Exploring Factors Associated with Changes in Pain and Function Following mHealth-Based Exercise Therapy for Chronic Musculoskeletal Pain: A Systematic Review with Meta-Analysis and Meta-Regression
Abstract
1. Introduction
2. Materials and Methods
2.1. Deviations from the Review Protocol
2.2. Data Sources and Search Strategies
2.3. Eligibility Criteria
- -
- P (population): adults (>18 years) diagnosed with chronic musculoskeletal pain or chronic spine pain according to the ACTTION-APS Pain Taxonomy [49]. This includes individuals with chronic low back pain, lumbosacral radiculopathy, fibromyalgia and myofascial widespread pain, gout, osteoarthritis, rheumatoid arthritis, and spondyloarthropathies.
- -
- E (exposure): participants underwent an mHealth-based exercise program, alone or within a multimodal intervention. mHealth was defined as ‘health practice supported by mobile devices such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices’ [32].
- -
- C (comparator): any control group, with no restriction, or no control group.
- -
- O (outcomes): pain-related measures (e.g., pain intensity) and physical function.
- -
- S (study design): observational studies and controlled clinical trials that provide results from a correlation or regression analysis between clinical or demographic variables and the impact of mHealth exercise-based therapy on pain and function.
- -
- Patients with chronic pain were analyzed together with participants with other chronic disorders.
- -
- Studies where exercise was provided using a digitally delivered modality other than mHealth, e.g., web-based tools.
- -
- Participants with cancer-related pain or with pain associated with the central or peripheral nervous systems.
- -
- Cluster analysis.
- -
- Outcome measures were evaluated before and after surgery, e.g., knee arthroplasty.
- -
- Studies written in a language other than English or Spanish.
- -
- Reviews, editorials, letters, commentaries, thesis dissertations, grey literature, and conference abstracts.
2.4. Study Selection
2.5. Data Extraction and Synthesis
2.6. Risk of Bias Appraisal
2.7. Description of Interventions
2.8. Spin of Information
2.9. Certainty in the Evidence
2.10. Meta-Analysis
2.11. Meta-Regression and Sensitivity Analyses
2.12. Publication Bias
3. Results
3.1. Study Selection
3.2. Description of Clinical Trials
3.3. Risk of Bias Assessment
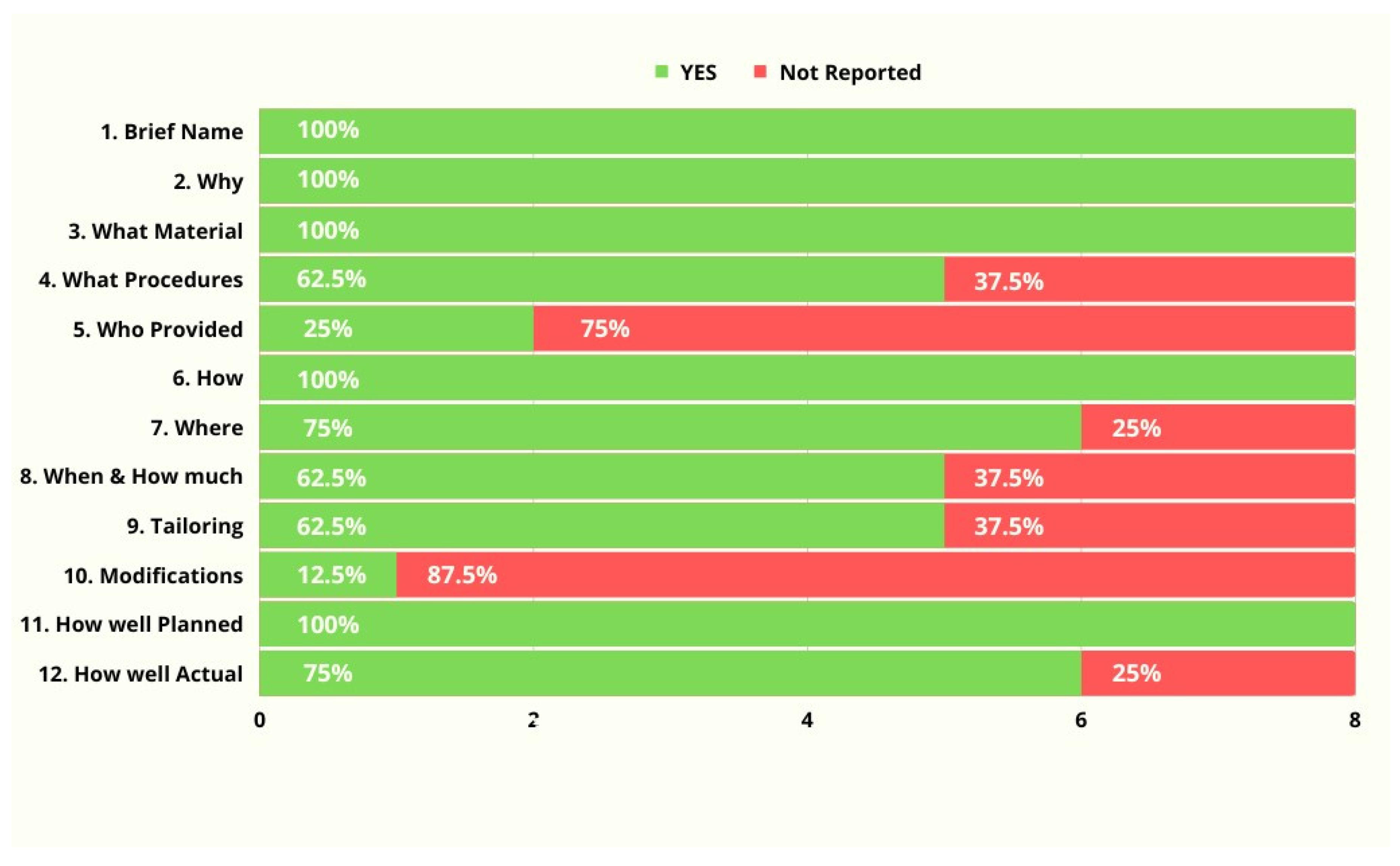
3.4. Completeness of Intervention Descriptions
3.5. Spin of Information
3.6. Certainty in the Evidence (GRADE)
3.7. Meta-Analysis of the Association between Pain and Physical Function (GRADE: Very Low)
3.8. Meta-Analysis of the Association between Pain and Anxiety (GRADE: Very Low)
4. Discussion
4.1. Methodological and Clinical Considerations
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, E.; Felten, R.; Sagez, F.; Sibilia, J.; Devilliers, H.; Arnaud, L. The world-wide burden of musculoskeletal diseases: A systematic analysis of the World Health Organization Burden of Diseases Database. Ann. Rheum. Dis. 2019, 78, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Kamper, S.J.; Wiggers, J.H.; O’brien, K.M.; Lee, H.; Wolfenden, L.; Yoong, S.L.; Robson, E.; McAuley, J.H.; Hartvigsen, J.; et al. Musculoskeletal conditions may increase the risk of chronic disease: A systematic review and meta-analysis of cohort studies. BMC Med. 2018, 16, 167. [Google Scholar] [CrossRef]
- Lin, I.; Wiles, L.; Waller, R.; Goucke, R.; Nagree, Y.; Gibberd, M.; Straker, L.; Maher, C.G.; O’sullivan, P.P.B. What does best practice care for musculoskeletal pain look like? Eleven consistent recommendations from high-quality clinical practice guidelines: Systematic review. Br. J. Sport. Med. 2020, 54, 79–86. [Google Scholar] [CrossRef]
- Conley, B.; Bunzli, S.; Bullen, J.; O’Brien, P.; Persaud, J.; Gunatillake, T.; Dowsey, M.M.; Choong, P.F.M.; Lin, I. Core Recommendations for Osteoarthritis Care: A Systematic Review of Clinical Practice Guidelines. Arthritis Care Res. 2023, 75, 1897–1907. [Google Scholar] [CrossRef]
- Booth, J.; Moseley, G.L.; Schiltenwolf, M.; Cashin, A.; Davies, M.; Hübscher, M. Exercise for chronic musculoskeletal pain: A biopsychosocial approach. Musculoskelet. Care 2017, 15, 413–421. [Google Scholar] [CrossRef]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Malmivaara, A.; van Tulder, M.W. Exercise therapy for chronic low back pain. Cochrane Database Syst. Rev. 2021, 2021, CD009790. [Google Scholar] [CrossRef]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Stewart, S.A.; Bagg, M.K.; Stanojevic, S.; Yamato, T.P.; Saragiotto, B.T. Some types of exercise are more effective than others in people with chronic low back pain: A network meta-analysis. J. Physiother. 2021, 67, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Castellini, G.; Pillastrini, P.; Vanti, C.; Bargeri, S.; Giagio, S.; Bordignon, E.; Fasciani, F.; Marzioni, F.; Innocenti, T.; Chiarotto, A.; et al. Some conservative interventions are more effective than others for people with chronic non-specific neck pain: A systematic review and network meta-analysis. J. Physiother. 2022, 68, 244–254. [Google Scholar] [CrossRef]
- Holden, M.A.; Metcalf, B.; Lawford, B.J.; Hinman, R.S.; Boyd, M.; Button, K.; Collins, N.J.; Cottrell, E.; Henrotin, Y.; Larsen, J.B.; et al. Recommendations for the delivery of therapeutic exercise for people with knee and/or hip osteoarthritis. An international consensus study from the OARSI Rehabilitation Discussion Group. Osteoarthr. Cartil. 2023, 31, 386–396. [Google Scholar] [CrossRef]
- Fransen, M.; Mcconnell, S.; Harmer, A.R.; Van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2015, 2015, CD004376. [Google Scholar] [CrossRef]
- Gross, A.; Kay, T.M.; Paquin, J.P.; Blanchette, S.; Lalonde, P.; Christie, T.; Dupont, G.; Graham, N.; Burnie, S.J.; Gelley, G.; et al. Exercises for mechanical neck disorders. Cochrane Database Syst. Rev. 2015, 2015, CD004250. [Google Scholar] [CrossRef]
- Hinman, R.; Jones, S.; Nelligan, R.; Campbell, P.K.; Hall, M.; Foster, N.E.; Russell, T.; Bennell, K.L. Absence of Improvement with Exercise in Some Patients with Knee Osteoarthritis: A Qualitative Study of Responders and Nonresponders. Arthritis Care Res. 2023, 75, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.; Takala, E.P.; Gerdle, B.; Larsson, B. Evaluation of pain and function after two home exercise programs in a clinical trial on women with chronic neck pain—With special emphasises on completers and responders. BMC Musculoskelet. Disord. 2014, 15, 6. [Google Scholar] [CrossRef]
- Jönsson, T.; Eek, F.; Ekvall-Hansson, E.; Dahlberg, L.; Dell’lsola, A. Who responds to first-line interventions delivered in primary care? a responder—Non-responder analysis on people with knee or hip osteoarthritis treated in Swedish primary cares. Osteoarthr. Cartil. 2020, 28, S466. [Google Scholar] [CrossRef]
- Gurung, T.; Ellard, D.R.; Mistry, D.; Patel, S.; Underwood, M. Identifying potential moderators for response to treatment in low back pain: A systematic review. Physiotherapy 2015, 101, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; Wilson, M.N.; Stewart, S.; Cartwright, J.L.; Smith, A.O.; Riley, R.D.; van Tulder, M.; Bendix, T.; Cecchi, F.; Costa, L.O.P.; et al. Exercise treatment effect modifiers in persistent low back pain: An individual participant data meta-analysis of 3514 participants from 27 randomised controlled trials. Br. J. Sport. Med. 2020, 54, 1277–1278. [Google Scholar] [CrossRef]
- Holden, M.A.; Hattle, M.; Runhaar, J.; Riley, R.; Healey, E.; Quicke, J.; van Der Windt, D.; Dziedzic, K.; van Middelkoop, M.; Burke, D.; et al. Moderators of the Effect of Therapeutic Exercise for People With Knee and/or Hip Osteoarthritis: An Individual Participant Data Meta-Analysis. Lancet Rheumatol. 2023, 5, 386–400. [Google Scholar] [CrossRef]
- Kroll, H.R. Exercise Therapy for Chronic Pain. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef]
- Bunting, J.W.; Withers, T.M.; Heneghan, N.R.; Greaves, C.J. Digital interventions for promoting exercise adherence in chronic musculoskeletal pain: A systematic review and meta-analysis. Physiotherapy 2021, 111, 23–30. [Google Scholar] [CrossRef]
- Mahmood, A.; Nayak, P.; Deshmukh, A.; English, C.; Manikandan, N.; John Solomon, M.; Unnikrishnan, B. Measurement, determinants, barriers, and interventions for exercise adherence: A scoping review. J. Bodyw. Mov. Ther. 2023, 33, 95–105. [Google Scholar] [CrossRef]
- Baroni, M.P.; Jacob, M.F.A.; Rios, W.R.; Fandim, J.V.; Fernandes, L.G.; Chaves, P.I.; Fioratti, I.; Saragiotto, B.T. The state of the art in telerehabilitation for musculoskeletal conditions. Arch. Physiother. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Vieira, L.M.S.M.d.A.; de Andrade, M.A.; Sato, T.d.O. Telerehabilitation for musculoskeletal pain—An overview of systematic reviews. Digit. Health 2023, 9, 20552076231164242. [Google Scholar] [CrossRef]
- Fonseca Dias, J.; Cunha Oliveira, V.; Tristão Borges, P.R.; Dutra, F.C.M.S.; Mancini, M.C.; Kirkwood, R.N.; Resende, R.A.; Sampaio, R.F. Effectiveness of exercises by telerehabilitation on pain, physical function and quality of life in people with physical disabilities: A systematic review of randomised controlled trials with GRADE recommendations. Br. J. Sport. Med. 2020, 15, 155–162. [Google Scholar] [CrossRef]
- Niknejad, N.; Ismail, W.; Bahari, M.; Nazari, B. Understanding Telerehabilitation Technology to Evaluate Stakeholders’ Adoption of Telerehabilitation Services: A Systematic Literature Review and Directions for Further Research. Arch. Phys. Med. Rehabil. 2021, 102, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- Suero-Pineda, A.; Oliva-Pascual-Vaca, Á.; Durán, M.R.-P.; Sánchez-Laulhé, P.R.; García-Frasquet, M.Á.; Blanquero, J. Effectiveness of a Telerehabilitation Evidence-Based Tablet App for Rehabilitation in Traumatic Bone and Soft Tissue Injuries of the Hand, Wrist, and Fingers. Arch. Phys. Med. Rehabil. 2023, 104, 932–941. [Google Scholar] [CrossRef]
- Kushnir, A.; Kachmar, O.; Bonnechère, B. STASISM: A Versatile Serious Gaming Multi-Sensor Platform for Personalized Telerehabilitation and Telemonitoring. Sensors 2024, 24, 351. [Google Scholar] [CrossRef] [PubMed]
- Stark, C.; Cunningham, J.; Turner, P.; Johnson, M.A.; Bäcker, H.C. App-Based Rehabilitation in Back Pain, a Systematic Review. J. Pers. Med. 2022, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, A.; Nordstoga, A.L.; Bach, K.; Aasdahl, L.; Nilsen, T.I.L.; Bardal, E.M.; Boldermo, N.; Bertheussen, G.F.; Marchand, G.H.; Gismervik, S.; et al. Effect of an Artificial Intelligence—Based Self-Management App on Musculoskeletal Health in Patients With Neck and / or Low Back Pain Referred to Specialist Care A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2320400. [Google Scholar] [CrossRef]
- Pfeifer, A.C.; Uddin, R.; Schröder-Pfeifer, P.; Holl, F.; Swoboda, W.; Schiltenwolf, M. Mobile application-based interventions for chronic pain patients: A systematic review and meta-analysis of effectiveness. J. Clin. Med. 2020, 9, 3557. [Google Scholar] [CrossRef]
- World Health Organization. mHealth: New horizons for health through mobile technologies. Observatory 2011, 3, 66–71. [Google Scholar] [CrossRef]
- Du, S.; Liu, W.; Cai, S.; Hu, Y.; Dong, J. The efficacy of e-health in the self-management of chronic low back pain: A meta analysis. Int. J. Nurs. Stud. 2020, 106, 103507. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, D. Joint Expedition: Exploring Telehealth and the Digital Healthcare Landscape as a Team Integration. Healthcare 2024, 12, 585. [Google Scholar] [CrossRef]
- Thompson, D.; Rattu, S.; Tower, J.; Egerton, T.; Francis, J.; Merolli, M. Mobile app use to support therapeutic exercise for musculoskeletal pain conditions may help improve pain intensity and self-reported physical function: A systematic review. J. Physiother. 2023, 69, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ligero, M.; Moral-Munoz, J.A.; Salazar, A.; Failde, I. mHealth Intervention for Improving Pain, Quality of Life, and Functional Disability in Patients With Chronic Pain: Systematic Review. JMIR mHealth uHealth 2023, 11, e40844. [Google Scholar] [CrossRef]
- Rodríguez Sánchez-Laulhé, P.; Biscarri-Carbonero, Á.; Suero-Pineda, A.; Luque-Romero, L.G.; García, F.J.B.; Blanquero, J.; Heredia-Rizo, A.M. The effects of a mobile app-delivered intervention in people with symptomatic hand osteoarthritis: A pragmatic randomized controlled trial. Eur. J. Phys. Rehabil Med. 2023, 59, 54–64. [Google Scholar] [CrossRef]
- Rodríguez Sánchez-Laulhé, P.; Luque-Romero, L.G.; Barrero-García, F.J.; Biscarri-Carbonero, Á.; Blanquero, J.; Suero-Pineda, A.; Heredia-Rizo, A.M. An Exercise and Educational and Self-management Program Delivered With a Smartphone App (CareHand) in Adults With Rheumatoid Arthritis of the Hands: Randomized Controlled Trial. JMIR mHealth uHealth 2022, 10, e35462. [Google Scholar] [CrossRef]
- Nelligan, R.K.; Hinman, R.S.; McManus, F.; Lamb, K.E.; Bennell, K.L. Moderators of the effect of a self-directed digitally delivered exercise program for people with knee osteoarthritis: Exploratory analysis of a randomized controlled trial. J. Med. Internet Res. 2021, 23, e30768. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.; Costa, F.; Molinos, M.; Areias, A.; Janela, D.; Moulder, R.G.; Lains, J.; Bento, V.; Yanamadala, V.; Cohen, S.P.; et al. Racial and Ethnic Differences in Outcomes of a 12-Week Digital Rehabilitation Program for Musculoskeletal Pain: Prospective Longitudinal Cohort Study. J. Med. Internet Res. 2022, 24, e41306. [Google Scholar] [CrossRef]
- Beller, E.M.; Glasziou, P.P.; Altman, D.G.; Hopewell, S.; Bastian, H.; Chalmers, I.; Gøtzsche, P.C.; Lasserson, T.; Tovey, D.; For The PRISMA for Abstracts Group. PRISMA for Abstracts: Reporting Systematic Reviews in Journal and Conference Abstracts. PLoS Med. 2013, 10, e1001419. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Janela, D.; Costa, F.; Areias, A.C.; Molinos, M.; Moulder, R.G.; Lains, J.; Bento, V.; Scheer, J.K.; Yanamadala, V.; Cohen, S.P.; et al. Digital Care Programs for Chronic Hip Pain: A Prospective Longitudinal Cohort Study. Healthcare 2022, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Janela, D.; Molinos, M.; Moulder, R.G.; Lains, J.; Francisco, G.E.; Bento, V.; Yanamadala, V.; Cohen, S.P.; Correia, F.D. Digital rehabilitation for hand and wrist pain: A single-arm prospective longitudinal cohort study. Pain Rep. 2022, 7, e1026. [Google Scholar] [CrossRef] [PubMed]
- Sitges, C.; Terrasa, J.L.; García-Dopico, N.; Segur-Ferrer, J.; Velasco-Roldán, O.; Crespí-Palmer, J.; González-Roldán, A.M.; Montoya, P. An Educational and Exercise Mobile Phone-Based Intervention to Elicit Electrophysiological Changes and to Improve Psychological Functioning in Adults With Nonspecific Chronic Low Back Pain (BackFit App): Nonrandomized Clinical Trial. JMIR mHealth uHealth 2022, 10, e29171. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Bailey, J.F.; Yang, M.; Krauss, J. Older Adult Use and Outcomes in a Digital Musculoskeletal (MSK) Program, by Generation. Front. Digit. Health 2021, 3, 693170. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Lefevre, A.E.; Lee, J.; L’engle, K.; Mehl, G.; Sinha, C.; Labrique, A. Guidelines for reporting of health interventions using mobile phones: Mobile health (mHealth) Evidence reporting and assessment (mERA) checklist. BMJ 2016, 352, i1174. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Bruehl, S.; Fillingim, R.B.; Loeser, J.D.; Terman, G.W.; Turk, D.C. Multidimensional Diagnostic Criteria for Chronic Pain: Introduction to the ACTTION–American Pain Society Pain Taxonomy (AAPT). J. Pain 2016, 17, T1–T9. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Coté, P.; Bombardier, C. Assessing Bias in Studies of Prognostic Factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.P.; Costa, L.O.P.; Gonzalez, G.Z.; Maher, C.G.; Moseley, A.M. Abstracts of Low Back Pain Trials Are Poorly Reported, Contain Spin of Information, and Are Inconsistent With the Full Text: An Overview Study. Arch. Phys. Med. Rehabil. 2019, 100, 1976–1985.e18. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.; Brozek, J.; Guyatt, G.; Oxman, A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach; McMaster University GRADE Center: Hamilton, ON, Canada, 2013. [Google Scholar]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid.-Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting meta-analisys in R with metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D.D. dmetar: Companion R Package For The Guide ‘Doing Meta-Analysis in R’. R Package Version 0.0.9000. Available online: http://dmetar.protectlab.org/ (accessed on 27 June 2023).
- Peterson, R.A.; Brown, S.P. On the use of beta coefficients in meta-analysis. J. Appl. Psychol. 2005, 90, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Tianjing, L.; Page, M. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H.; Murad, M.H.; Hong, C.; Qu, Z.; Cole, S.R.; Chen, Y. Empirical Comparison of Publication Bias Tests in Meta-Analysis. J. Gen. Intern. Med. 2018, 33, 1260–1267. [Google Scholar] [CrossRef]
- Chen, Y.P.; Lin, C.-Y.; Tsai, M.J.; Chuang, T.Y.; Kuang Sheng Lee, O. Wearable motion sensor device to facilitate rehabilitation in patients with shoulder adhesive capsulitis: Pilot study to assess feasibility. J. Med. Internet Res. 2020, 22, e17032. [Google Scholar] [CrossRef]
- Selter, A.; Tsangouri, C.; Ali, S.B.; Freed, D.; Vatchinsky, A.; Kizer, J.; Sahuguet, A.; Vojta, D.; Vad, V.; Pollak, J.; et al. An mhealth app for self-management of chronic lower back pain (Limbr): Pilot study. JMIR mHealth uHealth 2018, 6, e179. [Google Scholar] [CrossRef]
- Burgess, R.; Mansell, G.; Bishop, A.; Lewis, M.; Hill, J. Predictors of functional outcome in musculoskeletal healthcare: An umbrella review. Eur. J. Pain 2020, 24, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Wippert, P.M.; Niederer, D.; Drießlein, D.; Beck, H.; Banzer, W.; Schneider, C.; Schiltenwolf, M.; Mayer, F. Psychosocial Moderators and Mediators of Sensorimotor Exercise in Low Back Pain: A Randomized Multicenter Controlled Trial. Front. Psychiatry 2021, 12, 629474. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.E.; Hill, J.C.; Knoop, J. Are we giving stratified care a fair trial? J. Physiother. 2023, 69, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Legha, A.; Burke, D.L.; Foster, N.E.; van der Windt, D.A.; Quicke, J.G.; Healey, E.L.; Runhaar, J.; Holden, M.A. Do comorbidities predict pain and function in knee osteoarthritis following an exercise intervention, and do they moderate the effect of exercise? Analyses of data from three randomized controlled trials. Musculoskelet. Care 2020, 18, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Quicke, J.G.; Runhaar, J.; van der Windt, D.A.; Healey, E.L.; Foster, N.E.; Holden, M.A. Moderators of the effects of therapeutic exercise for people with knee and hip osteoarthritis: A systematic review of sub-group analyses from randomised controlled trials. Osteoarthr. Cartil. Open 2020, 2, 100113. [Google Scholar] [CrossRef] [PubMed]
- Knoop, J.; de Joode, J.W.; Brandt, H.; Dekker, J.; Ostelo, R.W.J.G. Patients’ and clinicians’ experiences with stratified exercise therapy in knee osteoarthritis: A qualitative study. BMC Musculoskelet. Disord. 2022, 23, 559. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Sezgin, E.; Sanchez-Vazquez, A.; Ivory, C. Socio-technical Factors Impacting Patients’ Adoption of Mobile Health Tools: Systematic Literature Review and Narrative Synthesis. JMIR mHealth uHealth 2022, 10, e36284. [Google Scholar] [CrossRef] [PubMed]
- Moman, R.N.; Dvorkin, J.; Pollard, E.M.; Wanderman, R.; Murad, M.H.; Warner, D.O.; Hooten, W.M. A Systematic Review and Meta-analysis of Unguided Electronic and Mobile Health Technologies for Chronic Pain—Is It Time to Start Prescribing Electronic Health Applications? Pain Med. 2019, 20, 2238–2255. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.L.; Holden, M.A.; Mason, E.E.; Foster, N.E. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2010, 1, CD005956. [Google Scholar] [CrossRef]
- Yang, Y.; Boulton, E.; Todd, C. Measurement of Adherence to mHealth Physical Activity Interventions and Exploration of the Factors That Affect the Adherence: Scoping Review and Proposed Framework. J. Med. Internet Res. 2022, 24, e30817. [Google Scholar] [CrossRef]
- Hung, M.; Bounsanga, J.; Voss, M. Interpretation of correlations in clinical research. Postgrad. Med. 2018, 129, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.D. Cohort studies of aetiology and prognosis: They’re different. J. Physiother. 2014, 60, 241–244. [Google Scholar] [CrossRef]
- Arah, O.A. Bias Analysis for Uncontrolled Confounding in the Health Sciences. Annu. Rev. Public Health 2017, 38, 23–38. [Google Scholar] [CrossRef]
- Agnew, J.M.R.; Hanratty, C.E.; McVeigh, J.G.; Nugent, C.; Kerr, D.P. An Investigation Into the Use of mHealth in Musculoskeletal Physiotherapy: Scoping Review. JMIR Rehabil. Assist. Technol. 2022, 9, e33609. [Google Scholar] [CrossRef] [PubMed]
- Beukenhorst, A.L.; Druce, K.L.; De Cock, D. Smartphones for musculoskeletal research—Hype or hope? Lessons from a decennium of mHealth studies. BMC Musculoskelet. Disord. 2022, 23, 487. [Google Scholar] [CrossRef] [PubMed]
- Eysenbach, G. The law of attrition. J. Med. Internet Res. 2005, 7, e11. [Google Scholar] [CrossRef]
- Amagai, S.; Pila, S.; Kaat, A.J.; Nowinski, C.J.; Gershon, R.C. Challenges in Participant Engagement and Retention Using Mobile Health Apps: Literature Review. J. Med. Internet Res. 2022, 24, e35120. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Steyerberg, E.W. The number of subjects per variable required in linear regression analyses. J. Clin. Epidemiol. 2015, 68, 627–636. [Google Scholar] [CrossRef]

| Author(s), Year, and Country | Study Design | Participants (Sex, Mean Age), BMI, and Race/Ethnicity | Educational Stage, Employment, Family, and Financial Status | Diagnosis | mHealth Type | Symptom Duration; Previous History | mHealth Intervention Group | Comparison Group | Outcomes of Interest; Assessment Points | Treatment Completion Rate a | Main Findings of Interest |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al., 2020 Taiwan [62] | Two-arm parallel non-RCT | N = 15 (5 females) b: EG = 8, CG = 7. Mean age, EG: 53(6.2), CG: 56.1(13.3) BMI: UR Ethnicity: UR | Educational stage: senior high (EG, n = 2; CG, n = 4); bachelor’s degree or higher (EG, n = 5; CG, n = 3). Employment status: UR Family status: UR Financial status: UR | Adhesive capsulitis of the shoulder > 12 wks. | Mobile app and wearable inertial sensors | Symptom duration: 3 to 6 mo. (n = 7); 26 to 52 mo. (n = 7) Previous history: UR | Motion sensor-assisted home-based shoulder exercise program via the Patient App. Daily, 12 wks. | Home-based shoulder exercise program, with advices on sleep, posture, and pain relief. Daily (10 times per exercise per day), 12 wks. | Pain intensity (worst pain last week): VAS Function: QDASH Shoulder ROM (active and passive): motion sensor T0, T1 (week 4), T2 (week 8), T3 (week 12) | Overall: 93.3% (14/15) EG: 87.5% CG: 100% | Correlations: Significant correlation between passive internal rotation and changes in QDASH at T1 (r = −0.539, p < 0.05) |
| Costa et al., 2022 USA [44] | Single-arm cohort study | N = 189 (115 females): Mean age, 47.3(11.1) BMI: 28.7(6.8) Ethnicity: UR | Educational stage: UR Employment status: Full- or part-time (n = 174) Family status: UR Financial status: UR | Wrist or hand pain: CTS (n = 50), tendinopathy (n = 45), non-specific wrist pain (n = 28), DeQuervain tenosynovitis (n = 16), wrist/hand OA (n = 16), sprain or fracture (n = 14), systemic disease (n = 11), other (n = 9) | Tablet App, Inertial motion trackers and Cloud-based portal. | Symptom duration: <3 mo. (n = 69); >3 mo. (n = 120) Previous history: UR | SWORD Health’s digital treatment (CBT, exercise program, and education). Three times per week, 8 wks. | N/A | Anxiety: GAD-7 Depression: PHQ-9 Pain intensity (average pain last week): NRS Function: QDASH T0, T1 (week 4), T2 (week 8) | Overall: 78.8% (149/189) | OR (95% CI), p value Considering an MCID (30% change) in pain intensity at T2: Age, 1.03 (0.97 to 1.10), 0.34 BMI, 1.08 (0.96 to 1.26), 0.24 Female, 0.94 (0.25 to 3.58), 0.92 GAD-7, 0.99 (0.67 to 1.53), 0.96 PHQ-9, 1.04 (0.78 to 1.38), 0.80 Correlations: Significant (p < 0.001) between ↓ pain and ↑ function: r = 0.659, Latent growth curve model: Female sex was associated with faster-paced recovery for QDASH = −0.85 per week, p = 0.029 |
| Janela et al., 2022 USA [43] | Single-arm cohort study | N = 534 (363 females): Mean age, 50.2(11.3) BMI: 29.1(6.4) Ethnicity: UR | Educational stage: UR Employment status: Full or part time (n = 480) Family status: UR Financial status: UR | Hip pain > 12 wks.: hip OA (n = 106), other conditions, e.g., non-specific pain, bursitis, sprain/strain, tendinopathy (n = 428) | Tablet App, Inertial motion trackers, and Cloud-based portal. | Symptom duration: UR Previous history: UR | SWORD Health’s digital treatment: (CBT, exercise program, and education). Three times per week, 12 wks. | N/A | Anxiety: GAD-7 Pain intensity (average pain last week): NRS Pain: HOOS-pain Function: HOOS-function T0, T1 (week 4), T2 (week 8), T3 (week 12) | Overall: 74.2% (396/534) | Correlations: Significant (p < 0.001) between ↓ pain and positive change in HOOS-function, r = −0.404 HOOS-pain, r = −0.556 HOOS-QoL, r = −0.357 GAD-7, r = 0.265 Significant between change in surgery intent and ↓ Pain, r = 0.155, p = 0.033 ↑ HOOS-function, r = −0.28, p = 0.004 Latent growth curve model: Older age was associated with ↓ pain (NRS) = −0.01, p = 0.012. Female sex was associated with change in HOOS-pain = −1.00, p = 0.016. ↑ BMI was associated with ↓ in HOOS-function = 0.14, p = 0.003 |
| Rodríguez-Sánchez-Laulhé et al., 2023 Spain [37] | Two-arm parallel RCT | N = 74 (50 females): EG = 34, CG = 40. Mean age, EG: 62.2(8.8) CG: 64.3(7.7) BMI: UR Ethnicity: UR | Educational stage: UR Employment status: UR Family status: UR Financial status: UR | Unilateral or bilateral hand OA > 6 mo. | Mobile App | Symptom duration: UR Previous history: UR | CareHand app: exercise (15 to 20 min.), info about the disease, joint protection, and self-management advice. Four times per week, 12 wks. | Written home exercise program and regular medical visits (info about the disease). Four times per week, 12 wks. | Pain intensity: NRS, AUSCAN Function: AUSCAN, QDASH Overall status: AUSCAN total Pinch and grip strength: pinch gauge, dynamometer. T0, T1 (week 4), T2 (week 12), T3 (week 24) | Overall: 85.1% (63/74) EG: 85.3% CG: 85% | Regression β (95% CI), p value: Change AUSCAN function at T2: AUSCAN pain at T0: 0.995 (0.788 to 2.639), p = 0.001 AUSCAN total at T0: −1.054 (−1.060 to −0.206), p = 0.005 Change in AUSCAN pain at T2: 0.592 (0.406 to 1.431), p = 0.001. Change NRS at T2: AUSCAN pain at T0: −0.603 (−0.997 to −0.334), p = 0.001 QDASH at T0: 0.433 (0.008 to 0.106), p = 0.023 Change in QDASH at T2: 0.469 (0.016 to 0.112), p = 0.010 |
| Scheer et al., 2022 USA [40] | Single-arm cohort study | N = 9550 (5589 females): Mean age, 49.4 (12.9) BMI: 29.2 (6.7) Ethnicity: Asian (n = 910); Black (n = 1025); Hispanic (n = 913); non-Hispanic white (n = 6240); other (n = 462) | Educational stage: middle, elementary, high school, or college (n = 3649); bachelor or higher (n = 5763). Employment status: Full- or part-time (n = 8080); not employed (n = 414) Family status: UR Financial status: UR | Chronic pain > 12 wks.: ankle (n = 352), hip (n = 817), knee (n = 1275), low back (n = 4097), neck (n = 882), wrist and hand (n = 335), elbow (n = 191), shoulder (n = 1431). | Tablet App, Inertial motion trackers, and Cloud-based portal. | Symptom duration: UR Previous history: UR | SWORD tablet app: exercise program, education, and CBT (60 min total). Three times per week, 12 wks. | N/A | Anxiety: GAD-7 Depression: PHQ-9 Pain intensity (average pain last week): NRS T0, T1 (week 4), T2 (week 8), T3 (week 12) | Overall: 72.8% (6949/9550) | Logistic regression OR (95% CI), p value Clinically meaningful change in pain intensity at T3: Hispanic vs. non-Hispanic: 1.74 (1.24 to 2.45), p = 0.001 Men vs. women: N/S, p = 0.007 Prior upper limb pain vs. no prior: N/S, p < 0.001 |
| Selter et al., 2018 USA [63] | Single-arm cohort study | N = 35 (22 females) b : Mean age, 46(16). BMI: 25.4(4.0) Ethnicity: UR | Educational stage: UR Employment status: UR Family status: UR Financial statu: UR | LBP (discogenic) > 12 wks. with axial symptoms | Mobile app | Symptom duration: 19.6 (7.4) mo. Previous history: UR | Limbr app: self-report system of pain, medication/coping, and affect, self-exercise program via coach and Force Therapeutics app. Three times a week, 12 wks. | N/A | Function: ODI, YADL Visual report. T0, T1 (week 2), T2 (week 6), T3 (week 12) | Overall: 37.6% (35/93) | Correlations: Significant correlations between ODI and YADL visual report at T0 (r = 0.551, p < 0.001) Hierarchical linear modeling: ODI increased by 0.33 for every one-unit increase in YADL visual report. |
| Sitges et al., 2022 Spain [45] | Two-arm parallel non-RCT | N = 59 (33 females): EG = 27, CG = 32. Mean age, EG: 45(9.1); CG: 48.6(7.5) BMI: EG, n = 0.41(0.07); CG, n = 0.43(0.09) Ethnicity: UR | Educational stage: UR Employment status: UR Family status: UR Financial status: UR | LBP > 12 wks. Diagnoses: hernia or protrusion (n = 13), degenerative pathology (n = 2), anterolisthe- sis (n = 3), others (n = 12) | Mobile app | Symptom duration (yrs.): EG = 8.1(8.7); CG = 11.8 (7.5). Previous history: >3 LBP episo-des > 1 wk. prior yr. | BackFit App: self-managed home-based exercise sessions (50 min.), pain education video (4 min.). Two times per week, 4 wks. (approx. 50 min). | Face-to-face group exercise program (50 min), pain education video (4 min.) Two times per week, 4 wks. (approx. 50 min). | Anxiety: STAI Pain intensity (current): VAS Pain sensitivity: PPT at spinal erector muscle Function: ODI T0, T1 (week 4) | Overall: 84.7% (50/59) EG: 85.2% CG: 84.4% | Correlations: No significant correlations between changes in ODI at T1 and EEG-resting state data |
| Wang et al., 2021 United States [46] | Single-arm retrospective cohort study | N = 41,241 (50.8% females): gen Z, n = 13,535; gen X, n = 16,982; baby boomers, n = 9262; silent gen, n = 1462. Mean age, Gen Z: 31.3(4.3); Gen X: 46.1(4.7); baby boomers: 58.7 (2.9); silent gen: 68.5(4.2) BMI: overweight/obese (76.3%), normal/under-weight (23.7%) Ethnicity: UR | Educational stage: UR Employment status: UR Family status: UR Financial status: UR | Low back, knee, hip, shoulder, or neck pain > 12 wks. Back pain (56.6%), knee (34.3%), hip (7.7%) | Tablet App | Symptom duration: UR Previous history: UR | Tablet app with wearable motion sensors: guided exercise therapy sessions (animations and videos), health coaching, and education for chronic pain. Three sensor-guided exercise sessions and two education papers per week, 12 wks. | NA | Anxiety: GAD-7 Depression: PHQ-9 Pain intensity (average last 24 h): NRS T0, T1 (week 12) | Overall: 84.7% (36,142/ 41,241) Gen Z: 66.9% Gen X: 75.5% Baby boomer: 81.5% Silent Gen: 83.0% | Regression β (95% CI), p value (adjusted or unadjusted model): No association of age with change in pain score (all, p > 0.05) OR (95% CI), p value (adjusted model): Association between age and change in anxiety (all, p < 0.05) Baby boomer vs. Gen Z and Millennial: 2.05 (1.56 to 2.69) Baby boomer and Silent generation vs. Gen Z and Millennial: 2.71 (1.19 to 6.20) Association between age and change in depression (p < 0.05) Baby boomer vs. Gen Z and Millennial: 1.31 (1.01 to 1.71) |
| Summary of Findings | Certainty in the Evidence Based on the GRADE Approach | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Studies (k) | Participants (N) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Certainty in the Evidence | Importance |
| Pain–Function | 5 | 871 | −1: Serious a | −1: Serious c | −2: Very Serious d | No f | Undetected | Very Low ⨁◯◯◯ h | Critical |
| Pain–Anxiety | 2 | 723 | −1: Serious b | −1: Serious c | −2: Very Serious e | No f | Not possible g | Very Low ⨁◯◯◯ h | Critical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sánchez-Laulhé, P.; Heredia-Rizo, A.M.; Salas-González, J.; Piña-Pozo, F.; Fernández-Seguín, L.M.; García-Muñoz, C. Exploring Factors Associated with Changes in Pain and Function Following mHealth-Based Exercise Therapy for Chronic Musculoskeletal Pain: A Systematic Review with Meta-Analysis and Meta-Regression. Appl. Sci. 2024, 14, 6632. https://doi.org/10.3390/app14156632
Rodríguez-Sánchez-Laulhé P, Heredia-Rizo AM, Salas-González J, Piña-Pozo F, Fernández-Seguín LM, García-Muñoz C. Exploring Factors Associated with Changes in Pain and Function Following mHealth-Based Exercise Therapy for Chronic Musculoskeletal Pain: A Systematic Review with Meta-Analysis and Meta-Regression. Applied Sciences. 2024; 14(15):6632. https://doi.org/10.3390/app14156632
Chicago/Turabian StyleRodríguez-Sánchez-Laulhé, Pablo, Alberto Marcos Heredia-Rizo, Jesús Salas-González, Fernando Piña-Pozo, Lourdes María Fernández-Seguín, and Cristina García-Muñoz. 2024. "Exploring Factors Associated with Changes in Pain and Function Following mHealth-Based Exercise Therapy for Chronic Musculoskeletal Pain: A Systematic Review with Meta-Analysis and Meta-Regression" Applied Sciences 14, no. 15: 6632. https://doi.org/10.3390/app14156632
APA StyleRodríguez-Sánchez-Laulhé, P., Heredia-Rizo, A. M., Salas-González, J., Piña-Pozo, F., Fernández-Seguín, L. M., & García-Muñoz, C. (2024). Exploring Factors Associated with Changes in Pain and Function Following mHealth-Based Exercise Therapy for Chronic Musculoskeletal Pain: A Systematic Review with Meta-Analysis and Meta-Regression. Applied Sciences, 14(15), 6632. https://doi.org/10.3390/app14156632










