Crestal Sinus Lift with the Hydrodynamic Technique: Prospective Clinical Study at 3 Years Follow-Up
Abstract
1. Introduction
2. Materials and Methods
- Age > 18 years old;
- Single edentulous at the posterior maxilla with a residual bone height less than 5 mm;
- Requiring implant-prosthetic rehabilitation;
- The absence of any contraindication to dental implant placement or sinus augmentation [21];
- Compliance with follow-up monitoring and professional oral hygiene maintenance protocol.
- Absolute contraindications to dental implant placement;
- Bisphosphonate medication [22];
- Head and neck radiotherapy within the past year [23];
- Uncompensated systemic disorders [24];
- Smoker patients [25];
- Inability to adhere to protocol checks;
- Failure to maintain regular oral hygiene sessions;
- Economic infeasibility in affording the treatment.
- 1.
- The Implant Survival Rate
- 2.
- Marginal Bone Loss (MBL)
- 3.
- Surgical Complications
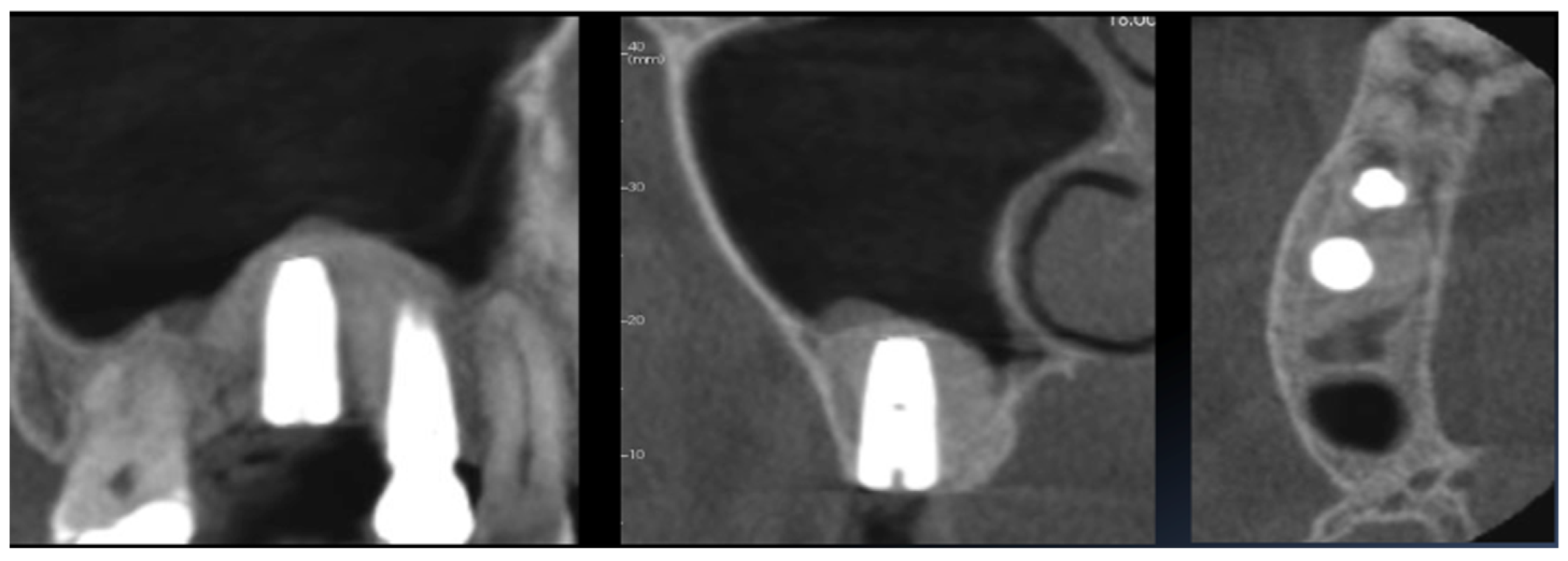
3. Results
- 1.
- The Implant Survival Rate
- 2.
- Marginal Bone Loss (MBL)
- 3.
- Surgical Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capparè, P.; Tetè, G.; D’Orto, B.; Nagni, M.; Gherlone, E.F. Immediate Loaded Full-Arch Mandibular Rehabilitations in Younger vs. Elderly Patients: A Comparative Retrospective Study with 7-Year Follow-Up. J. Clin. Med. 2023, 12, 4524. [Google Scholar] [CrossRef] [PubMed]
- Nagni, M.; Pirani, F.; D’Orto, B.; Ferrini, F.; Cappare, P. Clinical and Radiographic Follow-Up of Full-Arch Implant Prosthetic Rehabilitations: Retrospective Clinical Study at 6-Year Follow-Up. Appl. Sci. 2023, 13, 11143. [Google Scholar] [CrossRef]
- D’Orto, B.; Chiavenna, C.; Leone, R.; Longoni, M.; Nagni, M.; Capparè, P. Marginal Bone Loss Compared in Internal and External Implant Connections: Retrospective Clinical Study at 6-Years Follow-Up. Biomedicines 2023, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Al Amri, M.D. Influence of interimplant distance on the crestal bone height around dental implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2016, 115, 278–282.e1. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Barootchi, S.; Avila-Ortiz, G.; Urban, I.A.; Giannobile, W.V.; Wang, H.L. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: A systematic review and network meta-analysis. J. Periodontol. 2021, 92, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Taschieri, S.; Del Fabbro, M. Long-term outcomes for the treatment of atrophic posterior maxilla: A systematic review of literature. Clin. Implant. Dent. Relat. Res. 2015, 17, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Alghamdi, R.; Guallart, I.F.; Bergamini, M.; Yu, P.Y.; Froum, S.J.; Cho, S.C. Patient-Related Risk Factors for Maxillary Sinus Augmentation Procedures: A Systematic Literature Review. Int. J. Periodontics Restor. Dent. 2021, 41, e121–e128. [Google Scholar] [CrossRef]
- Lie, S.A.N.; Claessen, R.M.M.A.; Leung, C.A.W.; Merten, H.A.; Kessler, P.A.W.H. Non-grafted versus grafted sinus lift procedures for implantation in the atrophic maxilla: A systematic review and meta-analysis of randomized controlled trials. Int. J. Oral. Maxillofac. Surg. 2022, 51, 122–132. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Xu, J.; Hua, Z. Clinical evaluation of maxillary sinus floor elevation with or without bone grafts: A systematic review and meta-analysis of randomised controlled trials with trial sequential analysis. Arch. Med. Sci. 2024, 20, 384–401. [Google Scholar] [CrossRef]
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral. Surg. 1980, 38, 613–616. [Google Scholar]
- Tatum, H., Jr. Maxillary and sinus implant reconstructions. Dent. Clin. N. Am. 1986, 30, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Andrés-García, R.; Ríos-Santos, J.V.; Herrero-Climent, M.; Bullón, P.; Fernández-Farhall, J.; Gómez-Menchero, A.; Fernández-Palacín, A.; Ríos-Carrasco, B. Sinus Floor Elevation via an Osteotome Technique without Biomaterials. Int. J. Environ. Res. Public Health 2021, 18, 1103. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.V.; Suvvari, N.; Reddy, A.A. Trephine Core Procedure Versus Bone-Added Osteotome Sinus Floor Elevation in the Augmentation of the Sinus Floor: A Comparative Clinical and Radiographic Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Cosci, F.; Luccioli, M. A new sinus lift technique in conjunction with placement of 265 implants: A 6-year retrospective study. Implant. Dent. 2000, 9, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ye, G.; Zhao, F.; Wang, B.; Yu, M.; Wang, H. Endoscope-controlled maxillary sinus floor elevation: A review of the literature. Br. J. Oral. Maxillofac. Surg. 2022, 60, 113–119. [Google Scholar] [CrossRef]
- Lumbau, A.I.; Meloni, S.M.; Tallarico, M.; Melis, L.; Spano, G.; Baldoni, E.; Koshovari, A.; Pisano, M. Implant Placement Following Crestal Sinus Lift with Sequential Drills and Osteotomes: Five Years after Final Loading Results from a Retrospective Study. J. Funct. Biomater. 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Pommer, B.; Watzek, G. Gel-pressure technique for flapless transcrestal maxillary sinus floor elevation: A preliminary cadaveric study of a new surgical technique. Int. J. Oral Maxillofac. Implant. 2009, 24, 817–822. [Google Scholar]
- Danesh-Sani, S.A.; Loomer, P.M.; Wallace, S.S. A comprehensive clinical review of maxillary sinus floor elevation: Anatomy, techniques, biomaterials and complications. Br. J. Oral Maxillofac. Surg. 2016, 54, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Manekar, V.S. Graftless crestal hydraulic sinus lift with simultaneous implant insertion. Natl. J. Maxillofac. Surg. 2020, 11, 213–218. [Google Scholar] [CrossRef]
- Elghobashy, M.T.M.; Shaaban, A.M.; Melek, L.N.F. Radiographic comparison between Densah burs and osteotome for graftless internal sinus lifting with simultaneous implant placement: A randomized clinical trial. Int. J. Oral Maxillofac. Surg. 2023, 52, 388–395. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Aaboe, M.; Araujo, M.; Carrión, J.B.; Cavalcanti, R.; Cionca, N.; Cochran, D.; Darby, I.; Funakoshi, E.; Gierthmuehlen, P.C.; et al. Group 4 ITI Consensus Report: Risks and biologic complications associated with implant dentistry. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 351–358. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; Cicciù, M.; Tözüm, T.F.; D’Amico, C.; Oteri, G.; Cervino, G. Impact of bisphosphonate drugs on dental implant healing and peri-implant hard and soft tissues: A systematic review. BMC Oral Health. 2022, 22, 291. [Google Scholar] [CrossRef] [PubMed]
- Smith Nobrega, A.; Santiago, J.F., Jr.; de Faria Almeida, D.A.; Dos Santos, D.M.; Pellizzer, E.P.; Goiato, M.C. Irradiated patients and survival rate of dental implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2016, 116, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.; Pi-Anfruns, J.; Moshaverinia, A.; Sim, D.; Grogan, T.; Hadaya, D. The Effects of Systemic Diseases and Medications on Implant Osseointegration: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2019, 34, s35–s49. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, A.D.; Salame, Z.; Chrcanovic, B.R. Smoking and Dental Implants: A Systematic Review and Meta-Analysis. Medicina 2021, 58, 39. [Google Scholar] [CrossRef] [PubMed]
- Tetè, G.; Cattoni, F.; Polizzi, E. Anti-discoloration system: A new chlorhexidine mouthwash. J. Biol. Regul. Homeost. Agents 2021, 35 (Suppl. S1), 113–118. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, E.; Tetè, G. Manual vs Mechanical Oral Hygiene Procedures: Has the Role of the Dental Hygienist in Phase 2 Post-lockdown Really Changed? Oral Health Prev. Dent. 2020, 18, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Tetè, G.; D’orto, B.; Ferrante, L.; Polizzi, E.; Cattoni, F. Role of mast cells in oral inflammation. J. Biol. Regul. Homeost. Agents 2021, 35 (Suppl. S1), 65–70. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35, 216–240. [Google Scholar] [CrossRef]
- Stacchi, C.; Spinato, S.; Lombardi, T.; Bernardello, F.; Bertoldi, C.; Zaffe, D.; Nevins, M. Minimally Invasive Management of Implant-Supported Rehabilitation in the Posterior Maxilla, Part II. Surgical Techniques and Decision Tree. Int. J. Periodontics Restor. Dent. 2020, 40, e95–e102. [Google Scholar] [CrossRef]
- Lombardi, T.; Lamazza, L.; Bernardello, F.; Ziętek, G.; Stacchi, C.; Troiano, G. Clinical and radiographic outcomes following transcrestal maxillary sinus floor elevation with injectable xenogenous bone substitute in gel form: A prospective multicenter study. Int. J. Implant. Dent. 2022, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Antonaya-Mira, R.; Barona-Dorado, C.; Martínez-Rodríguez, N.; Cáceres-Madroño, E.; Martínez-González, J.M. Meta-analysis of the increase in height in maxillary sinus elevations with osteotome. Med. Oral. Patol. Oral Cir. Bucal. 2012, 17, e146–e152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsai, C.F.; Pan, W.L.; Pan, Y.P.; Chan, C.P.; Ju, Y.R.; Wang, Y.M.; Lin, C.Y.; Chang, C.C. Comparison of 4 sinus augmentation techniques for implant placement with residual alveolar bone height ≤3 mm. Medicine 2020, 99, e23180. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Lombardi, T.; Ottonelli, R.; Berton, F.; Perinetti, G.; Traini, T. New bone formation after transcrestal sinus floor elevation was influenced by sinus cavity dimensions: A prospective histologic and histomorphometric study. Clin. Oral Implant. Res. 2018, 29, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Dellavia, C.; Speroni, S.; Pellegrini, G.; Gatto, A.; Maiorana, C. A new method to evaluate volumetric changes in sinus augmentation procedure. Clin. Implant. Dent. Relat. Res. 2014, 16, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Borrone, A.; Leigheb, M.; Shastri, V.P.; Cannas, M. Injectable Graft Substitute Active on Bone Tissue Regeneration. Tissue Eng. Part A 2017, 23, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Fricain, J.C.; Aid, R.; Lanouar, S.; Maurel, D.B.; Le Nihouannen, D.; Delmond, S.; Letourneur, D.; Amedee Vilamitjana, J.; Catros, S. In-vitro and in-vivo design and validation of an injectable polysaccharide-hydroxyapatite composite material for sinus floor augmentation. Dent. Mater. 2018, 34, 1024–1035. [Google Scholar] [CrossRef]
- Tormos, M.; Morales, J.; Guevara, N.; Elías, A.R.; López, L.; García, O. Sinus Augmentation and Simultaneous Implant Placement Success: Pilot Study (2008–2011). Puerto Rico Health Sci. J. 2016, 35, 197–202. [Google Scholar]
- Praveen, A.A.; Venkadassalapathy, S.; Victor, D.J.; Prakash, P.S.G.; Umesh, S.G.; Ali Baeshen, H.; Balaji, T.M.; Patil, S.; Reda, R.; Testarelli, L. Efficacy of Two Different Hydrodynamic Sinus Lift Systems for Atraumatic Elevation in Immediate Implant Placement. Patient Prefer. Adherence 2023, 17, 1197–1207. [Google Scholar] [CrossRef]
- Tamer, Y. Evaluation of immediate implant placement with osteotome sinus floor elevation without graft material. Niger. J. Clin. Pract. 2023, 26, 274–279. [Google Scholar] [CrossRef]
- Bernardello, F.; Lombardi, T.; Stacchi, C. Clearance of Bone Substitute in Gel Form Accidentally Dispersed into the Sinus Cavity during Transcrestal Maxillary Sinus Floor Elevation: Two-Case Report. Sinusitis 2021, 5, 132–140. [Google Scholar] [CrossRef]
- Andreasi Bassi, M.; Lopez, M.A.; Confalone, L.; Carinci, F. Hydraulic sinus lift technique in future site development: Clinical and histomorphometric analysis of human biopsies. Implant. Dent. 2015, 24, 117–124. [Google Scholar] [CrossRef] [PubMed]







| Sample features | |
|---|---|
| Number of patients | 54 |
| Females | 25 |
| Males | 29 |
| Average age (range) | 54.5 (31–78) |
| Implant details | |
| Number of implants | 54 |
| TTi 3.3 × 9 | 9 |
| TTi 3.3 × 11 | 11 |
| TTi 3.8 × 9 | 17 |
| TTi 3.8 × 11 | 15 |
| Implant site | |
| 16 | 8 |
| 17 | 19 |
| 26 | 21 |
| 27 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speroni, S.; Polizzi, E.; Giuffrè, M.; Antonelli, L.; Coccoluto, L.; Gherlone, E.F. Crestal Sinus Lift with the Hydrodynamic Technique: Prospective Clinical Study at 3 Years Follow-Up. Appl. Sci. 2024, 14, 6204. https://doi.org/10.3390/app14146204
Speroni S, Polizzi E, Giuffrè M, Antonelli L, Coccoluto L, Gherlone EF. Crestal Sinus Lift with the Hydrodynamic Technique: Prospective Clinical Study at 3 Years Follow-Up. Applied Sciences. 2024; 14(14):6204. https://doi.org/10.3390/app14146204
Chicago/Turabian StyleSperoni, Stefano, Elisabetta Polizzi, Marco Giuffrè, Luca Antonelli, Luca Coccoluto, and Enrico Felice Gherlone. 2024. "Crestal Sinus Lift with the Hydrodynamic Technique: Prospective Clinical Study at 3 Years Follow-Up" Applied Sciences 14, no. 14: 6204. https://doi.org/10.3390/app14146204
APA StyleSperoni, S., Polizzi, E., Giuffrè, M., Antonelli, L., Coccoluto, L., & Gherlone, E. F. (2024). Crestal Sinus Lift with the Hydrodynamic Technique: Prospective Clinical Study at 3 Years Follow-Up. Applied Sciences, 14(14), 6204. https://doi.org/10.3390/app14146204






