Stratification of Older Adults According to Frailty Status and Falls Using Gait Parameters Explored Using an Inertial System
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Frailty Assessment by Standardized Frailty Phenotype Criteria (S-FPC)
2.2. Gait Analysis: The G-STRIDE System
2.3. Classification Model
- True Positive (TP): The number of correctly predicted positive cases.
- True Negative (TN): The number of correctly predicted negative cases.
- False Positive (FP): The number of incorrectly predicted positive cases (Type I error).
- False Negative (FN): The number of incorrectly predicted negative cases (Type II error).
2.4. Main Parameters Configuration
- max_depth: 5
- n_estimators: 100
- min_samples_split: 3
- min_samples_leaf: 1
2.5. Statistical Analysis
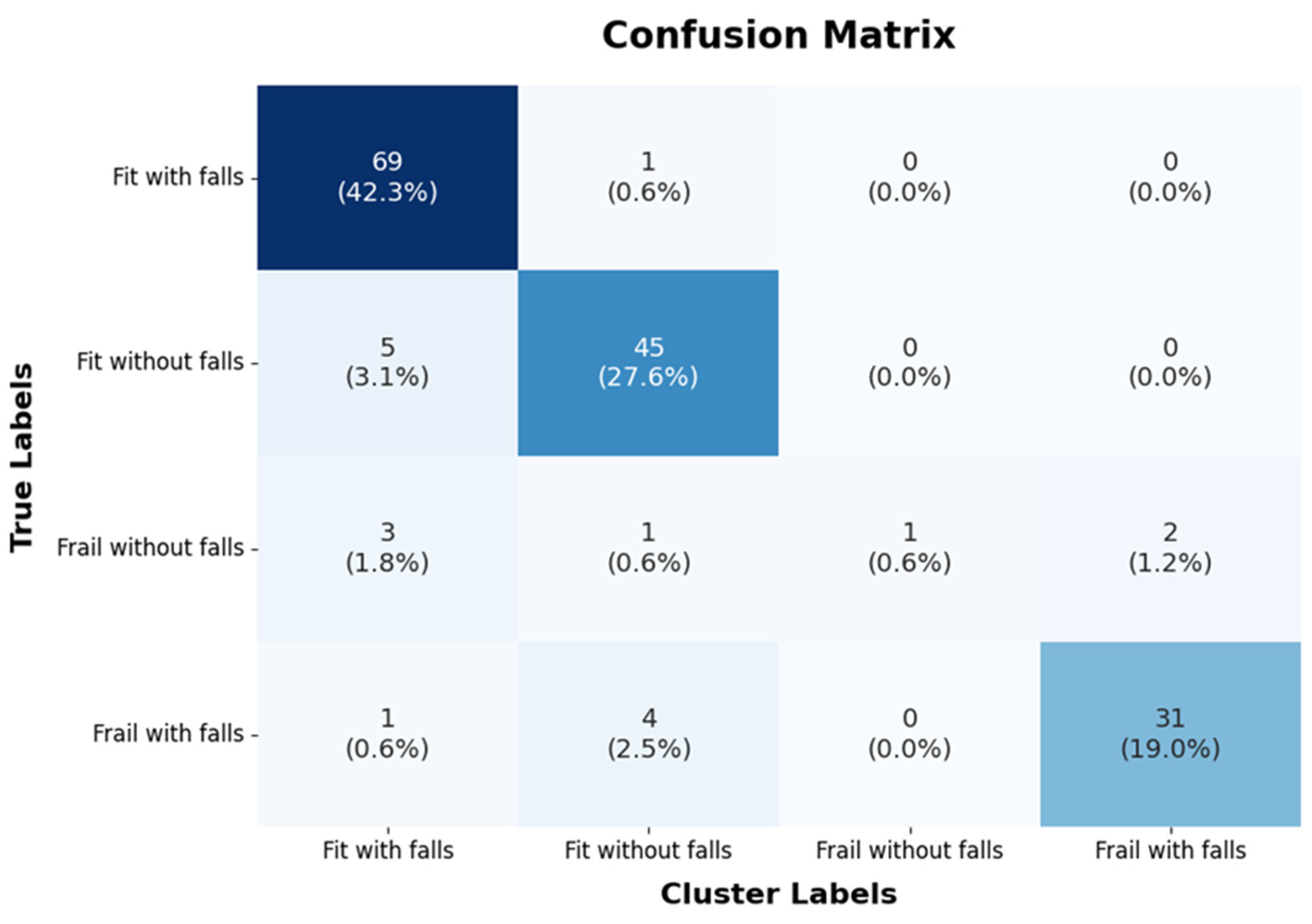
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Statistical Institute. 2018 Population Projections. 2018. Available online: https://www.ine.es/prensa/pp_2018_2068.pdf (accessed on 1 June 2024).
- Mitchell, E.; Walker, R. Global ageing: Successes, challenges and opportunities. Br. J. Hosp. Med. 2020, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 56. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age Ageing 2006, 35, ii37–ii41. [Google Scholar] [CrossRef] [PubMed]
- Bergen, G.; Stevens, M.R.; Burns, E.R. Falls and Fall Injuries Among Adults Aged ≥65 Years—United States. 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 993–998. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Frailty defined by FRAIL scale as a predictor of mortality: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2018, 19, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Chang, S.F.; Ho, H.Y. Adverse health effects of frailty: Systematic review and meta-analysis of middle-aged and older adults with implications for evidence-based practice. Worldviews Evid.-Based Nurs. 2021, 18, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Harmand, M.G.-C.; Meillon, C.; Bergua, V.; Teguo, M.T.; Dartigues, J.-F.; Avila-Funes, J.A.; Amieva, H. Comparing the predictive value of three definitions of frailty: Results from the Three-City study. Arch. Gerontol. Geriatr. 2017, 72, 153–163. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Rodríguez-Sánchez, I.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Biomarkers of frailty. Mol. Asp. Med. 2024, 97, 101271. [Google Scholar] [CrossRef]
- Lu, Z.; Er, Y.; Zhan, Y.; Deng, X.; Jin, Y.; Ye, P.; Duan, L. Association of Frailty Status with Risk of Fall among Middle-Aged and Older Adults in China: A Nationally Representative Cohort Study. J. Nutr. Health Aging 2021, 25, 985–992. [Google Scholar] [CrossRef]
- Chittrakul, J.; Siviroj, P.; Sungkarat, S.; Sapbamrer, R. Physical Frailty and Fall Risk in Community-Dwelling Older Adults: A Cross-Sectional Study. J. Aging Res. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a Predictor of Future Falls Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Chang, S.F. Frailty as a Risk Factor for Falls Among Community Dwelling People: Evidence from a Meta-Analysis. J. Nurs. Scholarsh. 2017, 49, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Gallo da Silva, T.T.; Filho, J.M.; Biesek, S.; Vojciechowski, A.S.; Borba, V.Z.C.; Gomes, A.R.S. Accuracy of Tools to Differentiate Single From Recurrent Fallers Pre-Frail Older Women. Front. Public. Health 2022, 10, 716851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, X.; Liu, H.; Ding, H. Frailty as a predictor of future falls and disability: A four-year follow-up study of Chinese older adults. BMC Geriatr. 2020, 20, 388. [Google Scholar] [CrossRef]
- World Health Organization. Step Safely: Strategies for Preventing and Managing Falls across the Life-Course. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/publications/i/item/9789240021914 (accessed on 1 June 2024).
- Montero-Odasso, M.; Montero-Odasso, M.; van der Velde, N.; van der Velde, N.; Martin, F.C.; Martin, F.C.; Petrovic, M.; Petrovic, M.; Tan, M.P.; Tan, M.P.; et al. Task Force on Global Guidelines for Falls in Older Adults. World guidelines for falls prevention and management for older adults: A global initiative. Age Ageing 2022, 51, afac205. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Abizanda, P.; Romero, L.; Sanchez-Jurado, P.; Atienzar-Núñez, P.; Esquinas-Requena, J.; Garcia-Nogueras, I. Association between Functional Assessment Instruments and Frailty in Older Adults: The FRADEA Study. J. Frailty Aging 2012, 1, 162–168. [Google Scholar] [CrossRef]
- Marzetti, E.; Cesari, M.; Calvani, R.; Msihid, J.; Tosato, M.; Rodriguez-Mañas, L.; Lattanzio, F.; Cherubini, A.; Bejuit, R.; Di Bari, M.; et al. SPRINTT Consortium. 2018. The “Sarcopenia and Physical fRailty IN older people: Multi-componenT Treatment strategies” (SPRINTT) randomized controlled trial: Case finding, screening and characteristics of eligible participants. Exp. Gerontol. 2018, 113, 48–57. [Google Scholar] [CrossRef]
- Jung, H.-W.; Baek, J.Y.; Jang, I.-Y.; Guralnik, J.M.; Rockwood, K.; Lee, E.; Kim, D.H. Short Physical Performance Battery as a Crosswalk Between Frailty Phenotype and Deficit Accumulation Frailty Index. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2249–2255. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; López Sáez de Asteasu, M.; Morley, J.E.; Cano-Gutierrez, C.A.; Izquierdo, M. Performance of the Short Physical Performance Battery in Identifying the Frailty Phenotype and Predicting Geriatric Syndromes in Community-Dwelling Elderly. J. Nutr. Health Aging 2021, 25, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, H.; Yu, L.; Yeung, E.H.K.; Luo, J.; Tsui, K.-L.; Zhao, Y. A Systematic Review of Wearable Sensor-Based Technologies for Fall Risk Assessment in Older Adults. Sensors 2022, 22, 6752. [Google Scholar] [CrossRef]
- Brognara, L.; Mazzotti, A.; Di Martino, A.; Faldini, C.; Cauli, O. Wearable Sensor for Assessing Gait and Postural Alterations in Patients with Diabetes: A Scoping Review. Medicina 2021, 57, 1145. [Google Scholar] [CrossRef] [PubMed]
- García-Villamil, G.; Neira-Álvarez, M.; Huertas-Hoyas, E.; Jiménez, A.R.; Rodríguez-Sánchez, C. A Pilot Study to Validate a Wearable Inertial Sensor for Gait Assessment in Older Adults with Falls. Sensors 2021, 21, 4334. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, L.; Jimenez, A.R.; Garcia-Villamil, G.; Seco, F. Detecting Fall Risk and Frailty in Elders with Inertial Motion Sensors: A Survey of Significant Gait Parameters. Sensors 2021, 21, 6918. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, J.; Zhao, Q.; Chen, M.; Luo, J.; Wang, H.; Yu, L.; Tsui, K.-L.; Zhao, Y. Identifying sensors-based parameters associated with fall risk in community-dwelling older adults: An investigation and interpretation of discriminatory parameters. BMC Geriatr. 2024, 24, 125. [Google Scholar] [CrossRef]
- Greene, B.R.; McManus, K.; Redmond, S.J.; Caulfield, B.; Quinn, C.C. Digital assessment of falls risk, frailty, and mobility impairment using wearable sensors. Npj Digit. Med. 2019, 2, 125. [Google Scholar] [CrossRef]
- Li, K.-J.; Wong, N.L.-Y.; Law, M.-C.; Lam, F.M.-H.; Wong, H.-C.; Chan, T.-O.; Wong, K.-N.; Zheng, Y.-P.; Huang, Q.-Y.; Wong, A.Y.-L.; et al. Reliability, Validity, and Identification Ability of a Commercialized Waist-Attached Inertial Measurement Unit (IMU) Sensor-Based System in Fall Risk Assessment of Older People. Biosensors 2023, 13, 998. [Google Scholar] [CrossRef]
- Vavasour, G.; Giggins, O.M.; Doyle, J.; Kelly, D. How wearable sensors have been utilised to evaluate frailty in older adults: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Razjouyan, J.; Naik, A.D.; Horstman, M.J.; Kunik, M.E.; Amirmazaheri, M.; Zhou, H.; Sharafkhaneh, A.; Najafi, B. Wearable Sensors and the Assessment of Frailty among Vulnerable Older Adults: An Observational Cohort Study. Sensors 2018, 18, 1336. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, S.; Mohler, J.; Toosizadeh, N.; Grewal, G.S.; Najafi, B. Postural Transitions during Activities of Daily Living Could Identify Frailty Status: Application of Wearable Technology to Identify Frailty during Unsupervised Condition. Gerontology 2017, 63, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.; Howe, C.; Saleh, A.; Mohler, J.; Grewal, G.; Armstrong, D.; Najafi, B. Frailty and technology: A systematic review of gait analysis in those with frailty. Gerontology 2014, 60, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Nihey, F.; Ihara, K.; Fukushi, K.; Kajitani, H.; Nozaki, Y.; Nakahara, K. Healthcare Application of In-Shoe Motion Sensor for Older Adults: Frailty Assessment Using Foot Motion during Gait. Sensors 2023, 23, 5446. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.N.; Ruiz, A.R.J.; Neira, G.G.; Huertas-Hoyas, E.; Cerda, M.T.E.; Delgado, L.P.; Robles, E.R.; Del-Ama, A.J.; Ruiz-Ruiz, L.; García-de-Villa, S.; et al. Assessing falls in the elderly population using G-STRIDE foot-mounted inertial sensor. Sci. Rep. 2023, 13, 9208. [Google Scholar] [CrossRef] [PubMed]
- Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J. Am. Geriatr. Soc. 2011, 59, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Auer, S.; Reisberg, B. The GDS/FAST Staging System. Int. Psychogeriatr. 1997, 9, 167–171. Available online: https://www.cambridge.org/core/product/identifier/S1041610297004869/type/journal_article (accessed on 1 June 2024). [CrossRef]
- Kempen, G.I.J.M.; Yardley, L.; Van Haastregt, J.C.M.; Zijlstra, G.A.R.; Beyer, N.; Hauer, K.; Todd, C. The Short FES-I: A shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing 2008, 37, 45–50. [Google Scholar] [CrossRef]
- Alonso Bouzón, C.; Carnicero, J.A.; Turín, J.G.; García-García, F.J.; Esteban, A.; Rodríguez-Mañas, L. The Standardization of Frailty Phenotype Criteria Improves Its Predictive Ability: The Toledo Study for Healthy Aging. J. Am. Med. Dir. Assoc. 2017, 18, 402–408. [Google Scholar] [CrossRef]
- García-Villamil, G.; Neira-Álvarez, M.; Huertas-Hoyas, E.; Ruiz-Ruiz, L.; García-de-Villa, S.; Del-Ama, A.; Rodríguez-Sánchez, M.C.; Jiménez-Ruiz, A. GSTRIDE: A Database of Frailty and Functional Assessments with Inertial Gait Data from Elderly Fallers and Non-Fallers Populations. 2022. Available online: https://zenodo.org/records/6883292 (accessed on 1 June 2024). [CrossRef]
- Park, S.H. Tools for assessing fall risk in the elderly: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2018, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Beck Jepsen, D.; Robinson, K.; Ogliari, G.; Montero-Odasso, M.; Kamkar, N.; Ryg, J.; Freiberger, E.; Masud, T. Predicting falls in older adults: An umbrella review of instruments assessing gait, balance, and functional mobility. BMC Geriatr. 2022, 22, 615. [Google Scholar] [CrossRef]
- Esbri-Victor, M.; Huedo-Rodenas, I.; Lopez-Utiel, M.; Navarro-Lopez, J.L.; Martinez-Reig, M.; Serra-Rexach, J.A.; Romero-Rizos, L.; Abizanda, P. Frailty and Fear of Falling: The FISTAC Study. J. Frailty Aging 2017, 6, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Gazibara, T.; Kurtagic, I.; Kisic-Tepavcevic, D.; Nurkovic, S.; Kovacevic, N.; Gazibara, T.; Pekmezovic, T. Falls, risk factors and fear of falling among persons older than 65 years of age. Psychogeriatrics 2017, 17, 215–223. [Google Scholar] [CrossRef]
- Greene, B.R.; Doheny, E.P.; Kenny, R.A.; Caulfield, B. Classification of frailty and falls history using a combination of sensor-based mobility assessments. Physiol. Meas. 2014, 35, 2053–2066. [Google Scholar] [CrossRef]
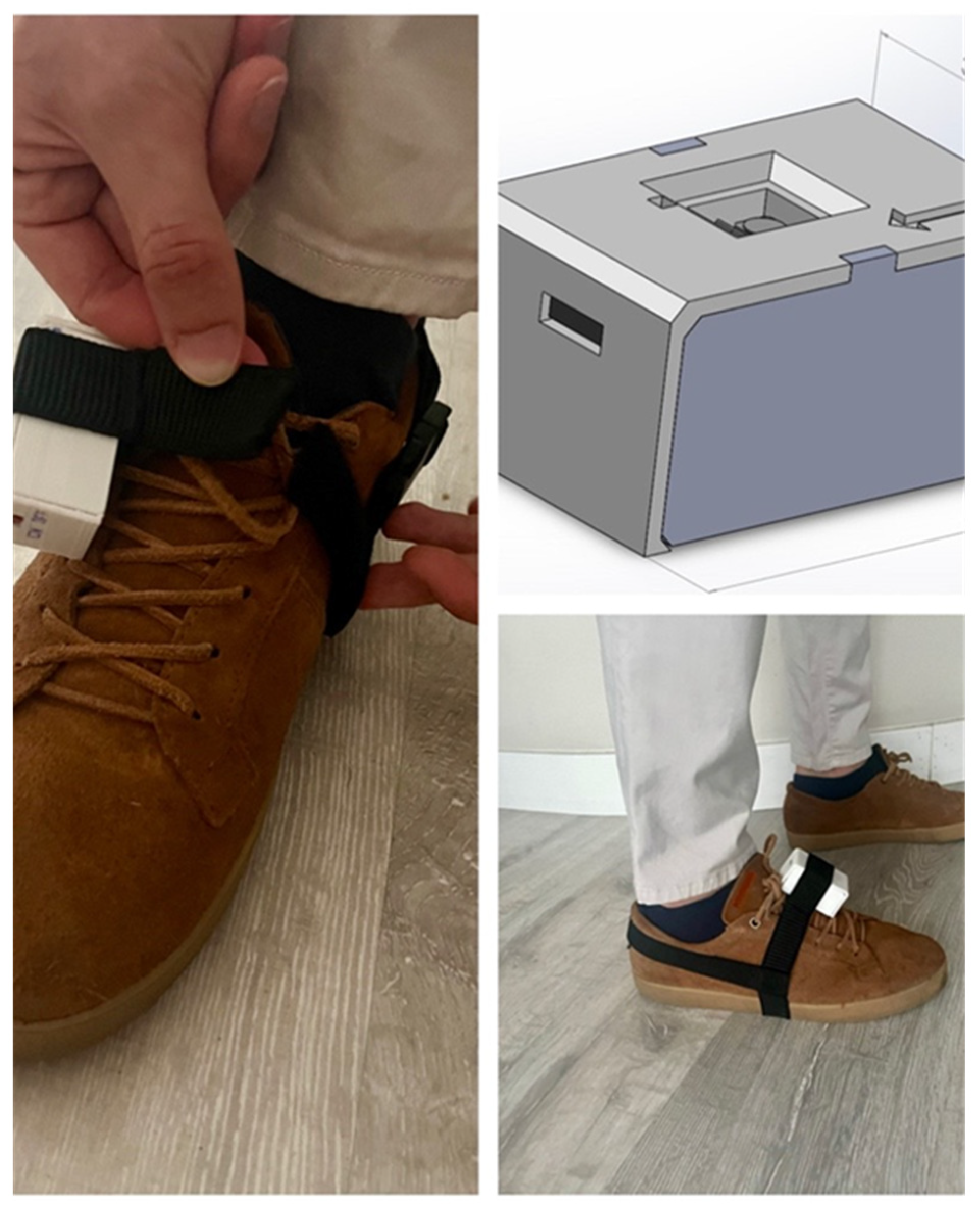
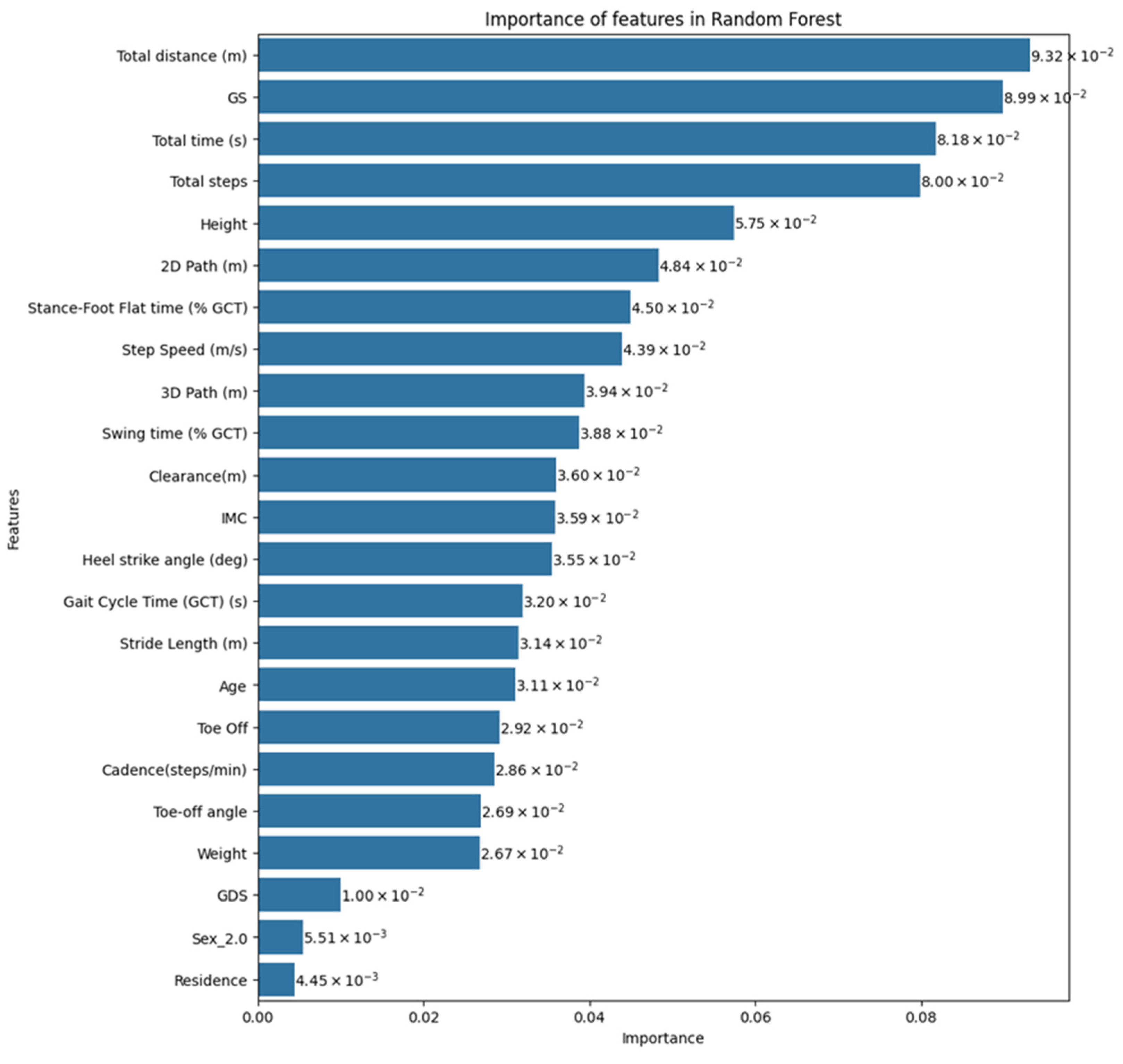
| Parameter | Description |
|---|---|
| Total distance (m) | The total distance covered during a long free walk. |
| Total time (s) | The total time spent in the free walk. |
| Total steps | The total number of steps in the free walk. |
| Gait cycle time (GCT) (s) | The time elapsed during a stride. |
| Velocity (m/s) | The mean walking speed computed over the total detected steps measured in meters per second. |
| Cadence (steps/min) | The number of steps per minute. |
| Swing time (% GCT) | Swing time (from toe-off to heel strike) as percentage of GCT. |
| Stance-foot flat time (% GCT) | Foot-flat (from start to end) time as percentage of GCT. It occurs between toe-strike and heel-off. |
| Toe Off (% GCT) | Percentage of the stride since the mid-stance until the foot is in the Toe Off instant, which corresponds to the Push Off phase. |
| Heel strike angle (deg) | The maximum pitch angle at heel strike measured in degrees. |
| Toe-off angle (deg) | The maximum pitch angle at toe off measured in degrees. |
| Stride Length (m) | Distance from one stance position to the next stance of the same foot. |
| Step Speed (m/s) | The forward speed of the foot only during the swing phase. |
| 2D Path (m) | The path length of the foot in the horizontal plane with respect to the stride length, which includes the lateral deviations of the strides. |
| 3D Path (m) | The path length of the foot in 3D space during a step with respect to the stride length, which includes the lateral deviations of strides and the foot elevation. |
| Clearance(m) | The clearance or maximum height of the foot with respect to the ground during the swing phase. |
| Class | Precision | Recall (TPR) | F1 Score | Specificity |
|---|---|---|---|---|
| Fit with falls | 0.88 | 0.98 | 0.93 | 0.98 |
| Fit without falls | 0.88 | 0.9 | 0.89 | 0.97 |
| Frail without falls | 1.0 | 0.14 | 0.25 | 1.0 |
| Frail with falls | 0.94 | 0.86 | 0.90 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neira Álvarez, M.; Huertas-Hoyas, E.; Novak, R.; Sipols, A.E.; García-Villamil-Neira, G.; Rodríguez-Sánchez, M.C.; Del-Ama, A.J.; Ruiz-Ruiz, L.; De Villa, S.G.; Jiménez-Ruiz, A.R. Stratification of Older Adults According to Frailty Status and Falls Using Gait Parameters Explored Using an Inertial System. Appl. Sci. 2024, 14, 6704. https://doi.org/10.3390/app14156704
Neira Álvarez M, Huertas-Hoyas E, Novak R, Sipols AE, García-Villamil-Neira G, Rodríguez-Sánchez MC, Del-Ama AJ, Ruiz-Ruiz L, De Villa SG, Jiménez-Ruiz AR. Stratification of Older Adults According to Frailty Status and Falls Using Gait Parameters Explored Using an Inertial System. Applied Sciences. 2024; 14(15):6704. https://doi.org/10.3390/app14156704
Chicago/Turabian StyleNeira Álvarez, Marta, Elisabet Huertas-Hoyas, Robert Novak, Ana Elizabeth Sipols, Guillermo García-Villamil-Neira, M. Cristina Rodríguez-Sánchez, Antonio J. Del-Ama, Luisa Ruiz-Ruiz, Sara García De Villa, and Antonio R. Jiménez-Ruiz. 2024. "Stratification of Older Adults According to Frailty Status and Falls Using Gait Parameters Explored Using an Inertial System" Applied Sciences 14, no. 15: 6704. https://doi.org/10.3390/app14156704
APA StyleNeira Álvarez, M., Huertas-Hoyas, E., Novak, R., Sipols, A. E., García-Villamil-Neira, G., Rodríguez-Sánchez, M. C., Del-Ama, A. J., Ruiz-Ruiz, L., De Villa, S. G., & Jiménez-Ruiz, A. R. (2024). Stratification of Older Adults According to Frailty Status and Falls Using Gait Parameters Explored Using an Inertial System. Applied Sciences, 14(15), 6704. https://doi.org/10.3390/app14156704












