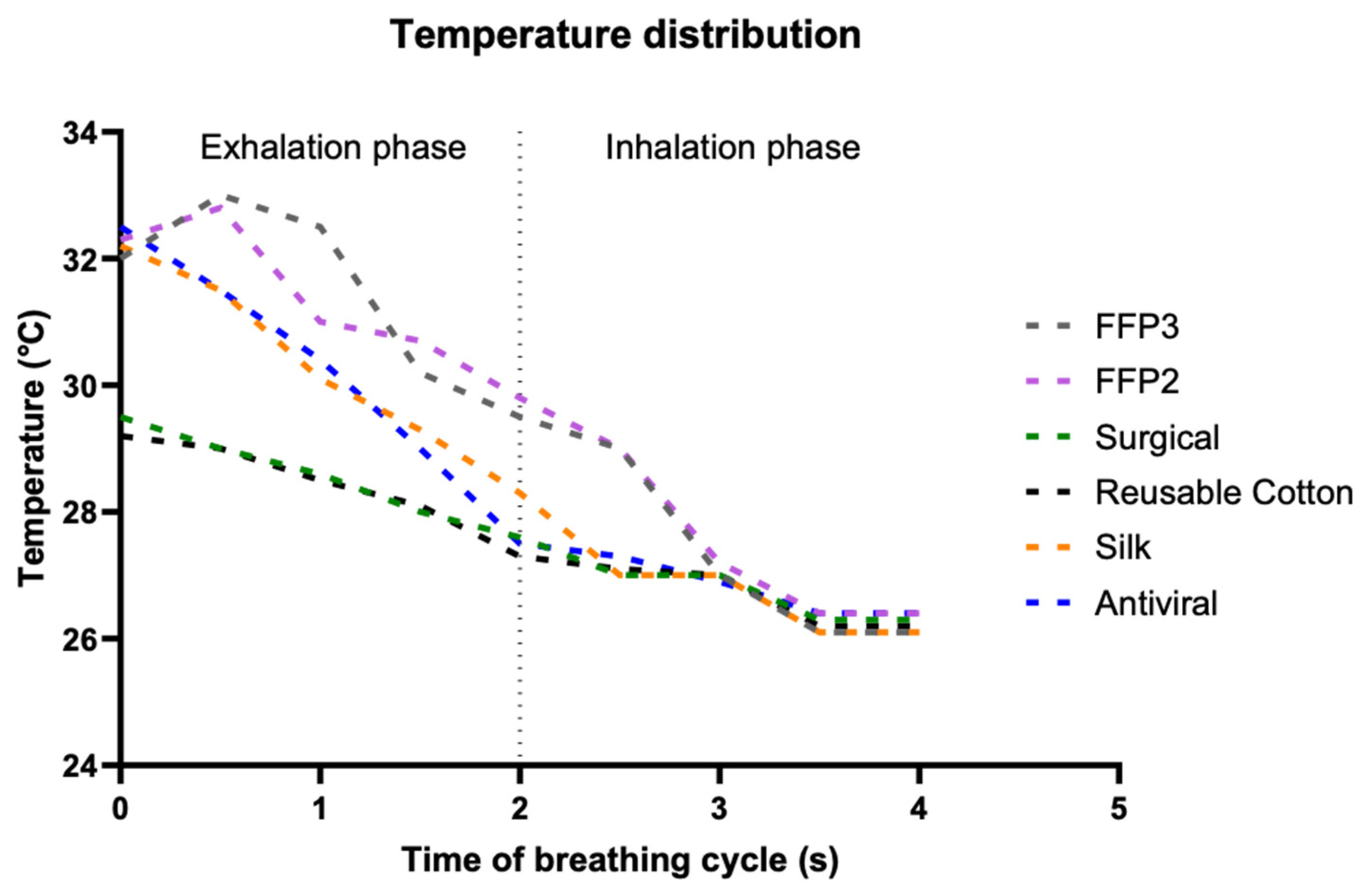
3.2. CFD Simulation Results
3.3. Darcy’s Law
3.4. Brownian Motion Effect
3.5. Prediction from Fluid Flow Mechanics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonald, F.; Horwell, C.J.; Wecker, R.; Dominelli, L.; Loh, M.; Kamanyire, R.; Ugarte, C. Facemask use for community protection from air pollution disasters: An ethical overview and framework to guide agency decision making. Int. J. Disaster Risk Reduct. 2020, 43, 101376. [Google Scholar] [CrossRef]
- Cheng, V.C.-C.; Wong, S.-C.; Chuang, V.W.-M.; So, S.Y.-C.; Chen, J.H.-K.; Sridhar, S.; To, K.K.-W.; Chan, J.F.-W.; Hung, I.F.-N.; Ho, P.-L.; et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020, 81, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Rab, S.; Javaid, M.; Haleem, A.; Vaishya, R. Face masks are new normal after COVID-19 pandemic. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1617–1619. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Bayham, J.; Richter, A.; Fenichel, E.P. Risk compensation and face mask mandates during the COVID-19 pandemic. Sci. Rep. 2021, 11, 3174. [Google Scholar] [CrossRef] [PubMed]
- Rasekh, M.; Pisapia, F.; Howkins, A.; Rees, D. Materials analysis and image-based modelling of transmissibility and strain behaviour in approved face mask microstructures. Sci. Rep. 2022, 12, 17361. [Google Scholar] [CrossRef] [PubMed]
- Sickbert-Bennett, E.E.; Samet, J.M.; Clapp, P.W.; Chen, H.; Berntsen, J.; Zeman, K.L.; Tong, H.; Weber, D.J.; Bennett, W.D. Filtration efficiency of hospital face mask alternatives available for use during the COVID-19 pandemic. JAMA Intern. Med. 2020, 180, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.W.; Eshbaugh, J.P.; Hofacre, K.C.; Gardner, P.D. Respirator Filter Efficiency Testing against Particulate and Biological Aerosols under Moderate to High Flow Rates; US Army Edgewood Chemical Biological Center Report for Contract No. SP0700-00-D-3180, Task (335); US Army Edgewood Chemical Biological Center: Edgewood, MD, USA, 2006. [Google Scholar]
- Ramarao, B.V.; Tien, C.; Mohan, S. Calculation of single fiber efficiencies for interception and impaction with superposed Brownian motion. J. Aerosol Sci. 1994, 25, 295–313. [Google Scholar] [CrossRef]
- Rasekh, M.; Pisapia, F.; Hafizi, S.; Rees, D. Filtering Efficiency and Design Properties of Medical and Non-Medical Grade Face Masks: A Multiscale Modeling Approach. Appl. Sci. 2024, 14, 4796. [Google Scholar] [CrossRef]
- Iitani, K.; Tyson, J.; Rao, S.; Ramamurthy, S.S.; Ge, X.; Rao, G. What do masks mask? A study on transdermal CO2 monitoring. Med. Eng. Phys. 2021, 98, 50–56. [Google Scholar] [CrossRef]
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano 2020, 14, 6339–6347. [Google Scholar] [CrossRef]
- Qian, Y.; Willeke, K.; Grinshpun, S.A.; Donnelly, J.; Coffey, C.C. Performance of N95 respirators: Filtration efficiency for airborne microbial and inert particles. Am. Ind. Hyg. Assoc. J. 1998, 59, 128–132. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, E.; Pirog, S.; Ward, J.; Clarkson, P.J. Ability of fabric face mask materials to filter ultrafine particles at coughing velocity. BMJ Open 2020, 10, e039424. [Google Scholar] [CrossRef]
- Brooks, J.T.; Beezhold, D.H.; Noti, J.D.; Coyle, J.P.; Derk, R.C.; Blachere, F.M.; Lindsley, W.G. Maximizing fit for cloth and medical procedure masks to improve performance and reduce SARS-CoV-2 transmission and exposure, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Kulmala, I.; Heinonen, K.; Salo, S. Improving filtration efficacy of medical face masks. Aerosol Air Qual. Res. 2021, 21, 200043. [Google Scholar] [CrossRef]
- Rengasamy, S.; Shaffer, R.; Williams, B.; Smit, S. A comparison of facemask and respirator filtration test methods. J. Occup. Environ. Hyg. 2017, 14, 92–103. [Google Scholar] [CrossRef]
- Tcharkhtchi, A.; Abbasnezhad, N.; Zarbini Seydani, M.; Zirak, N.; Farzaneh, S.; Shirinbayan, M. An overview of filtration efficiency through the masks: Mechanisms of the aerosols penetration. Bioact. Mater. 2021, 6, 106–122. [Google Scholar] [CrossRef]
- Lei, Z.; Yang, J.; Zhuang, Z.; Roberge, R. Simulation and evaluation of respirator faceseal leaks using computational fluid dynamics and infrared imaging. Ann. Occup. Hyg. 2013, 57, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Shen, S.; Cai, M. Investigation of the flow-field in the upper respiratory system when wearing N95 filtering facepiece respirator. J. Occup. Environ. Hyg. 2016, 13, 372–382. [Google Scholar] [CrossRef]
- Bai, H.; Qian, X.; Fan, J.; Qian, Y.; Liu, Y.; Duo, Y.; Guo, C.; Wang, X. Microscale layered structural filtration efficiency model: Probing filtration properties of non-uniform fibrous filter media. Sep. Purif. Technol. 2020, 236, 16037. [Google Scholar] [CrossRef]
- Monjezi, M.; Jamaati, H. The effects of face mask specifications on work of breathing and particle filtration efficiency. Med. Eng. Phys. 2021, 98, 36–43. [Google Scholar] [CrossRef]
- Wang, Q.; Maze, B.; Tafreshi, H.V.; Pourdeyhimi, B. A case study of simulating submicron aerosol filtration via lightweight spun-bonded filter media. Chem. Eng. Sci. 2006, 61, 4871–4883. [Google Scholar] [CrossRef]
- Polat, Y.; Calisir, M.; Gungor, M.; Sagirli, M.N.; Atakan, R.; Akgul, Y.; Demir, A.; Kiliç, A. Solution blown nanofibrous air filters modified with glass microparticles. J. Ind. Text. 2021, 51, 821–834. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.W.; Zhang, B.; Gong, W. Semi-analytical and computational investigation of different fibrous structures affecting the performance of fibrous media. Sci. Prog. 2020, 103, 0036850419874231. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Y. Enhanced spread of expiratory droplets by turbulence in a cough jet. Build. Environ. 2015, 93, 86–96. [Google Scholar] [CrossRef]
- Lee, J.H.W.; Chu, V. Turbulent Jets and Plumes: A Lagrangian Approach; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Wang, C.-S. Airflow in the respiratory system. Interface Sci. Technol. 2005, 5, 31–54. [Google Scholar] [CrossRef]
- Dbouk, T.; Drikakis, D. On respiratory droplets and face masks. Phys. Fluids 2020, 32, 063303. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, N.; Wei, J.; Yen, H.-L.; Li, Y. Short-range airborne route dominates exposure of respiratory infection during close contact. Build. Environ. 2020, 176, 106859. [Google Scholar] [CrossRef]
- Zhao, L.; Qi, Y.; Luzzatto-Fegiz, P.; Cui, Y.; Zhu, Y. COVID-19: Effects of Environmental Conditions on the Propagation of Respiratory Droplets. Nano Lett. 2020, 20, 7744–7750. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, Z.; Zhang, L.; Wang, S.; Bai, T.; Xiang, Y.; Long, E. Systematic review of the effects of environmental factors on virus inactivation: Implications for coronavirus disease 2019. Int. J. Environ. Sci. Technol. 2021, 18, 2865–2878. [Google Scholar] [CrossRef]
- Walker, F.C.; Sridhar, P.R.; Baldridge, M.T. Differential roles of interferons in innate responses to mucosal viral infections. Trends Immunol. 2021, 42, 1009–1023. [Google Scholar] [CrossRef]
- Whitaker, S. Flow in porous media I: A theoretical derivation of Darcy’s law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]
- Mittal, R.; Breuer, K.; Seo, J.H. The Flow Physics of Face Masks. Annu. Rev. Fluid Mech. 2023, 55, 193–211. [Google Scholar] [CrossRef]
- Ciofalo, M.; Collins, M.W.; Hennessy, T.R. Modelling nanoscale fluid dynamics and transport in physiological flows. Med. Eng. Phys. 1996, 18, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Chung, Y.-S.; Jo, H.J.; Lee, N.-J.; Kim, M.S.; Woo, S.H.; Park, S.; Kim, J.W.; Kim, H.M.; Han, M.-G. Identification of coronavirus isolated from a patient in korea with COVID-19. Osong Public Health Res. Perspect. 2020, 11, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Park, W.B.; Kwon, N.-J.; Choi, S.-J.; Kang, C.K.; Choe, P.G.; Kim, J.Y.; Yun, J.; Lee, G.-W.; Seong, M.-W.; Kim, N.J.; et al. Virus isolation from the first patient with SARS-CoV-2 in Korea. J. Korean Med. Sci. 2020, 35, e84. [Google Scholar] [CrossRef] [PubMed]
- White, F.M.; Majdalani, J. Viscous Fluid Flow; McGraw-Hill: New York, NY, USA, 2006; Volume 3, pp. 433–434. [Google Scholar]
- Tura, A.; Sarti, A.; Gaens, T.; Lamberti, C. Regularization of blood motion fields by modified Navier–Stokes equations. Med. Eng. Phys. 1999, 21, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D. Comparison of various turbulence models for unsteady flow around a finite circular cylinder at Re = 20000. J. Phys. Conf. Ser. 2017, 910, 012027. [Google Scholar] [CrossRef]
- Jaksic, D.; Jaksic, N. The porosity of masks used in medicine. Tekstilec 2004, 47, 301–304. [Google Scholar]
- Jaksic, N.; Jaksic, D. Novel theoretical approach to the filtration of nano particles through non-woven fabrics. In Woven Fabrics; IntechOpen: London, UK, 2012; pp. 205–238. [Google Scholar] [CrossRef]
- Anderson, J.D.; Wendt, J. Computational Fluid Dynamics; McGraw-Hill: New York, NY, USA, 1995. [Google Scholar]
- Osman, O.; Madi, M.; Ntantis, E.L.; Kabalan, K.Y. Displacement ventilation to avoid COVID-19 transmission through offices. Comput. Part. Mech. 2023, 10, 355–368. [Google Scholar] [CrossRef]
- Si, X.A.; Talaat, M.; Xi, J. SARS COV-2 virus-laden droplets coughed from deep lungs: Numerical quantification in a single-path whole respiratory tract geometry. Phys. Fluids 2021, 33, 023306. [Google Scholar] [CrossRef]
- Wedel, J.; Steinmann, P.; Štrakl, M.; Matjaž, H.; Jure, R. Can CFD establish a connection to a milder COVID-19 disease in younger people? Aerosol deposition in lungs of different age groups based on Lagrangian particle tracking in turbulent flow. Comput. Mech. 2021, 67, 1497–1513. [Google Scholar] [CrossRef] [PubMed]
- Meslema, A.; Bode, F.; Croitoru, C.; Nastase, I. Comparison of turbulence models in simulating jet flow from a cross-shaped orifice. Eur. J. Mech. B/Fluids 2014, 44, 100–120. [Google Scholar] [CrossRef]
- EN 149:2001+A1:2009 (E); Respiratory Protective Devices—Filtering Half Masks to Protect against Particles—Requirements, Testing, Marking. European Committee for Standardization (CEN): Brussels, Belgium, 2009.



| Time (s) | Δx (m) |
|---|---|
| 60 | 3 × 10−5 |
| 900 | 1 × 10−4 |
| 1800 | 1.5 × 10−4 |
| 2700 | 1.8 × 10−4 |
| 3600 | 2.1 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisapia, F.; Rees, D.; Rasekh, M. Design and Evaluation of Face Mask Filtration: Mechanisms, Formulas, and Fluid Dynamics Simulations. Appl. Sci. 2024, 14, 5432. https://doi.org/10.3390/app14135432
Pisapia F, Rees D, Rasekh M. Design and Evaluation of Face Mask Filtration: Mechanisms, Formulas, and Fluid Dynamics Simulations. Applied Sciences. 2024; 14(13):5432. https://doi.org/10.3390/app14135432
Chicago/Turabian StylePisapia, Francesca, David Rees, and Manoochehr Rasekh. 2024. "Design and Evaluation of Face Mask Filtration: Mechanisms, Formulas, and Fluid Dynamics Simulations" Applied Sciences 14, no. 13: 5432. https://doi.org/10.3390/app14135432





