Activated Biochar-Amended Phytoextraction of Selenium in Contaminated Soil under Cold Climate in Northern Québec (Canada)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Production and Characterization
2.2. Experimental Soil Collection, Analysis and Incubation
2.3. Pot Experiment and Plant Growth
2.4. Total Selenium Analysis in Soil and Plants
2.5. Bioaccumulation Factor (BAF)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Biochar Production and Characterization
3.2. Effect of the Addition of Biochar Materials on Selenium Mobility in Soil
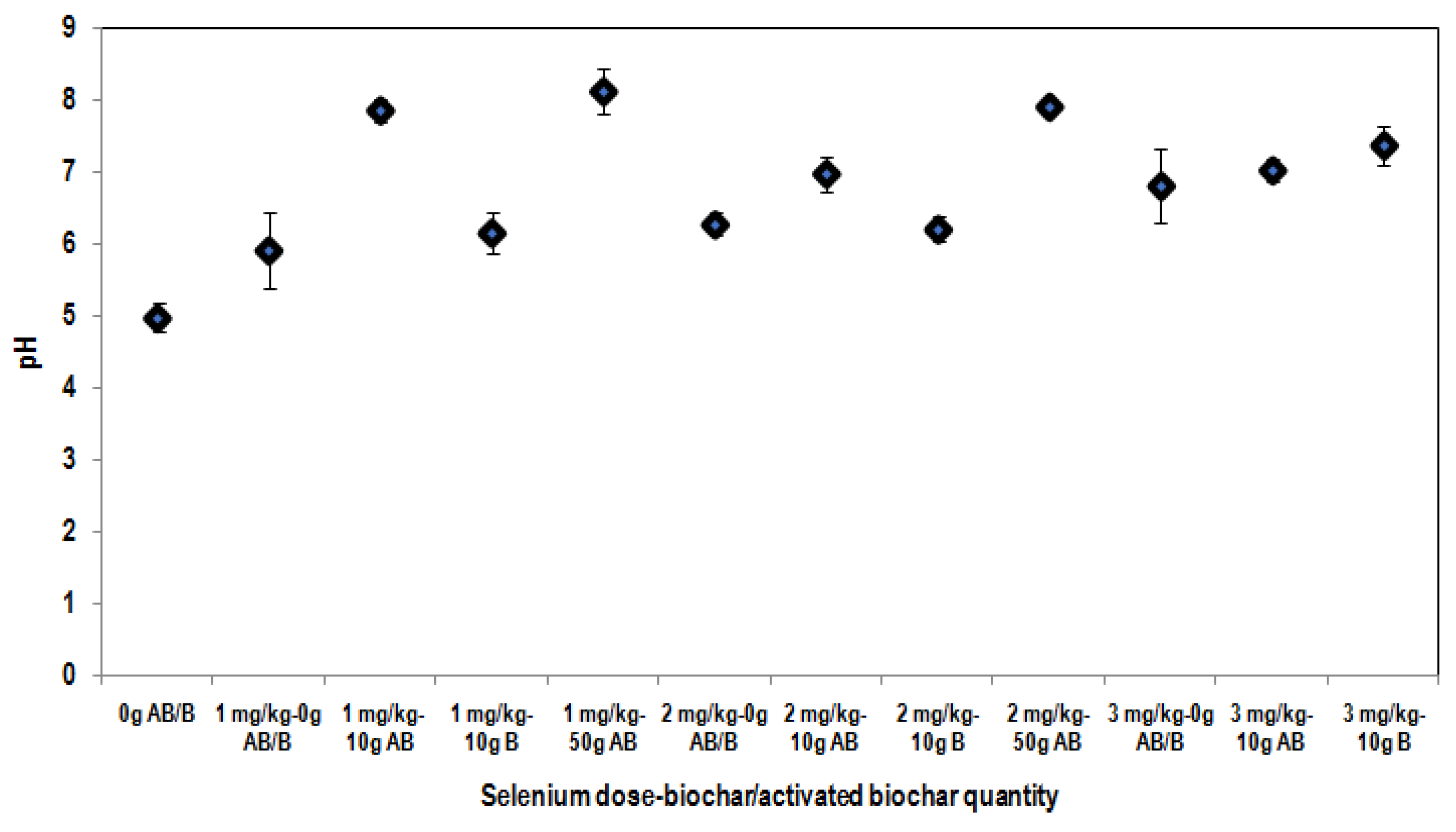
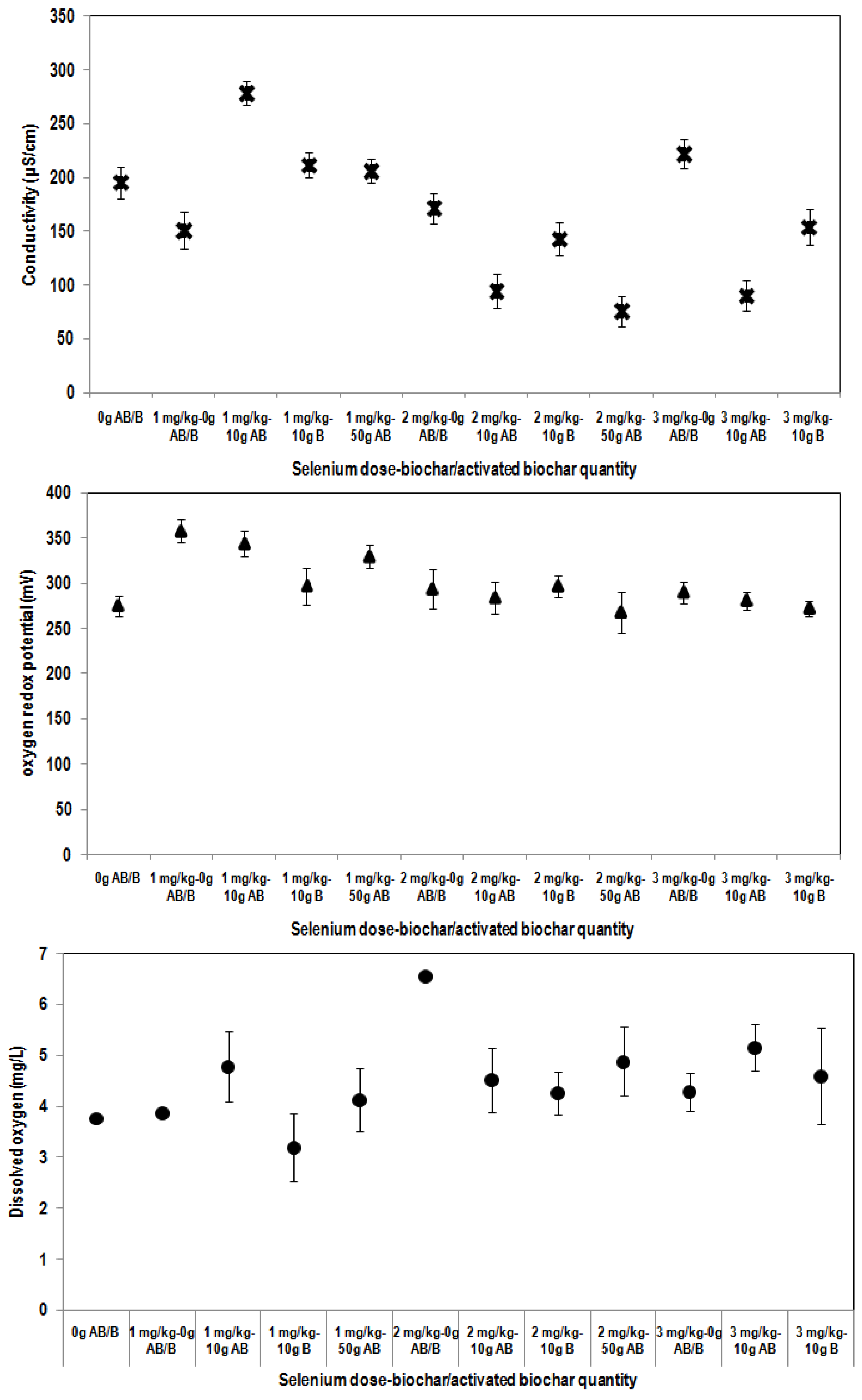
3.3. Effect of the Addition of Biochar Materials on Soil Parameters
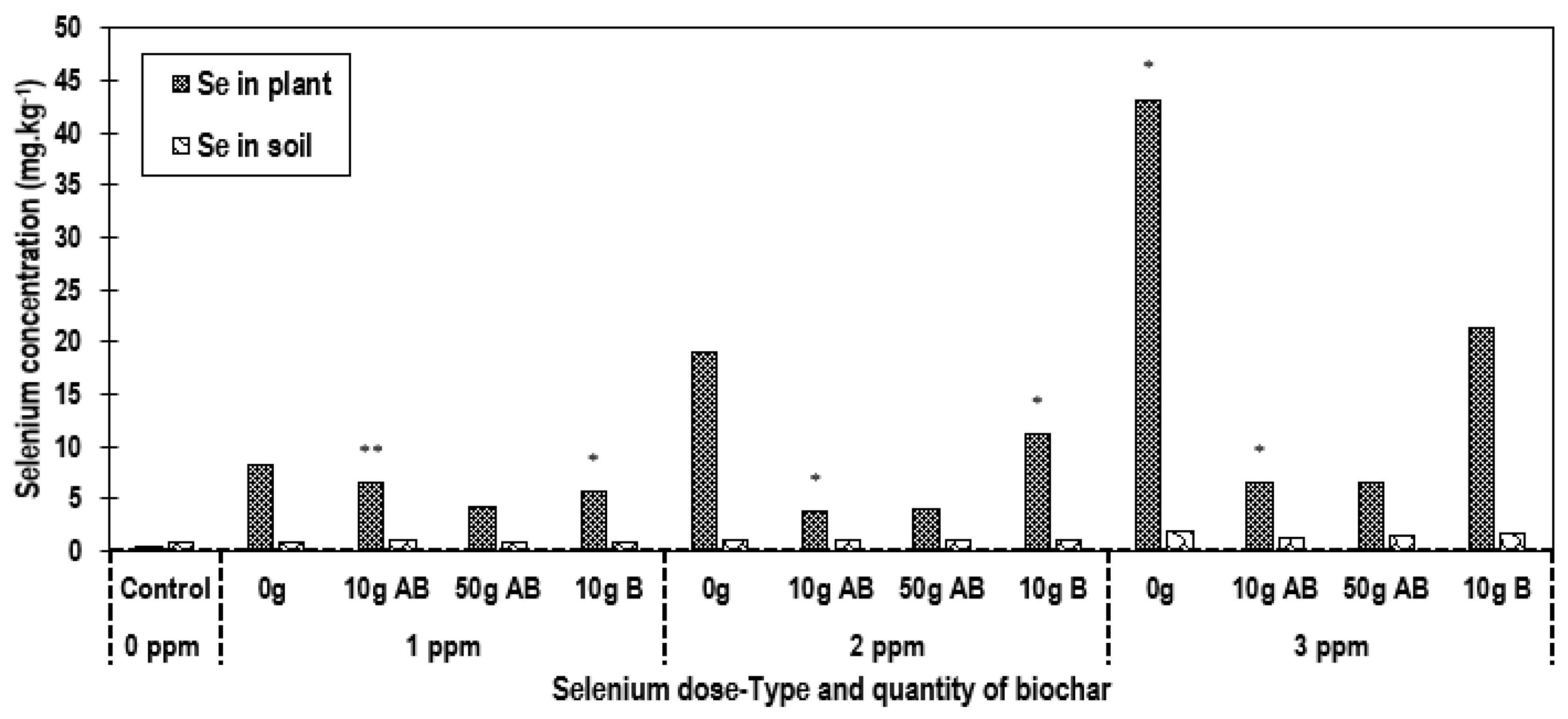
3.4. Effect of the Addition of Biochar Materials on Se Uptake from Soil and Its Phytoextraction
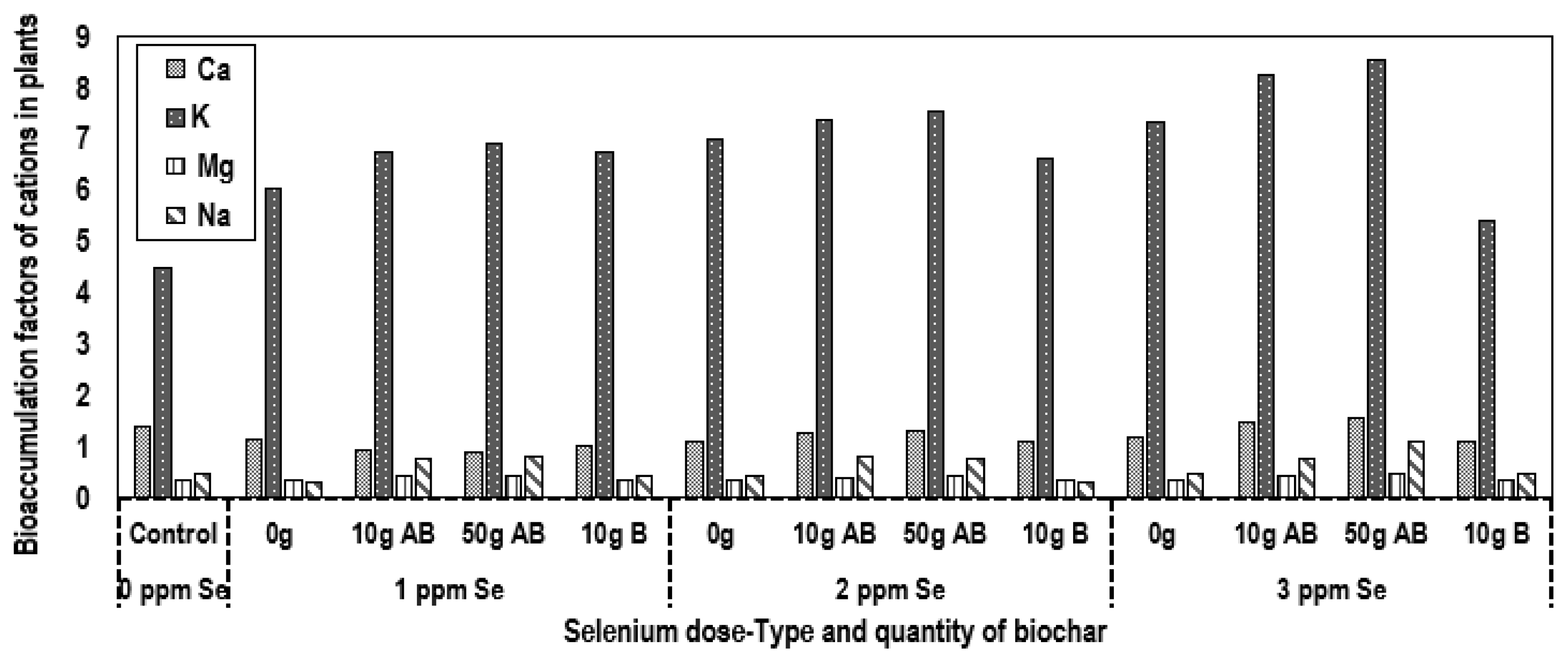
3.5. Potential for Heavy Metals and Cations Bioaccumulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MDDELCC. Directive 019 sur L’industrie Minière; Gouvernement du Québec: Québec City, QC, Canada, 2012.
- Anae, J.; Ahmad, N.; Kumar, V.; Thakur, V.K.; Gutierrez, T.; Yang, X.J.; Cai, C.; Yang, Z.; Coulon, F. Recent advances in biochar engineering for soil contaminated with complex chemical mixtures: Remediation strategies and future perspective. Sci. Total Environ. 2021, 767, 144351. [Google Scholar] [CrossRef]
- Li, K.; Yang, B.; Wang, H.; Xu, X.; Gao, Y.; Zhu, Y. Dual effects of biochar and hyperaccumulator Solanum nigrum L. on the remediation of Cd-contaminated soil. PeerJ 2019, 7, e6631. [Google Scholar] [CrossRef]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A Green Approach to Contaminant Containment. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2011; Volume 112, pp. 145–204. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Rasooli, S.; Yousefirad, M.; Taghavi, L. Selenium phytoremediation study of contaminated soils by Vetiveria zizanioides and Medicago sativa. Environ. Conserv. J. 2015, 16, 429–434. [Google Scholar] [CrossRef]
- Supriatin, S.; Weng, L.; Comans, R.N.J. Selenium-rich dissolved organic matter determines selenium uptake in wheat grown on Low-selenium arable land soils. Plant Soil 2016, 408, 73–94. [Google Scholar] [CrossRef]
- Fellet, G.; Marmiroli, M.; Marchiol, L. Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci. Total Environ. 2014, 468–469, 598–608. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a con-taminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef]
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef]
- Ahmed, A.; Kurian, J.; Raghavan, V. Biochar influences on agricultural soils, crop production, and the environment: A review. Environ. Rev. 2016, 24, 495–502. [Google Scholar] [CrossRef]
- He, T.; Meng, J.; Chen, W.; Liu, Z.; Cao, T.; Cheng, X.; Huang, Y.; Yang, X. Effects of biochar on cadmium accumulation in rice and cadmium fractions of soil: A three-year pot experiment. BioResources 2016, 12, 622–642. [Google Scholar] [CrossRef]
- Pourret, O.; Houben, D. Characterization of metal binding sites onto biochar using rare earth elements as a fingerprint. Heliyon 2018, 4, e00543. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.-L.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Zhang, X.; Qi, H.; Ma, J.; Xiao, Y.; et al. Adsorption characteristics of Pb(II) using biochar derived from spent mushroom substrate. Sci. Rep. 2019, 9, 15999. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, P.; Bogusz, A.; Dobrzyńska, J.; Dobrowolski, R.; Oleszczuk, P. Engineered biochar modified with iron as a new adsorbent for treatment of water contaminated by selenium. J. Saudi Chem. Soc. 2020, 24, 824–834. [Google Scholar] [CrossRef]
- Winkel, L.; Vriens, B.; Jones, G.; Schneider, L.; Pilon-Smits, E.; Bañuelos, G. Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, H.; Tang, X.; Guan, Z.; Reid, B.J.; Rajapaksha, A.U.; Ok, Y.S.; Sun, H. Biochar increased water holding capacity but accelerated organic carbon leaching from a sloping farmland soil in China. Environ. Sci. Pollut. Res. 2016, 23, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Chen, S.; Wang, R.; Xu, P.; Cheng, M.; Zhang, C.; Xue, W. Biochar facilitated the phytoremediation of cadmium contaminated sediments: Metal behavior, plant toxicity, and microbial activity. Sci. Total Environ. 2019, 666, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A review of adsorbents for heavy metal decontamination: Growing approach to wastewater treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- ASTM D3838-05; Test Method for pH of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M. Fundamentals of the theory of adsorption in micropores of carbon adsorbents: Characteristics of their adsorption properties and microporous structures. Carbon 1989, 27, 457–467. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity, 2nd ed.; Academic Press: London, UK; New York, NY, USA, 1982. [Google Scholar]
- U.S. EPA. Method 200.2: Sample Preparation Procedure for Spectrochemical Determination of Total Recoverable Elements; Revision 2.8; Environmental Protection Agency: Cincinnati, OH, USA, 1994.
- U.S. EPA. Method 200.3: Sample Preparation Procedure For Spectrochemical Determination of Total Recoverable Elements In Biological Tissues; Revision 1.0; Environmental Protection Agency: Cincinnati, OH, USA, 1991.
- U.S. EPA. Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-Mass Spectrometry; Revision 5.4; Environmental Protection Agency: Cincinnati, OH, USA, 1994.
- Aladesanmi, O.T.; Oroboade, J.G.; Osisiogu, C.P.; Osewole, A.O. Bioaccumulation factor of selected heavy metals in Zea mays. J. Health Pollut. 2019, 9, 191207. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, F.L.; Bouafif, H.; Hamza, N.; Bouslimi, B.; Neculita, C.M.; Koubaa, A. The influence of pilot-scale pyro-gasification and activation conditions on porosity development in activated biochars. Biomass Bioenergy 2018, 118, 105–114. [Google Scholar] [CrossRef]
- Zhang, W.; Cho, Y.; Vithanage, M.; Shaheen, S.M.; Rinklebe, J.; Alessi, D.S.; Hou, C.-H.; Hashimoto, Y.; Withana, P.A.; Ok, Y.S. Arsenic removal from water and soils using pristine and modified biochars. Biochar 2022, 4, 55. [Google Scholar] [CrossRef]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J.C. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.-S.; Wu, J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Uchimiya, M.; Klasson, K.T.; Wartelle, L.H.; Lima, I.M. Influence of soil properties on heavy metal sequestration by biochar amendment: 1. Copper desorption isotherms. Chemosphere 2011, 82, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, Y.; Jin, F.; McMillan, O.; Al-Tabbaa, A. Qualitative and quantitative characterisation of adsorption mechanisms of lead on four biochars. Sci. Total Environ. 2017, 609, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Břendová, K.; Tlustoš, P.; Száková, J. Biochar immobilizes cadmium and zinc and improves phytoextraction potential of willow plants on extremely contaminated soil. Plant Soil Environ. 2015, 61, 303–308. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef] [PubMed]
- Saha, U. Selenium in the soil-plant environment: A review. Int. J. Appl. Agric. Sci. 2017, 3, 1. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Q.; Liang, D.; Song, W.; Lei, L.; Yu, D. Effects of nitrogen application on selenium uptake, translocation and distribution in winter wheat. Environ. Sci. 2017, 38, 825–831. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Etteieb, S.; Magdouli, S.; Komtchou, S.P.; Zolfaghari, M.; Tanabene, R.; Brar, K.K.; Calugaru, L.L.; Brar, S.K. Selenium speciation and bioavailability from mine discharge to the environment: A field study in Northern Quebec, Canada. Environ. Sci. Pollut. Res. 2021, 28, 50799–50812. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium biofortification and interaction with other elements in plants: A review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef]
- Hartley, W.; Dickinson, N.M.; Riby, P.; Lepp, N.W. Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ. Pollut. 2009, 157, 2654–2662. [Google Scholar] [CrossRef]
- Lebrun, M.; Macri, C.; Miard, F.; Hattab-Hambli, N.; Motelica-Heino, M.; Morabito, D.; Bourgerie, S. Effect of biochar amendments on As and Pb mobility and phytoavailability in contaminated mine technosols phytoremediated by Salix. J. Geochem. Explor. 2017, 182, 149–156. [Google Scholar] [CrossRef]
- Narayanan, M.; Ma, Y. Influences of biochar on bioremediation/phytoremediation potential of metal-contaminated soils. Front. Microbiol. 2022, 13, 929730. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zeng, G.; Wu, H.; Liang, J.; Zhang, C.; Dai, J.; Xiong, W.; Song, B.; Wu, S.; Yu, J. The effects of activated biochar addition on remediation efficiency of co-composting with contaminated wetland soil. Resour. Conserv. Recycl. 2019, 140, 278–285. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]



| B | AB | |
|---|---|---|
| Textural properties | ||
| SBET (m2 g−1) | 177 | 590 |
| Vt (cm3 g−1) | - | 0.34 |
| Vµ (cm3 g−1) | 0.11 | 0.23 |
| Vm (cm3 g−1) | - | 0.11 |
| Physicochemical properties | ||
| pH | 5.9 | 8.6 |
| pHPZC | 6.6 | 9.0 |
| C (%) | 75.4 | 89.1 |
| H (%) | 3.5 | 0.6 |
| N (%) | 0.9 | 0.2 |
| S (%) | 0.5 | 0.0 |
| O (%) | 19.7 | 10.1 |
| Soil Parameters | Correlation Coefficient with Se in Plant |
|---|---|
| pH | 0.713 ** |
| Oxygen redox potential | 0.691 ** |
| Organic matter | 0.606 * |
| Calcium | 0.719 ** |
| Potassium | 0.675 ** |
| Magnesium | 0.72 ** |
| Sodium | 0.711 ** |
| Selenium | 0.759 ** |
| Aluminum | 0.688 ** |
| Chromium | 0.669 ** |
| Iron | 0.736 ** |
| Manganese | 0.691 ** |
| Cobalt | 0.698 ** |
| Lead | 0.634 * |
| Chloride | 0.538 * |
| Sulfate | 0.77 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etteieb, S.; Braghiroli, F.; Robert, É.; Magdouli, S.; Kaur Brar, S.; Blais, J.-F. Activated Biochar-Amended Phytoextraction of Selenium in Contaminated Soil under Cold Climate in Northern Québec (Canada). Appl. Sci. 2024, 14, 5596. https://doi.org/10.3390/app14135596
Etteieb S, Braghiroli F, Robert É, Magdouli S, Kaur Brar S, Blais J-F. Activated Biochar-Amended Phytoextraction of Selenium in Contaminated Soil under Cold Climate in Northern Québec (Canada). Applied Sciences. 2024; 14(13):5596. https://doi.org/10.3390/app14135596
Chicago/Turabian StyleEtteieb, Selma, Flavia Braghiroli, Émilie Robert, Sara Magdouli, Satinder Kaur Brar, and Jean-François Blais. 2024. "Activated Biochar-Amended Phytoextraction of Selenium in Contaminated Soil under Cold Climate in Northern Québec (Canada)" Applied Sciences 14, no. 13: 5596. https://doi.org/10.3390/app14135596





