A Preliminary Protocol of Radiographic Image Processing for Quantifying the Severity of Equine Osteoarthritis in the Field: A Model of Bone Spavin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
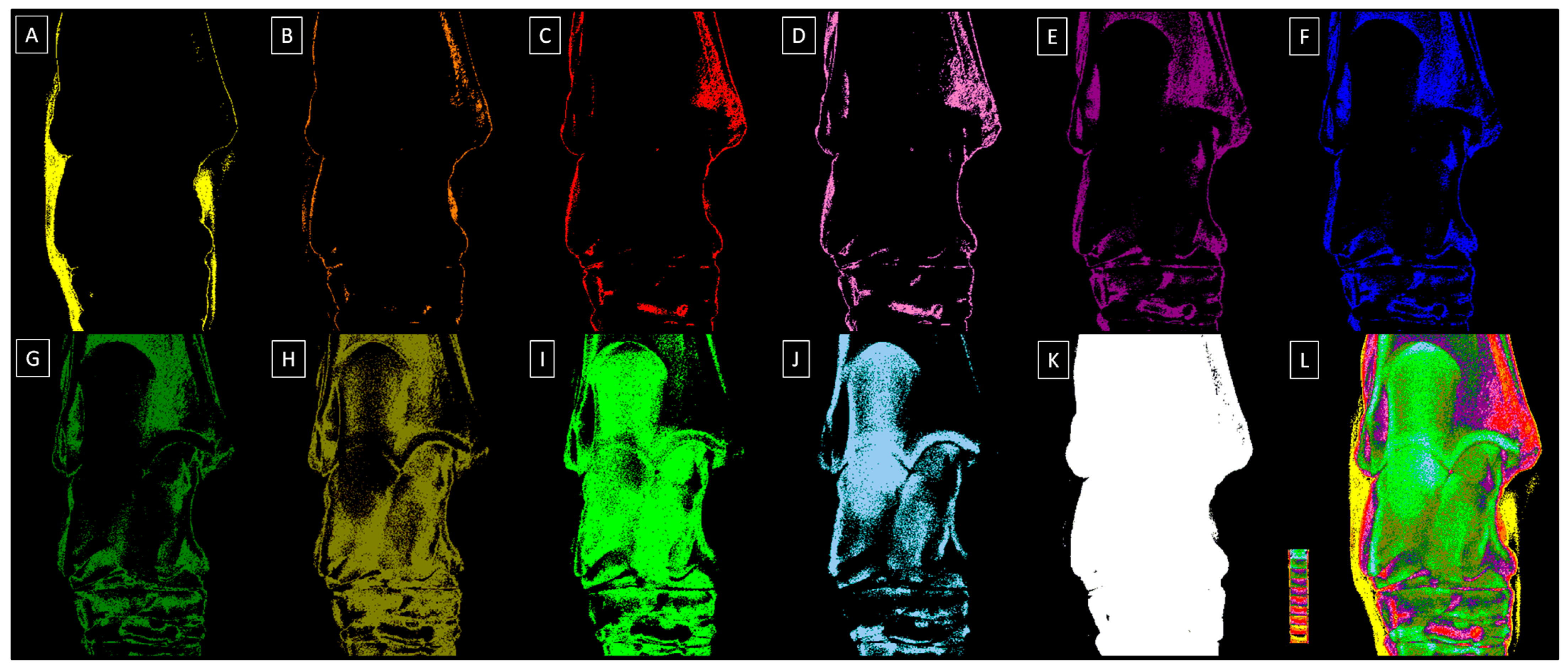
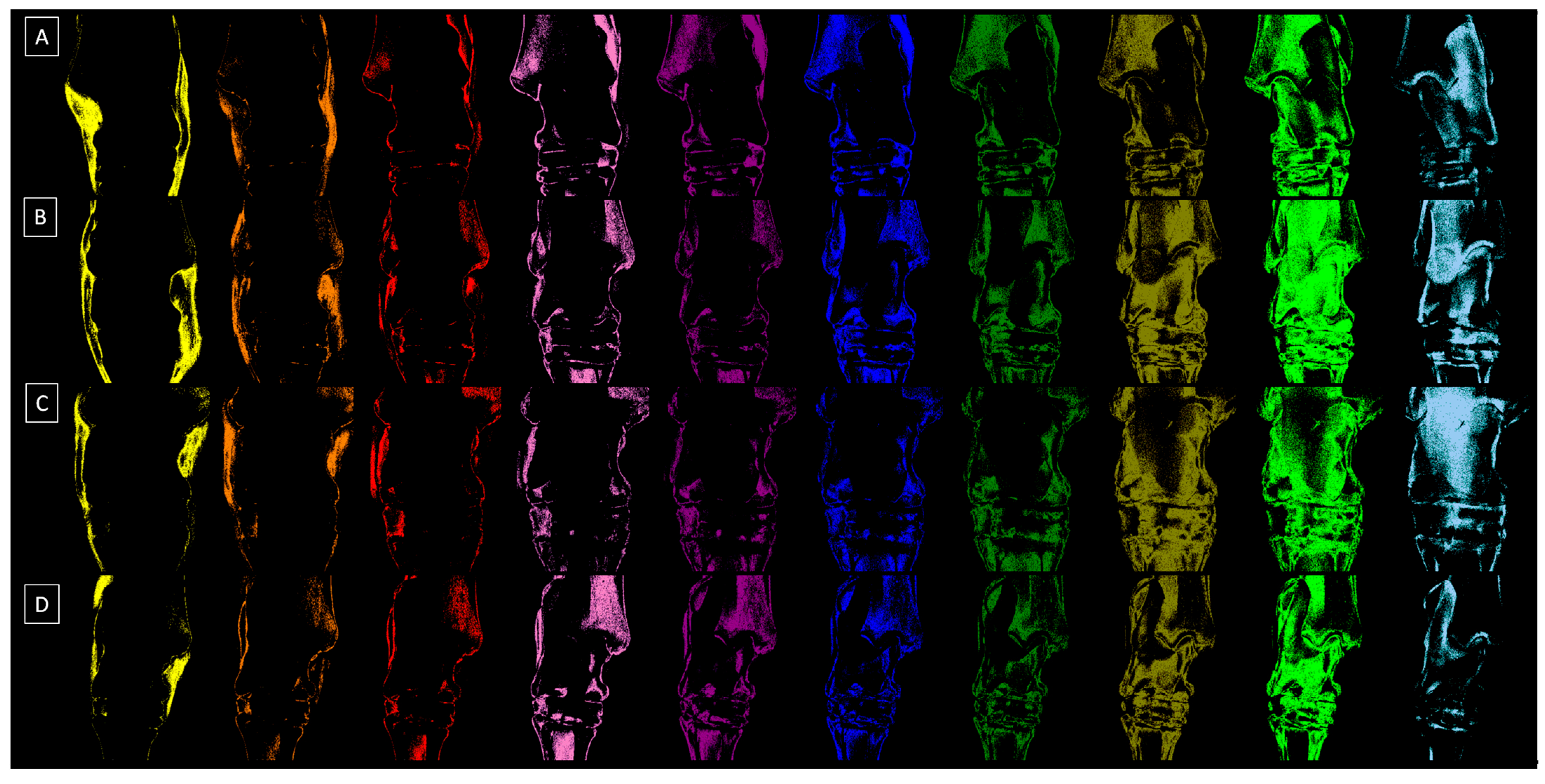
2.2. Radiograph Processing
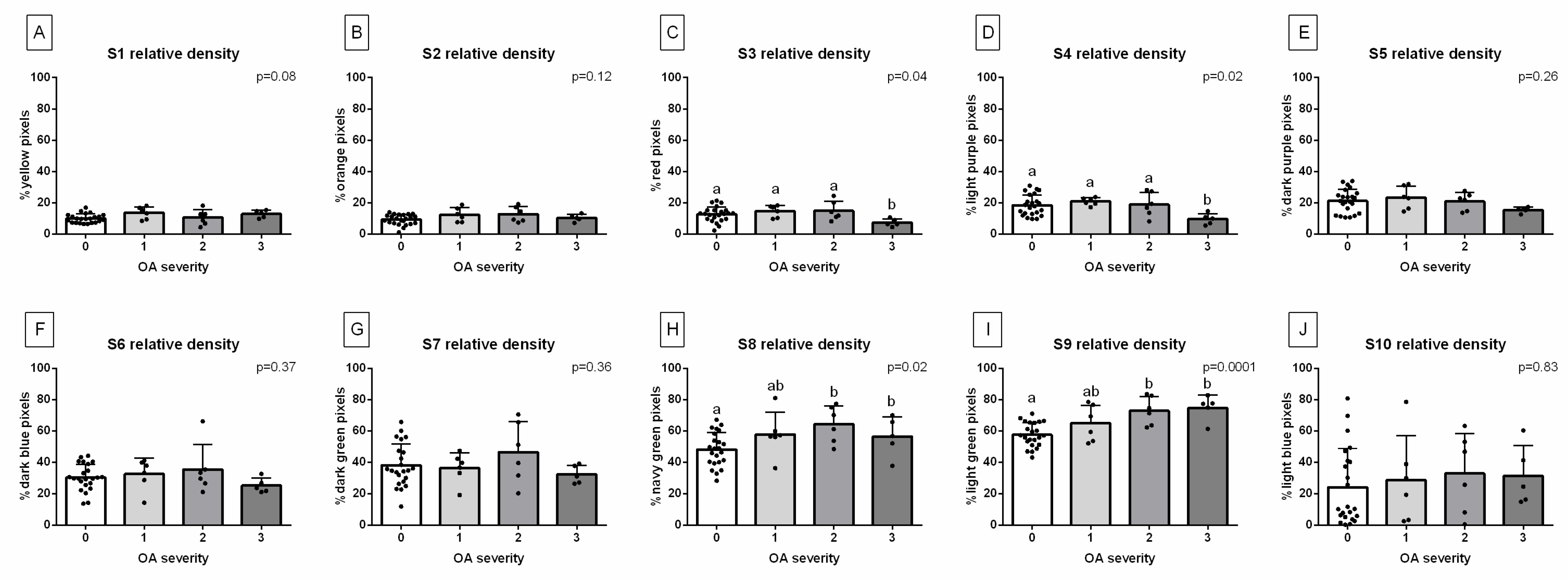
2.3. Radiograph Quantification
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations
4.2. Further Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lutz, W.; Sanderson, W.; Scherbov, S. The coming acceleration of global population ageing. Nature 2008, 451, 716–719. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Scimeca, M.; Piccirilli, E.; Tancredi, V.; Baldi, J.; Gasbarra, E.; Bonanno, E. Sarcopenia: A histological and immunohistochemical study on age-related muscle impairment. Aging Clin. Exp. Res. 2015, 27, 51–60. [Google Scholar] [CrossRef]
- Visconti, V.V.; Cariati, I.; Fittipaldi, S.; Iundusi, R.; Gasbarra, E.; Tarantino, U.; Botta, A. DNA methylation signatures of bone metabolism in osteoporosis and osteoarthritis aging-related diseases: An updated review. Int. J. Mol. Sci. 2021, 22, 4244. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.M.; Creevy, K.E.; Promislow, D.E.L. Mortality in North American dogs from 1984 to 2004: An investigation into age-, size-, and breed-related causes of death. J. Vet. Intern. Med. 2011, 25, 187–198. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Chen, F.L. Assessment and management of declining physical function in aging dogs. Top. Companion Anim. Med. 2022, 51, 100732. [Google Scholar] [CrossRef]
- Bellows, J.; Center, S.; Daristotle, L.; Estrada, A.H.; Flickinger, E.A.; Horwitz, D.F.; Shoveller, A.K. Aging in cats: Common physical and functional changes. J. Feline Med. Surg. 2016, 18, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Ladiges, W. The unrecognized potential of pet cats for studying aging and age-related diseases. Aging Pathobiol. Ther. 2021, 3, 134. [Google Scholar] [CrossRef]
- McGowan, C. Welfare of aged horses. Animals 2011, 1, 366–376. [Google Scholar] [CrossRef]
- Malalana, F.; McGowan, T.W.; Ireland, J.L.; Pinchbeck, G.L.; McGowan, C.M. Prevalence of owner-reported ocular problems and veterinary ocular findings in a population of horses aged ≥15 years. Equine Vet. J. 2019, 51, 212–217. [Google Scholar]
- Masko, M.; Domino, M.; Skierbiszewska, K.; Zdrojkowski, Ł.; Jasinski, T.; Gajewski, Z. Monitoring of the mare during the perinatal period at the clinic and in the stable. Equine Vet. Educ. 2020, 32, 654–663. [Google Scholar] [CrossRef]
- Ireland, J.L. Demographics, Management, Preventive Health Care and Disease in Aged Horses. Vet. Clin. N. Am. Equine Pract. 2016, 32, 195–214. [Google Scholar] [CrossRef]
- Welsh, C.E.; Duz, M.; Parkin, T.D.H.; Marshall, J.F. Prevalence, survival analysis and multimorbidity of chronic diseases in the general veterinarian-attended horse population of the UK. Prev. Vet. Med. 2016, 131, 137–145. [Google Scholar] [CrossRef]
- Innes, J.F.; Clegg, P. Comparative rheumatology: What can be learnt from naturally occurring musculoskeletal disorders in domestic animals? Rheumatology 2010, 49, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Van Weeren, P.R.; Back, W. Musculoskeletal disease in aged horses and its management. Vet. Clin. N. Am. Equine Pract. 2016, 32, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Brugha, T.S. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Long, M.; Dürnberger, C.; Jenner, F.; Kelemen, Z.; Auer, U.; Grimm, H. Quality of Life within Horse Welfare Assessment Tools: Informing Decisions for Chronically Ill and Geriatric Horses. Animals 2022, 12, 1822. [Google Scholar] [CrossRef]
- Grote, C.; Reinhardt, D.; Zhang, M.; Wang, J. Regulatory mechanisms and clinical manifestations of musculoskeletal aging. J. Orthopaed. Res. 2019, 37, 1475–1488. [Google Scholar] [CrossRef]
- Ireland, J.L.; Clegg, P.D.; McGowan, C.M. Factors associated with mortality of geriatric horses in the United Kingdom. Prev. Vet. Med. 2011, 101, 204–218. [Google Scholar] [CrossRef]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: The good, the bad and the promising. Osteoarthr. Cartil. 2019, 27, 230–239. [Google Scholar] [CrossRef]
- Gregory, M.H.; Capito, N.; Kuroki, K.; Stoker, A.M.; Cook, J.L.; Sherman, S.L. A review of translational animal models for knee osteoarthritis. Arthritis 2012, 2012, 764621. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; Cross, M.W.; McIlwraith, C.W. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet. Comp. Orthop. Traumatol. 2006, 19, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Benders, K.E.; Klein, T.J.; de Grauw, J.C.; Kik, M.J.; Hutmacher, D.W. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthr. Cartil. 2012, 20, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Little, C.B.; Zaki, S. What constitutes an “animal model of osteoarthritis”—The need for consensus? Osteoarthr. Cartil. 2012, 20, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.J.; Goodrich, L.R.; Chen, C.T.; Hidaka, C.; Nixon, A.J. Biochemical and biomechanical properties of lesion and adjacent articular cartilage after chondral defect repair in anequine model. Am. J. Sports Med. 2005, 33, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.W.; Van Sickle, D.C. Experimentally induced arthritis of the equine carpus: Histologic and histochemical changes in the articular cartilage. Am. J. Vet. Res. 1981, 42, 209–217. [Google Scholar] [PubMed]
- Eksell, P.; Axelsson, M.; Broström, H.; Ronéus, B.; Hägg-ström, J.; Carlsten, J. Prevalence and risk factors of bone spavin in Icelandic horses in Sweden: A radiographic field study. Acta Vet. Scand. 1998, 39, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.M.; Southwood, L.L.; Dechant, J.E. Diagnosis of distal tarsal osteoarthritis in horses. Comp. Cont. Ed. Pract. Vet. 2003, 2, 138–147. [Google Scholar]
- Bolam, C.J.; Hurtig, M.B.; Cruz, A.; McEwen, B.J. Characterization of experimentally induced post-traumatic osteoarthritis in the medial femorotibial joint of horses. Am. J. Vet. Res. 2006, 67, 433–447. [Google Scholar] [CrossRef]
- De Grauw, J.C.; van de Lest, C.H.; Brama, P.A.; Rambags, B.P.; van Weeren, P.R. In Vivo effects of meloxicam on inflammatory mediators, MMP activity and cartilage biomarkers in equine joints with acute synovitis. Equine Vet. J. 2009, 41, 693–699. [Google Scholar] [CrossRef]
- Bertoni, L.; Jacquet-Guibon, S.; Branly, T.; Legendre, F.; Desancé, M.; Mespoulhes, C.; Audigié, F. An experimentally induced osteoarthritis model in horses performed on both metacarpophalangeal and metatarsophalangeal joints: Technical, clinical, imaging, biochemical, macroscopic and microscopic characterization. PLoS ONE 2020, 15, e0235251. [Google Scholar] [CrossRef] [PubMed]
- Coppelman, E.B.; David, F.H.; Tóth, F.; Ernst, N.S.; Trumble, T.N. The association between collagen and bone biomarkers and radiographic osteoarthritis in the distal tarsal joints of horses. Equine Vet. J. 2020, 52, 391–398. [Google Scholar] [CrossRef]
- Carmalt, J.L.; Bell, C.D.; Panizzi, L.; Wolker, R.R.; Lanovaz, J.L.; Bracamonte, J.L.; Wilson, D.G. Alcohol-facilitated ankylosis of the distal intertarsal and tarsometatarsal joints in horses with osteoarthritis. J. Am. Vet. Med. Assoc. 2012, 240, 199–204. [Google Scholar] [CrossRef]
- Lamas, L.P.; Edmonds, J.; Hodge, W.; Zamora-Vera, L.; Burford, J.; Coomer, R.; Munroe, G. Use of ethanol in the treatment of distal tarsal joint osteoarthritis: 24 cases. Equine Vet. J. 2012, 44, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Björnsdóttir, S.; Axelsson, M.; Eksell, P.; Sigurdsson, H.; Carlsten, J. Radiographic and clinical survey of degenerative joint disease in the distal tarsal joints in Icelandic horses. Equine Vet. J. 2000, 32, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Ley, C.J.; Björnsdóttir, S.; Ekman, S.; Boyde, A.; Hans-son, K. Detection of early osteoarthritis in the centrodistal joints of I celandic horses: Evaluation of radiography and low-field magnetic resonance imaging. Equine Vet. J. 2016, 48, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Raes, E.; Bergman, H.J.; Van Ryssen, B.; Vanderperren, K.; Stock, E.; Saunders, J.H. Computed tomographic features of lesions detected in horses with tarsal lameness. Equine Vet. J. 2014, 46, 189–193. [Google Scholar] [CrossRef]
- Biggi, M.; Dyson, S.J. Use of high-field and low-field magnetic resonance imaging to describe the anatomy of the proximal portion of the tarsal region of nonlame horses. Am. J. Vet. Res. 2018, 79, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Mur, P.; Spriet, M.; Manso-Diaz, G.; Arndt, S.; Perez-Nogues, M.; Lopez-San Roman, J.; Galuppo, L.D. 18F-sodium fluoride positron emission tomography provides pertinent additional information to computed tomography for assessment and management of tarsal pain in horses. J. Am. Vet. Med. Assoc. 2023, 261, 1638–1645. [Google Scholar] [CrossRef]
- Branch, M.V.; Murray, R.C.; Dyson, S.J.; Goodship, A.E. Alteration of distal tarsal subchondral bone thickness pattern in horses with tarsal pain. Equine Vet. J. 2023, 39, 101–105. [Google Scholar] [CrossRef]
- St George, L.B.; Spoormakers, T.J.; Smit, I.H.; Hobbs, S.J.; Clayton, H.M.; Roy, S.H.; Serra Bragança, F.M. Adaptations in equine appendicular muscle activity and movement occur during induced fore-and hindlimb lameness: An electromyographic and kinematic evaluation. Front. Vet. Sci. 2022, 9, 989522. [Google Scholar] [CrossRef]
- Bell, R.A.; Nielsen, B.D.; Waite, K.; Rosenstein, D.; Orth, M. Daily access to pasture turnout prevents loss of mineral in the third metacarpus of Arabian weanlings. J. Anim. Sci. 2001, 79, 1142–1150. [Google Scholar] [CrossRef]
- Firth, E.C.; Rogers, C.W. Musculoskeletal responses of 2–year–old Thoroughbred horses to early training. 7. Bone and articular cartilage response in the carpus. N. Z. Vet. J. 2005, 53, 113–122. [Google Scholar] [CrossRef]
- Vaccaro, C.; Busetto, R.; Bernardini, D.; Anselmi, C.; Zotti, A. Accuracy and precision of computer–assisted analysis of bone density via conventional and digital radiography in relation to dual–energy X–ray absorptiometry. Am. J. Vet. Res. 2012, 73, 381–384. [Google Scholar] [CrossRef]
- Bowen, A.J.; Burd, M.A.; Craig, J.J.; Craig, M. Radiographic calibration for analysis of bone mineral density of the equine third metacarpal bone. J. Equine Vet. Sci. 2013, 33, 1131–1135. [Google Scholar] [CrossRef]
- Yamada, K.; Sato, F.; Higuchi, T.; Nishihara, K.; Kayano, M.; Sasaki, N.; Nambo, Y. Experimental investigation of bone mineral density in Thoroughbreds using quantitative computed tomography. J. Equine Sci. 2015, 26, 81–87. [Google Scholar] [CrossRef]
- Górski, K.; Borowska, M.; Turek, B.; Pawlikowski, M.; Jankowski, K.; Bereznowski, A.; Polkowska, I.; Domino, M. An application of the density standard and scaled–pixel–counting protocol to assess the radiodensity of equine incisor teeth affected by resorption and hypercementosis: Preliminary advancement in dental radiography. BMC Vet. Res. 2023, 19, 116. [Google Scholar] [CrossRef]
- McClure, S.R.; Glickman, L.T.; Glickman, N.W.; Weaver, C.M. Evaluation of dual energy x–ray absorptiometry for in situ measurement of bone mineral density of equine metacarpi. Am. J. Vet. Res. 2001, 62, 752–756. [Google Scholar] [CrossRef] [PubMed]
- El Maghraoui, A.; Roux, C. DXA scanning in clinical practice. QJM 2008, 101, 605–617. [Google Scholar] [PubMed]
- Johnson, T.R. Dual–energy CT: General principles. Am. J. Roentgenol. 2012, 199, S3–S8. [Google Scholar] [CrossRef]
- Ulivieri, F.M.; Rinaudo, L. Beyond bone mineral density: A new dual X–ray absorptiometry index of bone strength to predict fragility fractures, the bone strain index. Front. Med. 2021, 7, 590139. [Google Scholar] [CrossRef]
- Byam-Cook, K.L.; Singer, E.R. Is there a relationship between clinical presentation, diagnostic and radiographic findings and outcome in horses with osteoarthritis of the small tarsal joints? Equine Vet. J. 2009, 41, 118–123. [Google Scholar] [CrossRef]
- Ramos, S.; Pinto, A.; Cardoso, M.; Alexandre, N.; Bettencourt, E.; Monteiro, S.; Gama, L.T. Prevalence of Radiographic Signs of Osteoarthritis in Lusitano Purebred Horses. J. Equine Vet. Sci. 2020, 94, 103196. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ando, K.; Kaneko, M.; Inoue, Y.; Asai, Y.; Taniyama, H. Measurement of equine bone mineral content by radiographic absorptiometry using CR and ortho systems. J. Equine Sci. 2006, 17, 105–112. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ando, K.; Kaneko, M.; Inoue, Y.; Asai, Y.; Taniyama, H. Clinical usefulness of the measurement of bone mineral content by radiographic absorptiometry in the young thoroughbred. J. Equine Sci. 2007, 18, 99–106. [Google Scholar] [CrossRef]
- Taguchi, T.; Morales Yniguez, F.J.; Takawira, C.; Andrews, F.M.; Lopez, M.J. Agmatine Administration Effects on Equine Gastric Ulceration and Lameness. J. Clin. Med. 2022, 11, 7283. [Google Scholar] [CrossRef]
- American Association of Equine Practitioners. LAMENESS EXAMS: Evaluating the Lame Horse. Available online: https://aaep.org/horsehealth/lameness-exams-evaluating-lame-horse (accessed on 15 October 2023).
- Borowska, M.; Turek, B.; Lipowicz, P.; Jasiński, T.; Skierbiszewska, K.; Domino, M. The quantification of the radiological features of osteoarthritis using scaled–pixel–counting protocol on the model of spavin in the horse’s tarsal joint. In Proceedings of the 33rd Polish Conference on Biocybernetics and Biomedical Engineering, Łódź, Polska, 27–29 September 2023; p. 109. [Google Scholar]
- Driesang, I.; Böhm, D. Spavin in horses--clinical, radiological and scintigraphic findings. Tierarztl. Prax. 1993, 21, 141–148. [Google Scholar]
- Denoix, J.M. Imaging the hock. J. Equine Vet. Sci. 2000, 20, 713. [Google Scholar]
- Ross, M.W.; Dyson, S.J. Diagnosis and Management of Lameness in the Horse; Elsevier Health Sciences: Philadelphia, PA, USA, 2010; pp. 59–60. [Google Scholar]
- Maśko, M.; Borowska, M.; Sikorska, U.; Ciesielska, A.; Zdrojkowski, Ł.; Domino, M. Quantification of the Area of the Highest Temperature in Equine Infrared Images. Appl. Sci. 2023, 13, 11006. [Google Scholar] [CrossRef]
- Sharma, G.; Bala, R. Digital Color Imaging Handbook; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-351-83597-8. [Google Scholar]
- Labens, R.; Voûte, L.C.; Mellor, D.J. Retrospective study of the effect of intra-articular treatment of osteoarthritis of the distal tarsal joints in 51 horses. Vet. Rec. 2007, 161, 611–616. [Google Scholar] [CrossRef]
- Brounts, S.H.; Henry, T.; Lund, J.R.; Whitton, R.C.; Ergun, D.L.; Muir, P. Use of a novel helical fan beam imaging system for computed tomography of the head and neck in sedated standing horses: 120 cases (2019–2020). J. Am. Vet. Med. Assoc. 2022, 260, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Stieger-Vanegas, S.M.; Hanna, A.L. The role of computed tomography in imaging non-neurologic disorders of the head in equine patients. Front. Vet. Sci. 2022, 9, 798216. [Google Scholar] [CrossRef]
- Dechant, J.E.; Baxter, G.M.; Southwood, L.L.; Crawford, W.H.; Jackman, B.R.; Stashak, T.S.; Trotter, G.W.; Hendrickson, D.A. Use of a three-drill-tract technique for arthrodesis of the distal tarsal joints in horses with distal tarsal osteoarthritis: 54 cases (1990–1999). J. Am. Vet. Med. Assoc. 2003, 223, 1800–1805. [Google Scholar] [CrossRef]
- Zubrod, C.J.; Schneider, R.K.; Hague, B.A.; Ragle, C.A.; Gavin, P.R.; Kawcak, C.E. Comparison of three methods for arthrodesis of the distal intertarsal and tarsometatarsal joints in horses. Vet. Surg. 2005, 34, 372–382. [Google Scholar] [CrossRef]
- Labens, R.; Innocent, G.T.; Voûte, L.C. Reliability of a quantitative rating scale for assessment of horses with distal tarsal osteoarthritis. Vet. Radiol. Ultrasound 2007, 48, 204–211. [Google Scholar] [CrossRef]
- Chougule, V.N.; Mulay, A.; Ahuja, B.B. Clinical case study: Spine modeling for minimum invasive spine surgeries (MISS) using rapid prototyping. Bone 2018, 226, 3071. [Google Scholar]
- Borowska, M.; Lipowicz, P.; Daunoravičienė, K.; Turek, B.; Jasiński, T.; Pauk, J.; Domino, M. Three-Dimensional Segmentation of Equine Paranasal Sinuses in Multidetector Computed Tomography Datasets: Preliminary Morphometric Assessment Assisted with Clustering Analysis. Sensors 2024, 24, 3538. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, T.; Turek, B.; Kaczorowski, M.; Brehm, W.; Skierbiszewska, K.; Bonecka, J.; Domino, M. Equine Models of Temporomandibular Joint Osteoarthritis: A Review of Feasibility, Biomarkers, and Molecular Signaling. Biomedicines 2024, 12, 542. [Google Scholar] [CrossRef]
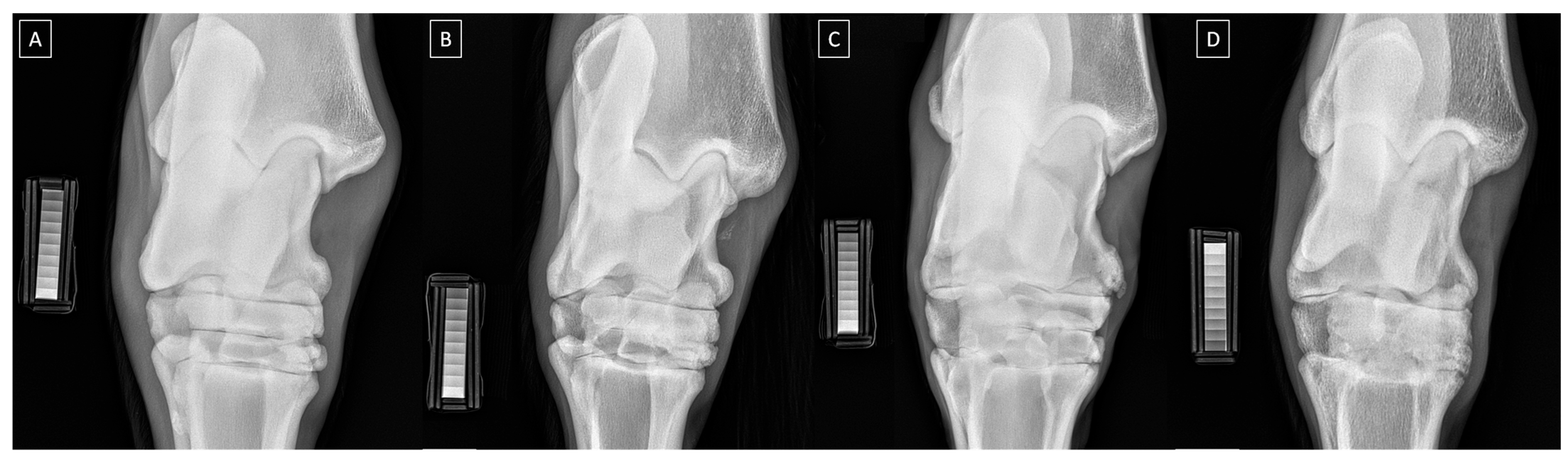

| Grade | Clinical Symptoms |
|---|---|
| 0 | Lameness that is not perceptible |
| 1 | Lameness that is difficult to observe regardless of the circumstances |
| 2 | Lameness that is difficult to observe at a walk; lameness that is difficult to observe when trotting in a straight line; lameness that is observable when trotting in a circle, on an incline, on a hard surface, or under weight-carrying conditions |
| 3 | Lameness that is observable at a trot regardless of the circumstances |
| 4 | Lameness that is observable at a walk |
| 5 | Lameness that is strongly observable at a walk; minimal weight bearing in motion and/or at rest or a complete inability to move |
| Grade | Severity | Radiographic Signs |
|---|---|---|
| 0 | Normal | Normal width and shape of the joint space; smooth cortical bone surface; normal subchondral bone pattern; no periosteal proliferation; no intra-articular mineralization |
| 1 | Mild | Narrow and irregular joint space with osteophytes; irregular cortical bone surface with well-defined protuberance; smooth subchondral bone pattern; flat periosteal proliferation; mild intra-articular mineralization |
| 2 | Moderate | Narrow and irregular joint space with multiple osteophytes, enthesiophytes, and marked asymmetry; irregular cortical bone surface with well-defined bone proliferation; subchondral bone cyst; flat periosteal proliferation; moderate intra-articular mineralization |
| 3 | Severe | Completely narrow joint space with large osteophytes and enthesiophytes; severe deformation of cortical bone surface; subchondral bone sclerosis; flat or intense periosteal proliferation; severe intra-articular mineralization |
| Decomposition | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Relative density (HU) | 1009 ± 163 | 1212 ± 111 | 1407 ± 98 | 1600 ± 134 | 1804 ± 112 | 2011 ± 99 | 2204 ± 107 | 2400 ± 133 | 2607 ± 129 | 2803 ± 147 |
| Color | Yellow | Orange | Red | Light purple | Dark purple | Dark blue | Dark green | Navy green | Light green | Light blue |
| HEX code | #FFFF00 | #E08000 | #FF0000 | #E080C0 | #800080 | #0000FF | #008000 | #808000 | #00FF00 | #A6CAF0 |
| No | Sex | Breed | Lameness Score * | Age | Training Background | OA Severity Scores |
|---|---|---|---|---|---|---|
| 1 | M | FTH | Left 2; Right 2 | 34 years | Leisure work for 18 years | Left 0; Right 0 |
| 2 | M | SH | Left 3; Right 3 | 35 years | Leisure work for 13 years | Left 0; Right 0 |
| 3 | M | MBW | Left 4; Right 4 | 28 years | Leisure work for 14 years | Left 0; Right 3 |
| 4 | M | PHH | Left 2; Right 2 | 28 years | Leisure work for 15 years | Left 2; Right 0 |
| 5 | M | SH | Left 3; Right 3 | 33 years | Leisure work for 14 years | Left 0; Right 0 |
| 6 | M | PHH | Left 2; Right 2 | 19 years | Leisure work for 12 years | Left 0; Right 2 |
| 7 | M | SH | Left 3; Right 3 | 21 years | Leisure work for 11 years | Left 2; Right 2 |
| 8 | G | PHH | Left 3; Right 3 | 26 years | Leisure work for 19 years | Left 0; Right 3 |
| 9 | G | PHH | Left 4; Right 4 | 22 years | Leisure work for 16 years | Left 3; Right 3 |
| 10 | M | PHH | Left 2; Right 2 | 15 years | Leisure work for 10 years | Left 0; Right 0 |
| 11 | M | MBW | Left 3; Right 3 | 25 years | Leisure work for 14 years | Left 0; Right 0 |
| 12 | M | FTH | Left 2; Right 2 | 23 years | Leisure work for 16 years | Left 0; Right 3 |
| 13 | M | PHH | Left 3; Right 3 | 27 years | Leisure work for 12 years | Left 0; Right 0 |
| 14 | G | PHH | Left 2; Right 2 | 32 years | Leisure work for 17 years | Left 1; Right 1 |
| 15 | G | PHH | Left 2; Right 2 | 26 years | Leisure work for 15 years | Left 0; Right 0 |
| 16 | G | PHH | Left 4; Right 4 | 28 years | Leisure work for 15 years | Left 2; Right 2 |
| 17 | G | PHH | Left 3; Right 3 | 22 years | Leisure work for 16 years | Left 1; Right 1 |
| 18 | G | PHH | Left 2; Right 2 | 18 years | Leisure work for 11 years | Left 1; Right 1 |
| 19 | G | PHH | Left 3; Right 3 | 27 years | Leisure work for 14 years | Left 0; Right 0 |
| 20 | G | PHH | Left 2; Right 2 | 24 years | Leisure work for 15 years | Left 0; Right 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turek, B.; Borowska, M.; Jankowski, K.; Skierbiszewska, K.; Pawlikowski, M.; Jasiński, T.; Domino, M. A Preliminary Protocol of Radiographic Image Processing for Quantifying the Severity of Equine Osteoarthritis in the Field: A Model of Bone Spavin. Appl. Sci. 2024, 14, 5498. https://doi.org/10.3390/app14135498
Turek B, Borowska M, Jankowski K, Skierbiszewska K, Pawlikowski M, Jasiński T, Domino M. A Preliminary Protocol of Radiographic Image Processing for Quantifying the Severity of Equine Osteoarthritis in the Field: A Model of Bone Spavin. Applied Sciences. 2024; 14(13):5498. https://doi.org/10.3390/app14135498
Chicago/Turabian StyleTurek, Bernard, Marta Borowska, Krzysztof Jankowski, Katarzyna Skierbiszewska, Marek Pawlikowski, Tomasz Jasiński, and Małgorzata Domino. 2024. "A Preliminary Protocol of Radiographic Image Processing for Quantifying the Severity of Equine Osteoarthritis in the Field: A Model of Bone Spavin" Applied Sciences 14, no. 13: 5498. https://doi.org/10.3390/app14135498






