Heterocyclic Nitrogen Compounds as Potential PDE4B Inhibitors in Activated Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. Ethyl 5,6-Dimethoxy-1H-indole-2-carboxylate (1)
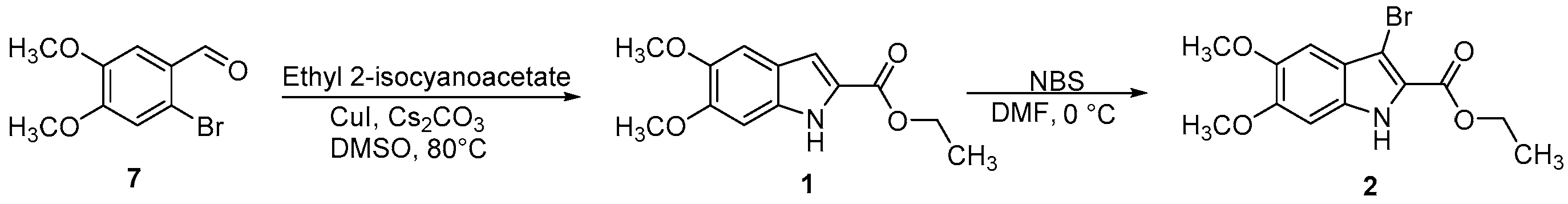
2.1.2. Ethyl 3-Bromo-5,6-dimethoxy-1H-indole-2-carboxylate (2)
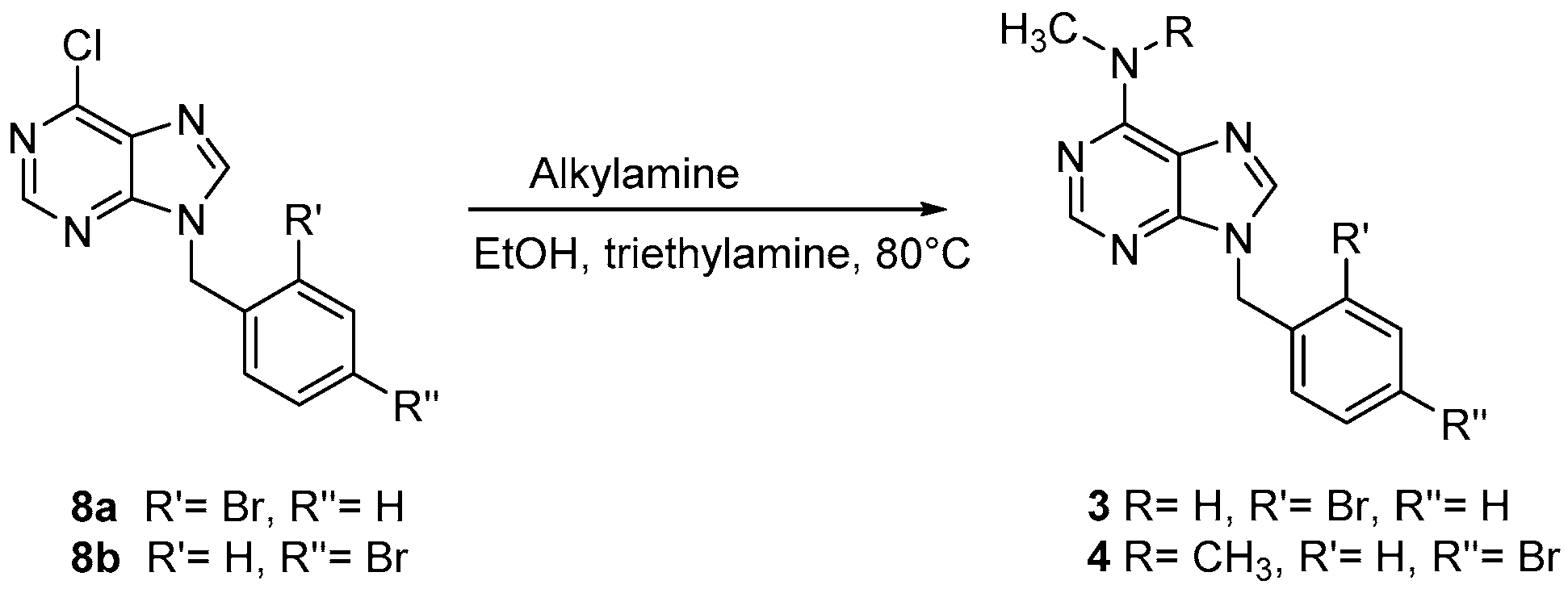
2.1.3. General Procedure for the Synthesis of Compounds 3 and 4
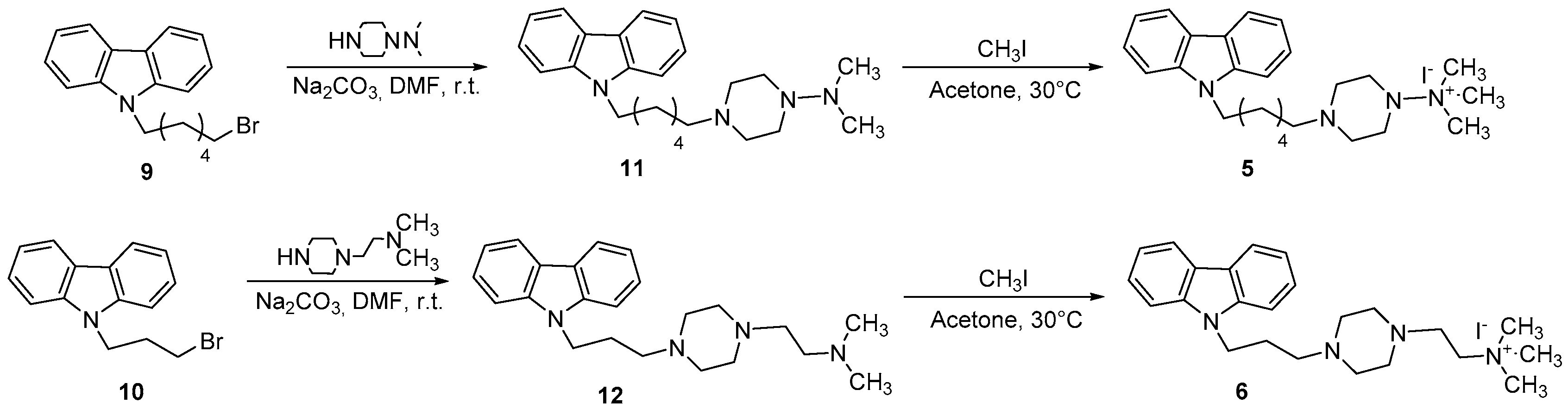
2.1.4. General Procedure for the Synthesis of Compounds 5 and 6
2.1.5. General Procedure for the Synthesis of Compounds 11 and 12
2.2. Human PDE4B ELISA Assay
2.3. Isolation of Human PBMCs and Differentiation of PBMC-Derived Macrophages
2.4. Cell Viability Assay
2.5. Reactive Oxygen Species (ROS) Detection
2.6. Statistical Analysis
3. Results and Discussion
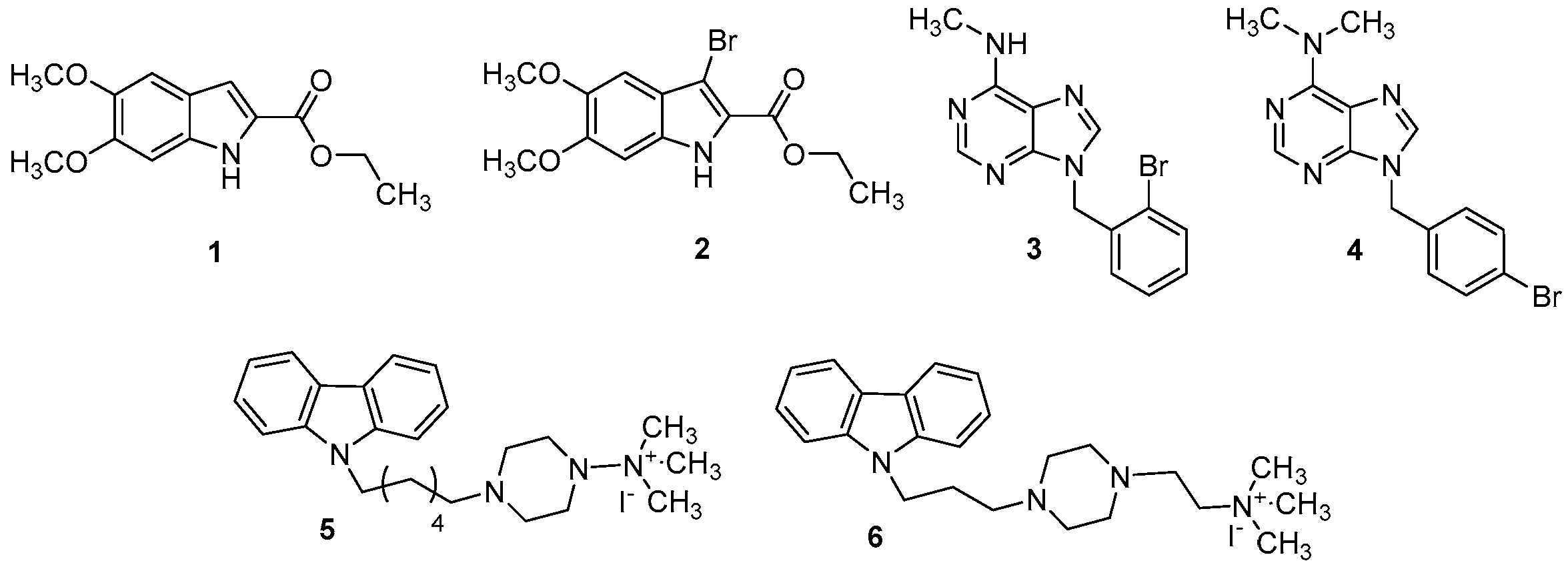
3.1. Chemistry
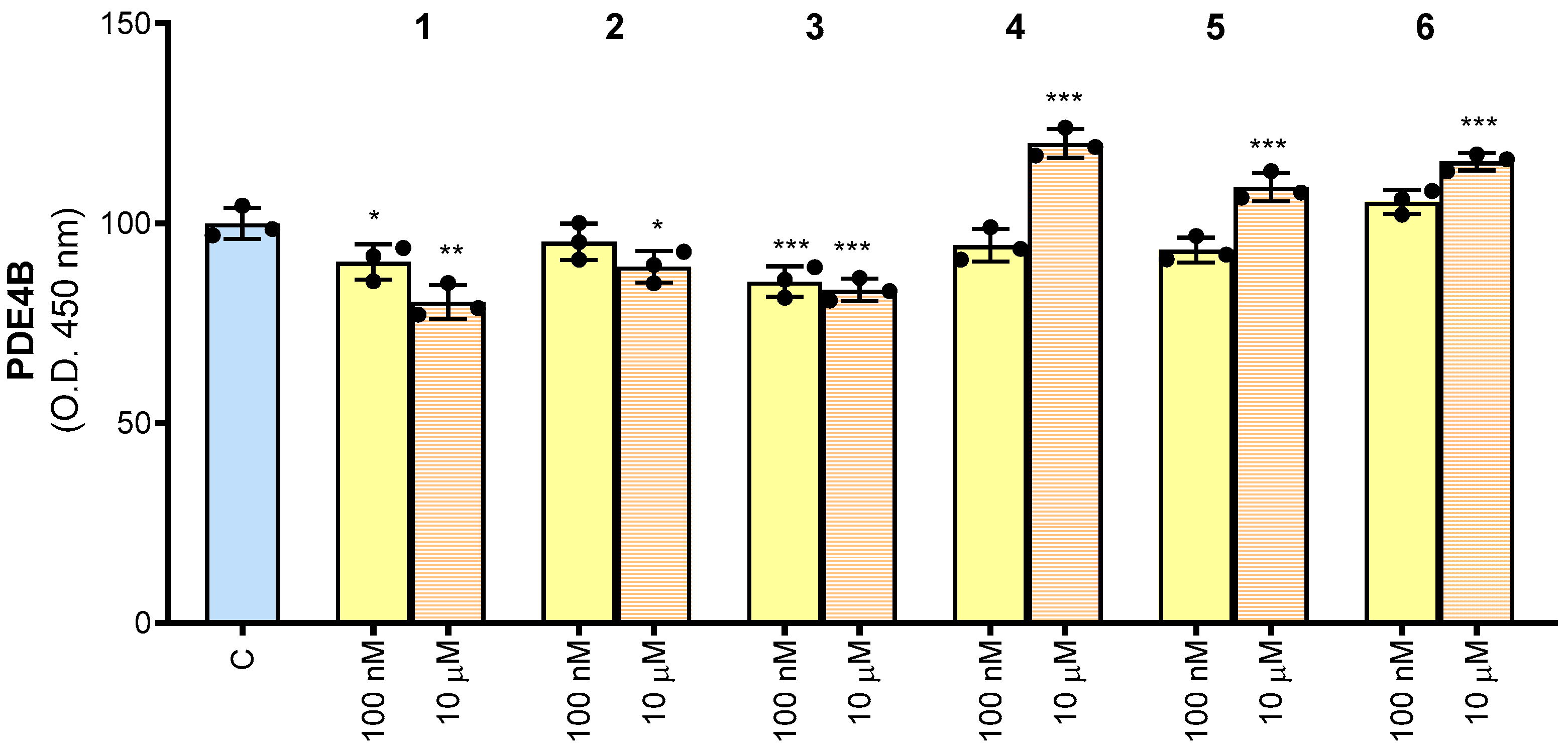
3.2. Human PDE4B Is Affected by Synthetic Molecules 1–6
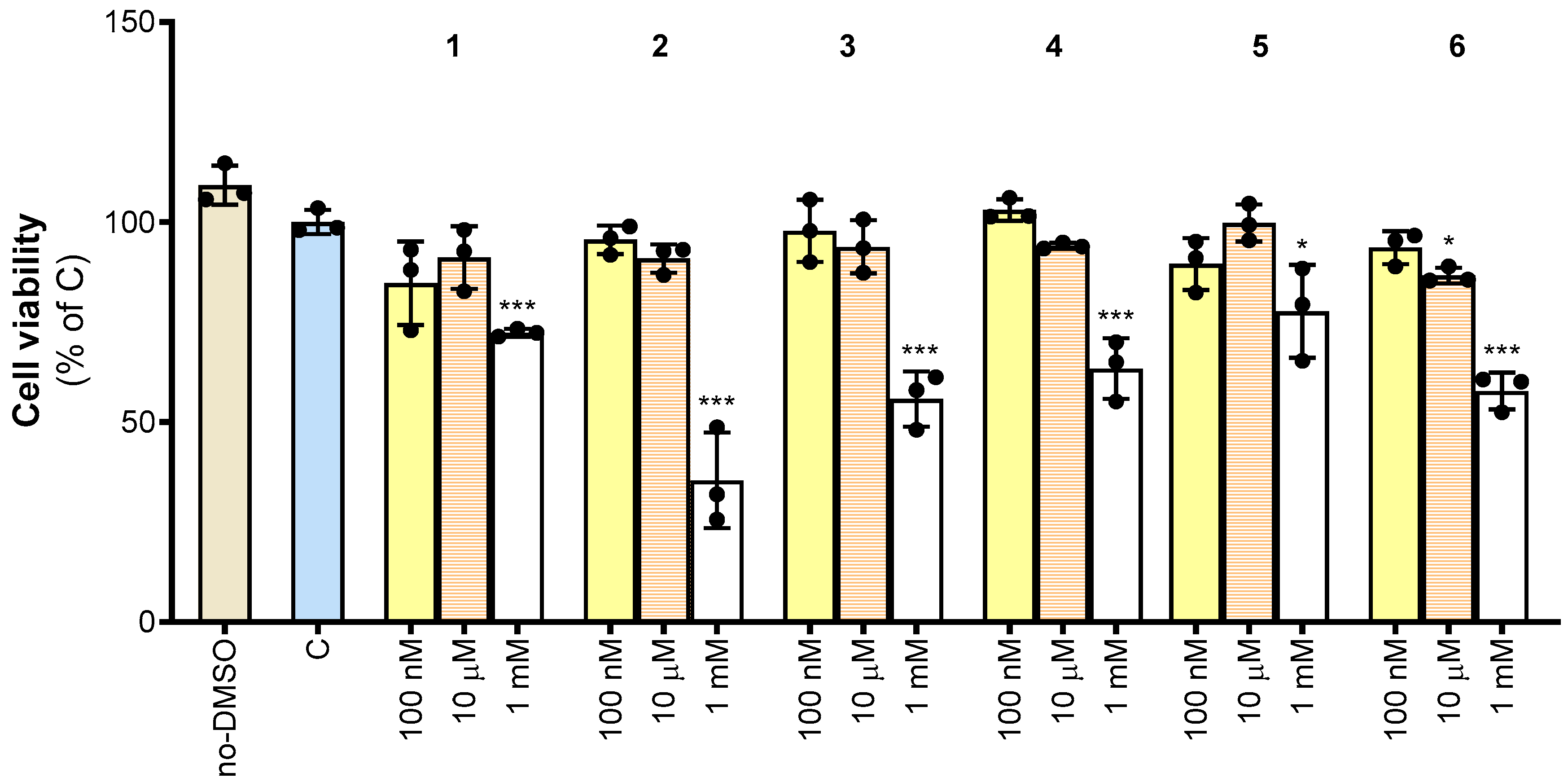
3.3. Effect of Molecules 1–6 on Cell Viability
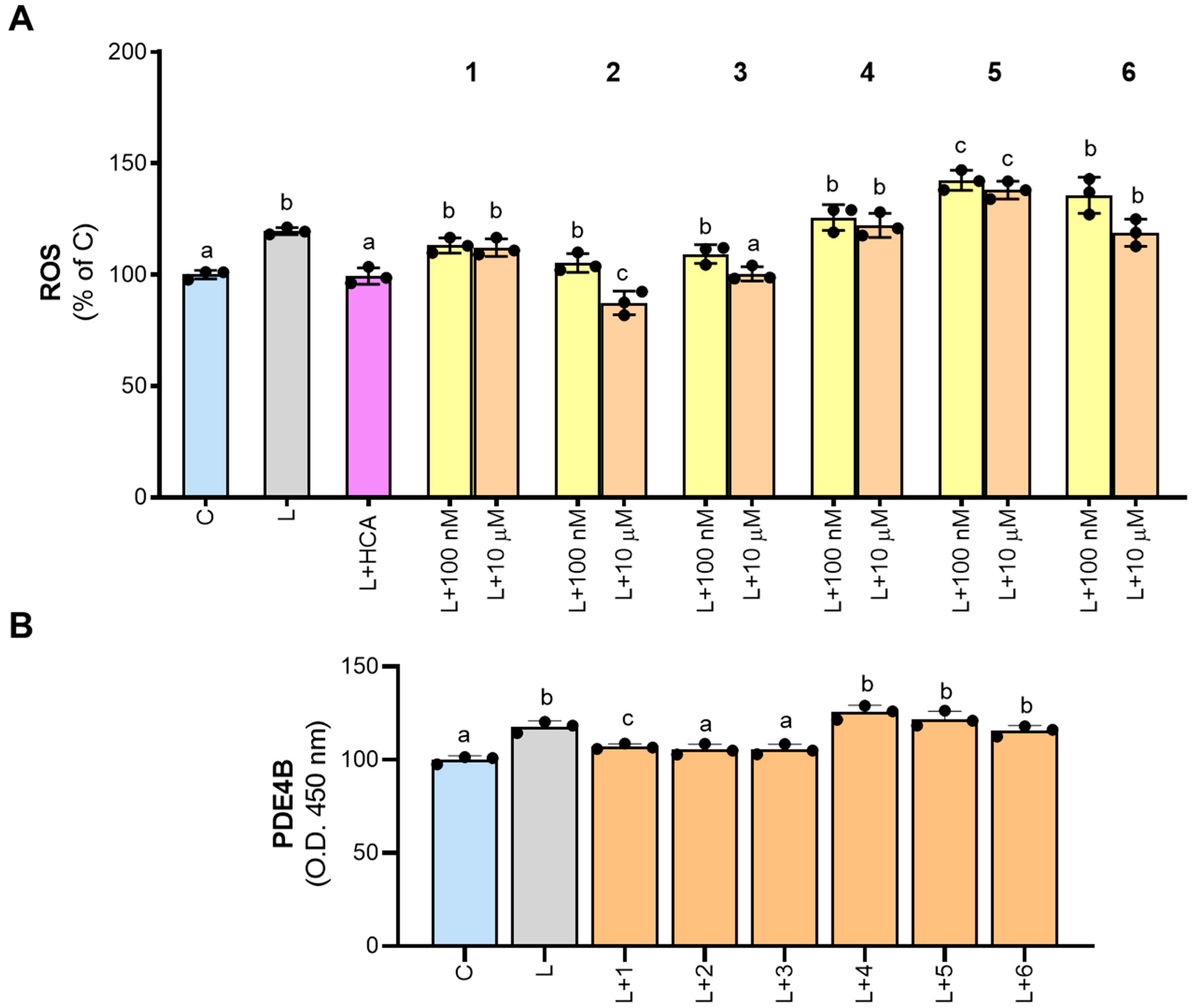
3.4. Compounds 1–3 Affect LPS-Induced PDE4B Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Peng, M.S.; Chen, Y.; Geng, J.; Robinson, H.; Houslay, M.D.; Cai, J.; Ke, H. Structures of the four subfamilies of phosphodiesterase-4 provide insight into the selectivity of their inhibitors. Biochem. J. 2007, 408, 193–201. [Google Scholar] [CrossRef]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef]
- Francis, S.H.; Morris, G.Z.; Corbin, J.D. Molecular mechanisms that could contribute to prolonged effectiveness of PDE5 inhibitors to improve erectile function. Int. J. Impot. Res. 2008, 20, 333–342. [Google Scholar] [CrossRef]
- Jin, S.L.; Ding, S.L.; Lin, S.C. Phosphodiesterase 4 and its inhibitors in inflammatory diseases. Chang. Gung Med. J. 2012, 35, 197–210. [Google Scholar] [CrossRef]
- Ma, D.; Wu, P.; Egan, R.W.; Billah, M.M.; Wang, P. Phosphodiesterase 4B gene transcription is activated by lipopolysaccharide and inhibited by interleukin-10 in human monocytes. Mol. Pharmacol. 1999, 55, 50–57. [Google Scholar] [CrossRef]
- Jin, S.L.; Lan, L.; Zoudilova, M.; Conti, M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J. Immunol. 2005, 175, 1523–1531. [Google Scholar] [CrossRef]
- Dhar, R.; Rana, M.N.; Zhang, L.; Li, Y.; Li, N.; Hu, Z.; Yan, C.; Wang, X.; Zheng, X.; Liu, H.; et al. Phosphodiesterase 4B is required for NLRP3 inflammasome activation by positive feedback with Nrf2 in the early phase of LPS- induced acute lung injury. Free Radic. Biol. Med. 2021, 176, 378–391. [Google Scholar] [CrossRef]
- Jin, S.L.; Conti, M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc. Natl. Acad. Sci. USA 2002, 99, 7628–7633. [Google Scholar] [CrossRef]
- Iacobazzi, D.; Convertini, P.; Todisco, S.; Santarsiero, A.; Iacobazzi, V.; Infantino, V. New Insights into NF-κB Signaling in Innate Immunity: Focus on Immunometabolic Crosstalks. Biology 2023, 12, 776. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef]
- Netea, M.G.; Nold-Petry, C.A.; Nold, M.F.; Joosten, L.A.; Opitz, B.; van der Meer, J.H.; van de Veerdonk, F.L.; Ferwerda, G.; Heinhuis, B.; Devesa, I.; et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 2009, 113, 2324–2335. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zhang, T.; Ma, Y.; Tong, X.; Fan, H. The role of immunometabolism in macrophage polarization and its impact on acute lung injury/acute respiratory distress syndrome. Front. Immunol. 2023, 14, 1117548. [Google Scholar] [CrossRef]
- Infantino, V.; Pierri, C.L.; Iacobazzi, V. Metabolic Routes in Inflammation: The Citrate Pathway and its Potential as Therapeutic Target. Curr. Med. Chem. 2019, 26, 7104–7116. [Google Scholar] [CrossRef]
- Convertini, P.; Santarsiero, A.; Todisco, S.; Gilio, M.; Palazzo, D.; Pappalardo, I.; Iacobazzi, D.; Frontuto, M.; Infantino, V. ACLY as a modulator of liver cell functions and its role in Metabolic Dysfunction-Associated Steatohepatitis. J. Transl. Med. 2023, 21, 568. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A. Master and commander: Epigenetic regulation of macrophages. Cell Res. 2016, 26, 145–146. [Google Scholar] [CrossRef]
- Santarsiero, A.; Onzo, A.; Pascale, R.; Acquavia, M.A.; Coviello, M.; Convertini, P.; Todisco, S.; Marsico, M.; Pifano, C.; Iannece, P.; et al. Hydrosol: Untargeted Metabolomic Analysis and Anti-Inflammatory Activity Mediated by NF-κB and the Citrate Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 4264815. [Google Scholar] [CrossRef]
- Santarsiero, A.; Convertini, P.; Todisco, S.; Pierri, C.L.; De Grassi, A.; Williams, N.C.; Iacobazzi, D.; De Stefano, G.; O’Neill, L.A.J.; Infantino, V. ACLY Nuclear Translocation in Human Macrophages Drives Proinflammatory Gene Expression by NF-κB Acetylation. Cells 2021, 10, 2962. [Google Scholar] [CrossRef]
- Saturnino, C.; Caruso, A.; Iacopetta, D.; Rosano, C.; Ceramella, J.; Muià, N.; Mariconda, A.; Bonomo, M.G.; Ponassi, M.; Rosace, G.; et al. Inhibition of Human Topoisomerase II by N,N,N-Trimethylethanammonium Iodide Alkylcarbazole Derivatives. ChemMedChem 2018, 13, 2635–2643. [Google Scholar] [CrossRef]
- Caruso, A.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Mauro, M.V.; Bruno, R.; Aquaro, S.; Sinicropi, M.S. Carbazole Derivatives as Antiviral Agents: An Overview. Molecules 2019, 24, 1912. [Google Scholar] [CrossRef]
- Giuzio, F.; Bonomo, M.G.; Catalano, A.; Infantino, V.; Salzano, G.; Monné, M.; Geronikaki, A.; Petrou, A.; Aquaro, S.; Sinicropi, M.S.; et al. Potential PDE4B inhibitors as promising candidates against SARS-CoV-2 infection. Biomol. Concepts 2023, 14, 20220033. [Google Scholar] [CrossRef]
- Saturnino, C.; Iacopetta, D.; Sinicropi, M.S.; Rosano, C.; Caruso, A.; Caporale, A.; Marra, N.; Marengo, B.; Pronzato, M.A.; Parisi, O.I.; et al. N-alkyl carbazole derivatives as new tools for Alzheimer’s disease: Preliminary studies. Molecules 2014, 19, 9307–9317. [Google Scholar] [CrossRef]
- Saturnino, C.; Grande, F.; Aquaro, S.; Caruso, A.; Iacopetta, D.; Bonomo, M.G.; Longo, P.; Schols, D.; Sinicropi, M.S. Chloro-1,4-dimethyl-9H-carbazole Derivatives Displaying Anti-HIV Activity. Molecules 2018, 23, 286. [Google Scholar] [CrossRef]
- Aebly, A.H.; Levy, J.N.; Steger, B.J.; Quirke, J.C.; Belitsky, J.M. Expedient synthesis of eumelanin-inspired 5,6-dihydroxyindole-2-carboxylate ethyl ester derivatives. RSC Adv. 2018, 8, 28323–28328. [Google Scholar] [CrossRef]
- Deng, H.; Fang, Y. Synthesis and Agonistic Activity at the GPR35 of 5,6-Dihydroxyindole-2-carboxylic Acid Analogues. ACS Med. Chem. Lett. 2012, 3, 550–554. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, Z.; Jiang, W.; Lin, D. C-H Amination of Purine Derivatives via Radical Oxidative Coupling. J. Org. Chem. 2018, 83, 3710–3718. [Google Scholar] [CrossRef]
- Marsico, M.; Santarsiero, A.; Pappalardo, I.; Convertini, P.; Chiummiento, L.; Sardone, A.; Di Noia, M.A.; Infantino, V.; Todisco, S. Mitochondria-Mediated Apoptosis of HCC Cells Triggered by Knockdown of Glutamate Dehydrogenase 1: Perspective for Its Inhibition through Quercetin and Permethylated Anigopreissin A. Biomedicines 2021, 9, 1664. [Google Scholar] [CrossRef]
- Su, Y.; Ding, J.; Yang, F.; He, C.; Xu, Y.; Zhu, X.; Zhou, H.; Li, H. The regulatory role of PDE4B in the progression of inflammatory function study. Front. Pharmacol. 2022, 13, 982130. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Barua, S.; Kim, J.Y.; Yenari, M.A.; Lee, J.E. The role of NOX inhibitors in neurodegenerative diseases. IBRO Rep. 2019, 7, 59–69. [Google Scholar] [CrossRef]
- Kaur, P.; Khan, H.; Grewal, A.K.; Dua, K.; Singh, T.G. Therapeutic potential of NOX inhibitors in neuropsychiatric disorders. Psychopharmacology 2023, 240, 1825–1840. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Spinelli, F.R.; Rosado, M.M.; Conti, F.; Laganà, B. Inhibition of Phosphodiesterase-4 in Psoriatic Arthritis and Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 2638. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todisco, S.; Infantino, V.; Caruso, A.; Santarsiero, A.; Convertini, P.; El-Kashef, H.; Giuzio, F.; Sinicropi, M.S.; Saturnino, C. Heterocyclic Nitrogen Compounds as Potential PDE4B Inhibitors in Activated Macrophages. Appl. Sci. 2024, 14, 6747. https://doi.org/10.3390/app14156747
Todisco S, Infantino V, Caruso A, Santarsiero A, Convertini P, El-Kashef H, Giuzio F, Sinicropi MS, Saturnino C. Heterocyclic Nitrogen Compounds as Potential PDE4B Inhibitors in Activated Macrophages. Applied Sciences. 2024; 14(15):6747. https://doi.org/10.3390/app14156747
Chicago/Turabian StyleTodisco, Simona, Vittoria Infantino, Anna Caruso, Anna Santarsiero, Paolo Convertini, Hussein El-Kashef, Federica Giuzio, Maria Stefania Sinicropi, and Carmela Saturnino. 2024. "Heterocyclic Nitrogen Compounds as Potential PDE4B Inhibitors in Activated Macrophages" Applied Sciences 14, no. 15: 6747. https://doi.org/10.3390/app14156747





