Abstract
Background: Osseointegration is fundamental to achieving successful implant therapy in dentistry. However, the heat generated during implant placement emerges as a critical factor predisposing to implant failure. Objective: This study aimed to analyze the different factors related to heat generation during implant placement, offering insights to clinicians in their daily clinical practice. Methods: Utilizing the PubMed, Web of Science, and Embase databases, we conducted an electronic search for articles published between January 2013 and December 2023. The analysis focused on several factors including bone type, drill shape, drill speed, drill material, drilling force, osteotomy depth, drill load, drilling technique (intermittent or continuous), presence of a surgical guide, irrigation methods, drill wear, and preparation tools available. Results: Initially, 2525 records were identified. After applying the inclusion and exclusion criteria and full-text assessment, 93 articles were included in this scoping review. Additionally, some articles published before 2013 were incorporated in the bibliography to ensure completeness of the review. Conclusions: Heat generation during implant placement arises from a complex interplay of multiple factors. While irrigation and bone hardness appear to be crucial determinants of heat generation during the osteotomy phase, the involvement of other factors remains less clear. Further studies are needed to better understand the precise contribution of these factors towards increasing temperature at the implant site.
1. Introduction
Bone tissue is a specialized connective tissue composed of cells embedded in a mineralized extracellular matrix. Ideal bone healing leads to complete restoration of both the morphology and function of the damaged tissue. In the realm of implant therapy, the successful integration of bone and implant without the interposition of soft tissue, known as osseointegration, is based on the principle of bone regeneration [1]. Failure of osseointegration due to interference in the healing process can result in what the literature refers to as “early failure” [2]. Extensive research has elucidated numerous factors that influence osseointegration, including the characteristics of the implant screw, factors related to the bone site, diverse surgical protocols, implant biomechanics, the use of adjuvant treatments such as bone regeneration, drug therapies, and the general health status of the patient [1,2,3].
Regarding surgical protocols, heat generation during implant site preparation has been recognized as a critical factor affecting bone tissue viability and subsequent implant success [4,5]. However, our understanding of the impact of heat generation during the osteotomy phase of implant placement remains incomplete.
While there seems to be consensus that surgical interventions cause cell death to some extent [5], preservation of bone viability is critical for osseointegration [5,6]. However, it is difficult to link biologic performance to one specific factor, i.e., temperature development vs. mechanical stress [7]. Based on an animal trial, it was concluded that the influence of drill speed and irrigation would be minimal in terms of the temperature of the cortical bone, primary and secondary implant stability, and osseointegration [5,6,7]. Given the widespread use of drilling as a technique for implant site preparation as well as the advanced devices available today, it has been questioned whether or not heat above the critical temperature for bone necrosis [8,9] can be generated at all, if accepted protocols are followed.
For conventional drilling, the following procedural parameters have been described [8,9,10] to affect temperature development: rotational speed, proceeding speed, contact pressure, drilling motion pattern, bone density [9,10,11,12], drill depth [13], and irrigation [14,15]. Variables related directly to drill design also seem to play a role, with the major parameters being the number of drill blades, drill design (tapered vs. straight) [16], drill fatigue, and drill material and its heat capacity and thermal conductivity [14,15,16,17].
In an attempt to reduce surgery times, abbreviated drilling protocols have been advocated, for instance, using multistepped drills for single-stage implant site preparation [17]. In an animal trial, a novel drill design led to lower osteotomy temperature values and shorter drill times but also improved the osseointegration of dental implants [8]. In this context, low-speed drilling without irrigation has been shown to result in a greater quantity of and more beneficial cellular and histomorphologic properties of harvested bone with even greater osteotomy precision [18].
Applying diamond-like carbon (DLC) coatings on drills has been claimed to optimize existing surgical approaches. DLC coatings have already been shown to bear superior tribological and mechanical properties, leading to improved wear properties as well as reduced friction between mechanical components [18,19,20].
With the goal of shortening overall treatment times, clinicians have been trying to insert implants with maximum primary stability in order to limit the risk of excessive micromotion at the implant–bone interface during healing in immediate loading cases [21]. The undersizing of an osteotomy as well as using a tapered implant resulting in bone compression have been described as effective approaches for reaching high insertion torque values [20,21]. This effect of bone compression has been verified in finite element simulations showing a clear trend towards greater stress levels in bone with increasing levels of compression under preparation of the osteotomy [21,22]. Temperature rise during implant insertion has also been shown in an animal trial and seems to be a phenomenon based on friction between bone and the implant body [8,9]. Several authors [11,12] have claimed that bone damage during implant insertion will cause cracks to varying extents, which leads to bone resorption followed by new bone formation during the healing phase, which, however, will take longer as compared to areas which have not been damaged [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120].
Despite the existing gap in knowledge, recent research sheds light on the potential consequences of heat generation on bone tissue. For instance, Erikson et al. conducted two studies using animal bone tissue to show that bone is sensitive to heating of around 47 °C, and that heating to a temperature of 53 °C for one minute can halt blood circulation and may result in irreversible bone damage [6,7,8]. Bone heating can lead to implant failure and crestal bone loss during implant healing [9].
Considering this, our study aimed to elucidate the interplay between heat generation and implant success during the osteotomy phase. In this regard, we chose to conduct a scoping review. Scoping reviews primarily aim to identify and map available evidence and can serve as a valuable precursor to a more focused systematic review. By conducting a scoping review, our objective was to comprehensively map the extant evidence on the role of heat generation during implant placement and its implications for osseointegration, with the aim of providing clinicians with valuable insights to optimize their daily practice and ultimately enhance patient outcomes.
2. Materials and Methods
2.1. Identifying Question
This scoping review was performed to deal with the following question:
What is the current state of scientific knowledge based on the available literature on the various factors involved in heat generation during implant placement?
2.2. Search Strategy
The search strategy encompassed articles published between January 2013 and December 2023 in PubMed, Web of Science, and Embase databases. We chose this timeframe to focus on the most recent literature within the last decade, ensuring our review would capture the most up-to-date evidence on the topic. To ensure comprehensive coverage, select articles published before 2013 were included. Two independent reviewers (V.C. and R.D) screened and selected articles following the framework for scoping reviews of the PRISMA-ScR (PRISMA Extension for Scoping Reviews) guidelines [10]. The screening process involved assessing titles, abstracts, and texts to determine eligibility for inclusion. Any disagreements were resolved through discussion and consensus.
The search strategy was structured using Medical Subject Heading (MeSH) terms, including (“Drills for dental implants”) OR (“speed and force during osteotomy”) OR (“heat generated by drills for implants”) OR (“external and internal irrigation of drills for dental implants”) OR (“heat generated during osteotomy”) OR (“effect of heat on osseointegration”).
2.2.1. Inclusion Criteria
Articles eligible for inclusion were published between January 2013 and December 2023 and adhered to the selection criteria established from the current literature and systematic reviews. The following study designs were considered: meta-analyses, systematic reviews, randomized controlled trials, non-randomized controlled trials, and cohort studies. Included studies focused on the surgical phase of endosseous implant placement and associated heat generation, encompassing complete in vivo and in vitro articles that had already been published at the time of review. Articles that did not meet the inclusion criteria were excluded.
2.2.2. Exclusion Criteria
Excluded from the final analysis were case reports, case series, expert opinions, and articles published prior to January 2013.
2.3. Materials and Methods in Various Studies
The main methods used to measure temperature variations in bone are real-time infrared thermography and thermocouple thermometers. In the first case, an image of the infrared heat is displayed on a screen and analyzed by digital systems. In the second case, thermocouples are inserted into the body to be analyzed and connected directly to a machine that detects the temperature.
2.4. Analyzed Values
Analysis was based on in vitro studies or samples, (Table 1). Some in vivo studies were also included, with corresponding Implant Stability Quotient (ISQ) values and polymorphonuclear counts.

Table 1.
Analyzed factors.
3. Results
A total of 2525 studies were identified through our database search (Figure 1), (Table 2). During the initial screening phase, titles and abstracts were independently assessed to see if they aligned with the study’s objective. After the removal of 1106 duplicates, 855 articles were excluded after title screening and 215 after abstract screening for being unrelated to implant dentistry, and a total of 349 unique articles remained. The full-text analysis showed 185 articles not focusing on the specific theme of heating during implant placement, 67 articles with insufficient details for the methodology, and 4 failing to meet the inclusion criteria. The remaining 93 articles were further analyzed for final inclusion and qualitative analysis.
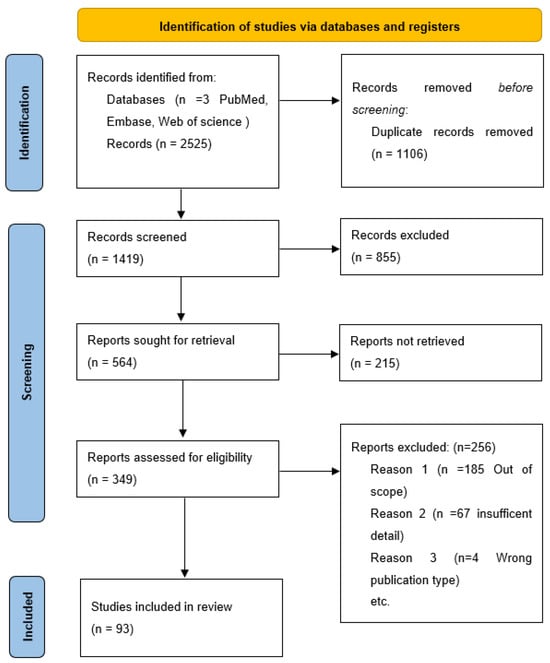
Figure 1.
PRISMA flow chart diagram of search strategy.

Table 2.
Database search.
The selection process and final inclusion of articles were summarized in a PRISMA flow diagram (Figure 1), (Table 2), illustrating the stages of the selection process. The final 93 articles were then reviewed and categorized based on the analyzed factors.
3.1. Type of Bone (Compact and Cancellous Bone)
One of the key factors widely analyzed in early implant research is the characteristics of the implant site. A distinction is made between cortical and cancellous bone. According to Misch et al. [11], bone can be classified according to its density, notably type D1 bone for predominantly compact and hard bone, and type D4 bone for less dense bone [12]. In general, anterior regions of the mandible and maxilla have higher densities compared to posterior regions [13].
Bone type significantly impacts heat generation during the osteotomy phase [14,15,16,17,18,19,20]. Compact or cortical bone, which has a lower water content than cancellous bone, has a higher thermal conductivity and therefore tends to develop higher temperatures for the same amount of heat supplied. Conversely, cancellous bone has better vascularization, making it superior at dissipating the heat generated during drilling [21]. The three-dimensional structure, higher water content, and presence of lipids in cancellous bone accentuates its ability to dissipate heat.
For these reasons, early implant failure in D1 bone is more prevalent than in less dense bone [22]. However, while it is clinically more difficult to achieve good primary stability in less dense bone, the superior healing capacities of cancellous bone have been reported [23], which enhances the possibility of osseointegration in this type of bone.
3.2. Cylindrical, Conical, and Trephine Drills
Another feature that can influence heat generation during implant preparation is the type of cutter employed. In general, milling cutters fall into two macro-categories: cylindrical and conical. Gehrke et al. conducted a study comparing the effects of cylindrical and conical cutters, examining both continuous and intermittent motion. They collected important data such as mean thermal variation, new bone formation, and polymorphonuclear cell count [24]. These findings revealed that the conical burr, whether used with continuous or intermittent motion, showed better results, with reduced thermal variation, increased new bone formation, and fewer polymorphonuclear cells after osteotomy. Measurements were carried out using K-type thermocouple sensors, positioning the gauge exactly 1 mm from the implant preparation area.
Soldatos et al. evaluated several variables in the context of implant preparation, also using K-type thermocouple sensors. They found that conical cutters generated heat without significant variations. In cylindrical drills, however, a relatively consistent increase in temperature was found with increasing depth [25].
In a different study by Omar et al., traditional burs were compared to trephine hollow burs and manual bone expanders. This study found that trephine burs produced the highest temperature values, followed by conventional burs and then bone expanders [26].
Gupta et al. compared conventional and hollow cutters (trephine drills) and found that the absence of irrigation led to much higher temperatures compared to the groups with irrigation. The only difference identified was that the effectiveness of irrigation was lower with hollow drills, likely due to their different shape and smaller cutting surface [27].
Notably, despite presenting significant differences in terms of heat generation among milling cutters, none of the cases analyzed exceeded the limit value set in the literature.
3.3. Speed (RPM)
The revolutions per minute (RPM) employed in osteotomies have always been a subject of debate. Several studies have analyzed this variable, yielding conflicting results. While it might seem intuitive to think that as speed increases there is a proportional increase in temperature, several studies have revealed the opposite.
A 1997 study by Lyer et al. demonstrated an inverse relationship between speed and heat generation. This study compared speeds of 2000, 30 k, and 400 k RPM, and found that as speed increased, heat generation decreased during osteotomy [28]. In a study by Raj et al., three different groups of milling speeds were analyzed: 1500, 2000, and 2500 RPM. This study examined different diameters (2 mm versus 2.8 mm) and forces applied during milling (1.2 kg and 2.4 kg). Regardless of diameter and force, maximum heat generation occurred at a milling speed of 2000 RPM [29]. Other studies, including the study by Soldatos et al., report no significant differences when comparing speeds of 1000, 1500, 2000 RPM [25].
In a study by Emir Benca et al., the authors reported that doubling instrument rotation speed did not result in significant temperature changes, suggesting a better execution practice and reduced operating time [30]. The study by Sharawi et al. found that higher speeds led to lower heat generation when comparing speeds of 1225, 1667 and 2500 rpm. According to the authors, this was also due to the speed at which the osteotomy was performed and therefore the real milling time. To reach the 8 mm depth point, the higher RPM drills took less time, causing less friction time and therefore generating less heat. The time required for the bone to return to basal temperature was also quantified in this study; this time was directly proportional to the time necessary for the milling phase. The authors of this study recommended waiting 30 to 60 s between drills to allow the bone to return to basal temperature [31].
Mazork et al. compared speeds of 1000, 1500, and 2000 RPM and found that, even when varying the diameters to 3, 3.5, and 4 mm, higher speeds led to lower heat generation, shorter working and milling times, as well as reduced temperature variations [32]. Similarly, Aldabagh et al. compared speeds of 1250, 2000, and 2500 RPM and also found that the lowest temperature values were achieved at 2500 RPM, accompanied by reduced milling times and heat generation compared to lower speeds [33].
Limmeechokchai et al. found safe a 50 RPM drilling protocol in their study, even without irrigation [34]. Similar results were found by Hideon-Ji et al. [35], Sàlomo-Coll et al. [36], and Chen et al. [37].
Lower milling speeds enable, at the expense of greater heat generation, better control of movement. This is particularly important when in proximity to vital structures like the maxillary sinus and the inferior alveolar nerve. However, all studies reviewed here reported temperature values below the literature-reported limit of 47 degrees for one minute. Given these findings, adhering to manufacturer-recommended speeds seems to be a reasonable approach for optimizing outcomes during osteotomy.
3.4. Drill Material
Titanium and zirconium are the primary materials used for constructing implant osteotomy drills. While zirconium is used relatively rarely, it is still available from several manufacturers as an alternative for the osteotomy stage.
Tur et al. conducted a study revealing significant bone heating differences based on drill material [38]. They found that drills made of zirconium dioxide cutters generated more heat in the bone compared to stainless steel. The same group confirmed these results in 2022, demonstrating that bone heat generation is lower when using stainless steel cutters compared to zirconium dioxide cutters [39]. These findings contradict previous studies, suggesting no difference or indicating that metal drills produce more heat than ceramic drills. In fact, studies by Scarano et al. [40], Oliveira et al. [41], and Hochscheidt et al. [42] showed that that metal drills produced more heat than zirconium drills. Conversely, studies by Koo et al. [43] and Er N et al. [44] found no significant differences between the two materials.
Despite these conflicting findings, more recent reviews from 2020 [45] and 2023 [46] suggest inconsistencies between study findings, preventing a firm conclusion.
3.5. Drilling Force
Drilling force, or the pressure applied during the osteotomy phase, also emerges as an important factor according to the literature. While the average force used by the operator during osteotomy is known to be around 1.2 kg [47], studies by Cordioli et al. [48] and Brisman et al. [22] suggest that a drilling force of 2.1 kg may be safe clinical practice.
As indicated in the literature, increasing the drilling force and hand pressure during the osteotomy phase may improve cutting capacity [22], reduce working and drilling times, and consequently, reduce bone heating [49]. When combined with a higher drilling speed, increasing the force can minimize the temperature and the time of exposure to heat [50].
However, some studies emphasize the need to control the force during osteotomy to reduce heat generation. In their study, Rashad compared different drilling forces with conventional burs and a piezon. They found no temperature increase with the conventional drill, even at the maximum drilling force (20 N) [51], though the piezon appeared to produce more heat. Similar results were reported by Stezle [52], who observed that piezon and trephine burs with a force of 1 kg generated temperatures close to the limit, underscoring a need for careful attention to the force applied by clinicians when using piezoelectric and trephine burs, and 400 g was identified as the ideal force limit when using the piezon [52].
3.6. Intermittent vs. Continuous Drilling
Continuous drilling and intermittent drilling are two methods used for osteotomy site preparation. Considering the significance of exposure time in bone heating [53], intermittent drilling aims to reduce bone heating at the base of the osteotomy site. According to the literature, heat generated by the bur is completely reabsorbed by the body after approximately 10 s [54]. This means that intermittent drilling may enable the osteotomy site to return to its initial temperature before the next drilling cycle.
Some studies have compared intermittent and continuous drilling techniques. Di Fiore et al. [55] found no statistical difference between the two techniques during the drilling phase. Their study highlighted the impact of cooled and non-cooled irrigants.
Additionally, cylindrical drills require intermittent drilling to a greater extent than tapered drills [24]. Irrespective of this, many authors have found better temperature control with intermittent drilling [56,57,58].
3.7. Osteotomy Depth
As demonstrated by Erikssonn et al. in 1983 [6], the duration of exposure to heat is as important as the temperature itself in causing bone damage. Prolonged exposure to traumatic stimuli at the same temperature has been reported to lead to more significant damage. Consequently, factors like the depth of osteotomy, increasing working time, and exposure time to heat could pose potential risks to the bone.
The correlation between timing, osteotomy depth, and heat generation was demonstrated in the studies by Cordioli et al. in 1997 [48] and Augustin et al. in 2012 [59].
In 2004, Kalidindi et al. [60] concluded that a greater depth of instrumentation results in a longer time to process the bone and therefore a greater amount of heat developed during osteotomy. The same conclusion was reached by Lee et al. in 2012 [61]. In their study, temperature increases between 3 mm and 7 mm osteotomies were evident. Again, increasing the friction time increased the overall heat in the bone.
Oliveira et al. [41] demonstrated the same in an in vitro study, working at milling depths of 8 mm and 10 mm, indicating the increased difficulty of irrigation reaching the active part of the drill as a possible cause. This difference remained unchanged even when varying the materials that made up the drills (metal versus ceramic).
Strbac et al. [62] were able to demonstrate a significant temperature increase in the case of 16 mm and 10 mm osteotomies. An innovative finding reported in the study was the temperature during the drill withdraw phase. Depth had a negative influence on heat generation even during the drill shrinkage phase. This new indication could have a clinical relevance regarding depth.
In a study by Tur et al. [38], different implant preparation lengths were compared. In the two reference groups, two preparations were performed, at 10 and 16 mm, using titanium and zirconium drills with different diameters. Standardized bovine bone samples and infrared thermography were used for the measurements. This study demonstrated how greater depths (16 mm vs. 10 mm) and longer working times (23 s vs. 43 s) lead to greater heat development during the osteotomy phase.
Again, in their 2022 study, Tur et al. [39] showed that performing a 10 mm osteotomy takes less time than performing a 16 mm osteotomy. Similarly, they demonstrated a strong correlation between osteotomy depth and increased intraosseous temperature. This finding complements another conclusion given in the study, namely, that the passive shrinkage time of the drill is also an important parameter in the development of heat. Thus, it appears that a longer passive shrinkage time causes more heating in the bone. Great importance is given to the passive withdrawal phase of the cutter.
Similar results were found by Sannino et al. in a 2018 study [63].
In conclusion, the literature agrees that the depth of the osteotomy is an important factor in the generation of intraosseous heat, and as the depth of the osteotomy increases, so does the heat generated [64,65,66,67]. Considering data on the survival rate of implants with a length of 10/12 mm [68], it does not seem reasonable, even if there is bone available vertically, to opt for much longer implants, especially in type 1 bone.
3.8. Preparation Tools
The critical temperature of 47 degrees during implant site preparation can lead to bone healing failure due to osteonecrosis. This challenge has motivated the development of different preparation protocols, including techniques such as piezoelectric and laser surgery, which minimize the applied force and friction generated at the implant site. Piezoelectric instruments work on bone with a vibration frequency ranging between 24 and 32 kHz. A key advantage of using these tools lies in their minimally invasive and safe nature: accidental contact with soft tissue does not result in damage because their vibration frequencies are selective for mineralized tissue [68].
Studies have shown that the piezoelectric approach to implant bed preparation yields results comparable to those of traditional preparation methods in terms of implant survival rates [69,70].
Lamazza et al. [71] investigated the maximum temperature reached by bone preparation with piezoelectric instruments for cycles of 6 and 4 s, consistently finding variations in temperature rise, though temperatures always remained below the critical threshold of 47 degrees. Temperature variations are influenced by factors including the force applied during bone preparation, movement management, and type of bone [72].
Conversely, Bhargava et al. [73] observed a significant temperature increase with the use of piezoelectric systems compared to conventional milling drills, attributing this difference to the longer working time of these tools.
Similar results were found by Lajolo et al. [74] and Szalma et al. [75], where a piezoelectric system showed higher temperatures than conventional drilling, especially at the apical portion.
To reduce the risk of bone overheating, this technique requires precise irrigation [76] and a light pressure load, coupled with a quarter-turn rotary movement, to enable enhanced bone cutting efficacy and the dissipation of potential energy.
Marques et al. found a medium pressure as the most favorable in terms of bone heating [77].
As for laser ablation, i.e., Erbium:yttrium–aluminium–garnet (Er:YAG), lasers work on bone with a wavelength of 2940 nm, causing evaporation of interstitial water upon irradiation. This technique has numerous advantages, including the absence of vibration and friction, a bactericidal effect, reduced tissue bleeding and injuries to adjacent tissue, and precise geometric cuts with regular edges. This method, which has a depth propagation of around 30 microns, is considered to have no influence on the bone healing process [78].
Using infrared thermography, Gabric et al. investigated [79] the overheating of bone produced by piezoelectric surgery, Er:YAG lasers in contact and non-contact modes, piezoelectric instruments, and conventional surgical drills on rat tibia. The highest temperatures measured never exceeded 40 degrees. Their findings revealed that both Er:YAG and piezoelectric surgery are safe alternative methods to conventional surgical drills in dentistry.
Similar results were obtained in Sagheb et al.’s study and Fugito et al.’s study, where they demonstrated no difference between twisted drills and piezoelectric instruments in terms of bone heating [19,80].
3.9. Surgical Guide and Design
Computer-assisted implant planning emerged in the late 1990s [81]. The use of three-dimensional imaging techniques for planning implant placement brought immediate advantages, including improved accuracy of placement, preservation of anatomical structures like nerves, vessels, and the roots of adjacent teeth, and the ability to avoid invasive surgical techniques such as bone augmentation by adjusting the implant angle within the available bone. Furthermore, the position of the restoration is visualized and determined prior to surgery, ensuring predictable aesthetics and function.
At the time of surgery, a surgical guide designed using implant planning software is used. Made from resin attached to the teeth adjacent to the surgical site, the axis of the implant is determined by the bur sleeve. Despite variations in design, the guide structure tends to cover the surgical site, limiting irrigation from reaching the surgical site.
Several studies have explored potential differences in terms of bone site heating between guided and freehand approaches.
A 2013 study by Migliorati et al. [82] found that preparation under surgical guidance resulted in an increase in bone temperature compared to conventional preparation techniques, although it did not lead to detrimental effects on bone healing.
Similar results were found by Dos Santos et al. in 2014, Markovic et al. in 2016, Alhroob et al. in 2021, and Frösch et al. in 2019 [83,84,85,86].
Waltenberger et al. [87] conducted a study to evaluate the influence of guide design on the volume of coolant reaching the surgical site. This study analyzed different designs, from the most to the least overhanging, and found variations in total irrigation volume and bone temperature across different guide designs, but also concluded that critical temperatures for bone healing were not reached.
Another study by Amal Ashry et al. [88] proposed using open or perforated bushings to improve irrigation during surgery. In fact, the use of conventional cylindrical bushings has been shown to lead to greater heat generation compared to other designs, hindering cooling fluid flow.
Another study [89] reported no obvious increases in bone temperature with variations in rail design and irrigation conditions.
A 2023 investigation by Abuhajar et al. [90] compared two main groups of surgical guides, open (limiting) and closed (non-limiting) surgical guides. This study found that the open guides led to greater heat production, especially in the initial phase of osteotomy, though this finding was deemed clinically irrelevant.
A different study [91] explored how the closed traditional guide can also be modified to improve irrigation in the operating site, resulting in significant reductions in heat generation.
Similar results were found in Gargallo et al.’s study, where the open guides showed better results in terms of intra-operatory bone temperatures [67].
Stocchero et al. found no significant difference between external irrigation and the modified internal irrigation added to the surgical guide [92].
Some studies have shown external irrigation sufficient to control bone temperature [93], even with the flapless technique [94], while using the surgical guide.
Teich et al. showed a possible way to modify the surgical guide to improve irrigation and reduce bone heating [95,96,97].
3.10. Irrigation (Internal Coolant, External Coolant, Temperature of the Coolant)
Irrigation during the osteotomy phase also plays an important role in controlling intraosseous temperature and preventing temperature peaks. The literature on this subject is extensive and reports few contradictory results [20,98,99,100,101,102,103].
Irrigation methods are categorized as external and internal irrigation. Several studies have shown that irrigation is one of the best methods, if not the best, to reduce heat production. External irrigation is considered sufficient to decrease the measured drilling temperature at the implant/bone tissue surface and avoid structural tissue damage [63,65,93,104]. On the other hand, internal coolant drills have shown varied outcomes in reducing bone temperature, with some studies supporting their effectiveness [105,106], while others do not [107].
External irrigation is particularly effective during the most superficial osteotomy phases and is widely used today. Internal irrigation, though less common, is still valid, especially in cases of deep osteotomies. However, an additional source of coolant at 1.5 mm from the bone site does not improve heat dispersion compared to the external irrigation alone [78].
Gehrke et al., in 2018, found that double irrigation gave better results in reducing bone heating [106], even when trephine drills were used [120].
Tur et al. [38] compared the two irrigation methods, revealing that the effect of external irrigation is less effective in deeper osteotomy positions.
While some studies have suggested internal irrigation is superior in controlling heat, even when used alone [107,108,109], other studies argue there is no significant difference when compared to external irrigation, mainly because the latter would act on bone that is less corticalized and therefore less susceptible to heat development [110].
Another advantage of internal irrigation is that it increases drill lubrication during osteotomy and reduces the accumulation of bone debridement at the osteotomy site [100,107,109,111].
The other variable frequently discussed in the context of irrigation is the temperature of the irrigant. The reference temperatures for cooled sprinklers range between 0 and 10 degrees Celsius. Intermediate options fall between 15 and 20 degrees, while room-temperature irrigants generally exceed 20 degrees.
Several studies have revealed a significant difference between room-temperature and cooled sprinklers [20,29,78,84,112,113,114,115], even in case of guided surgery [67,116]. In any case, data analysis indicates that even simple temperature control using room-temperature water prevents temperatures from exceeding the literature threshold of 47 degrees.
Another important factor to consider during the osteotomy phase is the presence and design of a surgical guide. Some studies indicate that irrigants below 20 degrees have been found to be more effective, even when using surgical guides [91]. The study by Yun-Feng Liu et al. [117] highlights that the body of the guide can obstruct irrigation flow, necessitating the use of a dedicated irrigation system or internal irrigation to ensure proper cooling of the bone.
After analyzing irrigation temperatures, some authors have questioned the flow and volume of irrigant used in the osteotomy phase. Some studies have shown that there is no significant difference between the different irrigant volumes [78,100]. Others have shown an important relationship between irrigation volume and temperature control [72,118].
Some protocols have also reported high survival rates and bone temperatures above the critical limit in the case of implant preparation without irrigation, though these studies challenge the prevailing literature [35,119,120,121].
3.11. Cutter Wear
Another important topic of discussion concerns the role of drill wear and the resulting loss of cutting capacity. Some researchers suggest that drill wear and reduced cutting efficiency result in a significant temperature increase during osteotomy [15,83,122].
Some studies, such as that by Tsiagadigui et al. [123], have shown that even minimal use of a drill (n = 3) leads to a significant increase in temperature between osteotomies, which progressively increases with each new use of the drills.
The study by Allain et al. [124] compared three groups of drills: unused, used 600 times, and used for several months (i.e., more than 600 times, without giving an exact number). The findings indicated a significant difference between the three groups of drills, with some values exceeding the limit considered to be the wear limit threshold. The author recommended a frequent change of osteotomy drills, suggesting no more than 300 uses, and reported a small cost against important benefits.
Reichart et al. [125] used each drill a total of 51 times, showing that with increased drill use came higher heat developed during the osteotomy phase. While no values exceeded the critical threshold, this study recommended a maximum of 40 uses per osteotomy drill. Electron microscope analyses also showed structural changes after autoclave cycles, which resulted in differences in the cutting capacity of the drill.
The study by Aquilanti et al. [72], which used drills 35 times without sterilization, also aligned with previous findings, demonstrating increasing heat development with drill use up to 35 osteotomies, considered safe without sterilization between uses.
The study by Fugito et al. demonstrated no significant difference after 30 implant bed preparations in terms of bone heating [80].
Similarly, the studies by Scarano et al. [126] and Alam et al. [127] all reported increased heat generation with drill wear and more osteotomies. Quaranta et al. [128], for instance, used a drill for 25 osteotomies with no significant differences in heat generation. On the other hand, the study by Allsobrook et al. [129] found no significant differences in final temperatures, and thus in heat generation, after testing the drills for 50 osteotomies.
Koo et al. [43] and Oliveira et al. [41] also found no significant temperature differences after 50 osteotomies. Both studies did not report temperature values above the critical value.
The study by Soldatos et al. showed no differences after 40 drill uses [130] and the study by Lorusso et al. found no differences in terms of bone heating after 30 uses [131].
The authors recommended adhering to the manufacturers’ guidelines, which typically recommend drill replacement after 40 to 50 uses. Importantly, they also recommend considering factors like drill type, manufacturers, and materials.
Further investigation is needed to establish a definitive threshold value for osteotomies beyond which drills should not be used. Many variables may affect this value, including drill type, shape, material, manufacturer, and autoclave cycles. In any case, 50 uses per drill should not be exceeded.
In daily clinical practice, managing the number of uses of drills can be challenging, especially given that drill wear may vary depending on the type of bone previously prepared. Nonetheless, it is advisable to use new drills, particularly for type I bone preparations, in clinical practice.
4. Discussion
Preparation and insertion of implants into bone tissue requires a surgical technique that minimizes trauma. The biomechanical implications of heat generation during implant site preparation are significant, as thermal injury on bone healing could hinder primary implant stability and increase the risk of implant failure [5,6,30,132,133,134,135]. In fact, heat generation during bone preparation can damage hard tissues, leading to osteonecrosis and cell death. Previous studies have defined the critical bone temperature thresholds above which bone necrosis can occur [136]. Eriksson et al. [133] noted that, when threaded titanium implants were placed in the rabbit tibia, heating the implants to a temperature of 50 °C for one minute was sufficient to reduce bone formation. This was not an immediate event, but a gradual process that took place over a 4-week period; the bone was replaced by fat cells, which prevented the implant from being incorporated. It only takes a temperature of 47 °C for 1 min to impair osseointegration of an implant [6].
In terms of bone type (compact or cancellous), most studies indicate that a challenging clinical scenario is linked to bone implantation on D1 bone, which has a higher thermal conductivity and develops higher temperatures for the same amount of heat supplied [14,15,16,17,18]. When faced with the demanding task of bone bed preparation on D1 bone, clinicians should consider adopting a few simple precautions. These include using a new or lightly used drill [127], applying a refrigerated coolant [20], implementing intermittent drilling with continuous irrigation even when drilling pauses, and combining internal and external cooling [23,100]. Specifically, for type 1 bone, it seems sensible to avoid prolonged use of the implant trephine drill. Internal and/or external irrigation [100,114] with adequate quantities [6] and low-temperature (below 10 degrees Celsius) saline solution has proven effective as a cooling agent. In general, external cooling is beneficial for both compact and cancellous bone [29,78,115,118,120]. The role of irrigation has already been partially discussed, and as far as temperature and flow rate are concerned, the simple precaution of lowering the saline temperature (to 20 degrees Celsius), which resulted in a significant drop in temperature, should be considered. Nevertheless, the average temperature rise was below the critical threshold when using either refrigerated or non-refrigerated cooling solutions. However, findings regarding flow rate are mixed: one study [78] suggests that using a flow rate greater than 120 mL/min does not necessarily result in a reduction in temperature. The combination of external and internal cooling appears to be advantageous, particularly in very dense and compact bones, given their heightened sensitivity to heat. In this case, an internal and external cooling system is a practical solution for all bone drilling and reaming systems [23,100]. The shape of the drill is among the various factors that can influence bone heating during implant osteotomies [26]. Heat generation can be reduced by using tapered drills [24], but in the dental literature, other shapes like cylindrical and trephine drills do not exceed the specified limit. The use of a piezoelectric device is both safe and effective [19,72,73], but attention must be paid to the load applied and the specific bed preparation protocol [53,54]. Although early studies suggested that drilling speed had a negative impact on heat generation [136,137], the current literature fails to identify an ideal milling speed [138]. Lower milling speeds enable better control of drill movement, at the expense of higher heat generation. This is particularly important when working close to delicate anatomic structures such as the maxillary sinus and inferior alveolar nerve. It is advisable to adhere to indications provided by manufacturers, including recommended RPM settings and regularly replacing drills. Additionally, recent advancements in surgical template design, such as adding an irrigation channel, also facilitate cooling during osteotomy preparation.
The dental literature remains divided on the choice of drilling material: metal or zirconia [38,39,40,41,42,43,44,45,46]. It is known that a prolonged thermal stimulus can inflict the greatest damage to bone at equivalent levels of heating [38,39], with a consensus on the direct correlation between osteotomy depth and heat generated. The average force used during bone preparation is 1.2 kg [47], and while the literature suggests that load may influence heat generation [50], reports have indicated that a small load of 1.2 kg and 2.4 kg can be used without problem [48]. When using a piezoelectric device, the load must be modified to no more than 400 g and, in clinical practice, the piezoelectric handpiece must be handled with extreme care [54,78], supported by a cooled irrigant [75].
Guided surgery is gaining popularity in modern implant dentistry due to its increasing precision and reliability. Despite the broad consensus that surgical guides can lead to an increase in bone temperature [83,90], studies such as that by Migliorati et al. [82] indicate that the use of these devices at the time of implant placement does not adversely affect bone implant integration, considering long-term implant survival results. Significant advancements include the development of open guides [67,91], the use of cooled irrigants [117], and the possibility of adding irrigation channels [95,96,97]. Modern implant planning software lacks tools for adding additional irrigation channels. In virtual planning, using a fixation pin as an additional channel is the easiest way to add this secondary irrigation [96]. Nevertheless, flapless surgery seems to limit this improvement. Further research is needed to better understand bone heating during guided surgery.
For temperature measurement methods, infrared thermography appears to be more accurate than thermocouples during drilling. At the same time, real-time temperature monitoring during implant placement could help clinicians better control bone temperature during surgery, reducing the risk of thermal injury [139].
Temperature monitoring is also relevant in others branches of medicine [140]. It is conceivable that one day the same technology could be adopted in the dental field. Real-time temperature monitoring could aid clinicians in reducing bone heating and associated complications.
Finally, conflicting indications exist regarding cutter wear. While Allain et al. suggested that a bur used for 600 times remains acceptable [124], other studies have reported signs of drill wear [125]. It is advisable to maintain a record card for each drill, documenting the number of uses and the type of bone it is used on. For instance, drills may wear out more quickly when used on denser bone types like type D1 compared to type D4 bone. Importantly, it is recommended not to exceed 50 uses per drill to ensure optimal performance and minimize the risk of heat generation.
5. Clinical Recommendations and Best Practices
Based on our review findings, clinical recommendations and best practices include the following:
- (1)
- Adherence to manufacturer guidelines, following recommended indications, including limiting speed and using the recommended RPM.
- (2)
- Adherence to manufacturer guidelines to replace drills regularly, typically recommending drill replacement after 40 to 50 uses. It also is important to considering factors like drill type and materials.
- (3)
- Use of an effective cooling agent like saline irrigation solution in adequate quantities and at a low temperature (below 10 degrees Celsius).
- (4)
- Addition of an irrigation channel to enhance and facilitate cooling during osteotomy preparation.
- (5)
- Removal of bone from the drilling site between each drill to maintain effective cooling and reduce heat buildup.
These seem to be reasonable clinical recommendations and best practices to manage heat generated during dental implant placement and optimize outcomes during osteotomy.
6. Conclusions
Heat generation during the osteotomy phase is a multifactorial event. Certain factors, including irrigation and bone hardness, appear to be indisputable variables in heat generation during the osteotomy phase. Current evidence calls for further clinical and in vitro studies to help better define the contribution of all factors involved in implant site preparation to improve bone metabolism, an essential aspect of implant therapy success.
7. Limitations
Several limitations warrant consideration. An aim of this scoping review was to serve as a first assessment of the broader literature and potentially serve as a precursor for a more in-depth systematic review, ensuring a more exhaustive review of the available evidence. Future research efforts could benefit from including sources of gray literature to ensure a more exhaustive review of the available evidence.
Additionally, this review focused exclusively on benchtop research, which may constrain the direct applicability of the findings to clinical practice. While this focus aims to mitigate the impact of clinical variability and confounding elements, incorporating clinical studies in future investigations could enhance our understanding of this topic and particularly of the clinical implications of heat generation during impact procedures.
The relatively limited number of studies also highlights a need for further research. Future studies with diverse methodologies may contribute to a more robust evidence base.
Author Contributions
Conceptualization, R.D.F., A.P. and V.C.; methodology, R.D.F., A.P. and V.C.; software, A.P. and V.C.; validation, R.D.F., A.P. and T.L.; investigation, V.C., R.D.F. and T.L.; writing—original draft preparation, V.C.; writing—review and editing, A.P., T.L. and R.D.F.; visualization, R.D.F., A.P. and V.C.; supervision, A.P., T.L. and R.D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Alessandro De Dominicis (Teramo, Italy) and Giovanna Marchesani (Osimo, Italy) for their constructive remarks.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surgery Suppl. 1977, 16, 1–132. [Google Scholar]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet Neuronal Interact 2009, 9, 61–71. [Google Scholar] [PubMed]
- Thiebot, N.; Hamdani, A.; Blanchet, F.; Dame, M.; Tawfik, S.; Mbapou, E.; Kaddouh, A.A.; Alantar, A. Implant failure rate and the prevalence of associated risk factors: A 6-year retrospective observational survey. J. Oral Med. Oral Surg. 2022, 28, 19. [Google Scholar] [CrossRef]
- Babbar, A.; Jain, V.; Gupta, D.; Agrawal, D. Histological evaluation of thermal damage to Osteocytes: A comparative study of conventional and ultrasonic-assisted bone grinding. Med. Eng. Phys. 2021, 90, 1–8. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.; Albrektsson, T.; Grane, B.; McQueen, D. Thermal injury to bone: A vital-microscopic description of heat effects. Int. J. Oral Surg. 1982, 11, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P.; Masciotra, L. Effect of 50 to 60 °C Heating on Osseointegration of Dental Implants in Dense Bone: An: In Vivo: Histological Study. Implant Dent. 2014, 23, 516–521. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. Effect of Temperature on the Dental Implant Osseointegration Development in Low-Density Bone: An In Vivo Histological Evaluation. Implant Dent. 2015, 24, 96–100. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E. Density of bone: Effect on treatment planning, surgical approach, and healing. In Contemporary Implant Dentistry; Misch, C.E., Ed.; Mosby: St. Louis, MO, USA, 1993; pp. 469–485. [Google Scholar]
- Katranji, A.; Misch, K.; Wang, H. Cortical bone thickness in dentate and edentulous human cadavers. J. Periodontol. 2007, 78, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Pagnutti, S.; Vinci, R.; Gherlone, E.F. Distribution of Trabecular Bone Density in the Maxilla and Mandible. Implant Dent. 2019, 28, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Wili, P.; Maquer, G.; Zysset, P. The thermal conductivity of cortical and cancellous bone. Eur. Cells Mater. 2018, 35, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xie, R.; Ren, N.; Li, Z.; Zhang, S.; Liu, Y.; Dong, Y.; Yin, A.; Zhao, Y.; Bai, S. Correlation between intraosseous thermal change and drilling impulse data during osteotomy within autonomous dental implant robotic system: An in vitro study. Clin. Oral Implant. Res. 2023, 35, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Farias Gomes, A.G.; Cadena Lins, N.; de Mello Soares Frauches, V.; Mendes Senna, P.; Heggendorn, F.L. Ex vivo porcine study of thermal changes in the bone marrow region during osseodensification and osteotomy. J. Osseointegr. 2023, 15, 40–47. [Google Scholar] [CrossRef]
- Yamaba, T.; Suganami, T.; Ikebe, K.; Sogo, M.; Maeda, Y.; Wada, M. The Evaluation of the Heat Generated by the Implant Osteotomy Preparation Using a Modified Method of the Measuring Temperature. Int. J. Oral Maxillofac. Implant. 2015, 30, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Möhlhenrich, S.; Abouridouane, M.; Heussen, N.; Hölzle, F.; Klocke, F.; Modabber, A. Thermal evaluation by infrared measurement of implant site preparation between single and gradual drilling in artificial bone blocks of different densities. Int. J. Oral Maxillofac. Surg. 2016, 45, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Sagheb, K.; Kumar, V.V.; Azaripour, A.; Walter, C.; Al-Nawas, B.; Kämmerer, P.W. Comparison of conventional twist drill protocol and piezosurgery for implant insertion: An ex vivo study on different bone types. Clin. Oral Implant. Res. 2017, 28, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kosior, P.; Nikodem, A.; Kozuń, M.; Dudek, K.; Janeczek, M.; Dobrzyński, M. The assessment of temperature amplitude arising during the implant bed formation in relation to variable preparation parameters. Acta Bioeng. Biomech. 2021, 23, 163–173. [Google Scholar] [CrossRef]
- Watanbe, F.; Tawada, Y.; Komatsu, S.; Hata, Y. Heat distribution in bone during preparation of implant sites: Heat analysis by real-time thermography. Int. J. Oral Maxillofac. Implant. 1992, 7, 212–219. [Google Scholar]
- Brisman, D.L. The effect of speed, pressure, and time on bone temperature during the drilling of implant sites. Int. J. Oral Maxillofac. Implant. 1996, 11, 35–37. [Google Scholar]
- Haider, R.; Watzek, G.; Plenk, H. Effects of drill cooling and bone structure on IMZ implant fixation. Int. J. Oral Maxillofac. Implant. 1993, 8, 83–91. [Google Scholar] [PubMed]
- Gehrke, S.A.; Treichel, T.L.E.; Aramburu Junior, J.; de Aza, P.N.; Prados-Frutos, J.C. Effects of the technique and drill design used during the osteotomy on the thermal and histological stimulation. Sci. Rep. 2020, 10, 20737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soldatos, N.; Nelson-Rabe, L.; Palanker, N.; Angelov, N.; Romanos, G.; Weltman, R. Temperature Changes during Implant Osteotomy Preparations in Fresh Human Cadaver Tibiae, Comparing Straight with Tapered Drills. Materials 2022, 15, 2369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Omar, S.; Jaiswal, H.; Mishra, S.; Bhargava, D.; Kumar, P. A comparative study to evaluate the heat generated during osteotomy with conventional drill, trephine and alveolar expander. Eur. Oral Res. 2023, 57, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gupta, A.S.; Chandu, G.S.; Jain, S. Infrared thermographic evaluation of rise in temperature with conventional versus trephine drills. J. Indian Prosthodont. Soc. 2021, 21, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Weiss, C.; Mehta, A. Effects of drill speed on heat production and the rate and quality of bone formation in dental implant osteotomies. Part I: Relationship between drill speed and heat production. Int. J. Prosthodont. 1997, 10, 411–414. [Google Scholar] [PubMed]
- Raj, R.; Manju, V.; Kumar-Gopal, V.; Eswar, M. Analysis of factors determining thermal changes at osteotomy site in dental implant placement—An in-vitro study. J. Clin. Exp. Dent. 2021, 13, e234–e239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benca, E.; Ferrante, B.; Zalaudek, M.; Hirtler, L.; Synek, A.; Kainberger, F.M.; Windhager, R.; Brånemark, R.; Hobusch, G.M.; Unger, E. Thermal Effects during Bone Preparation and Insertion of Osseointegrated Transfemoral Implants. Sensors 2021, 21, 6267. [Google Scholar] [CrossRef]
- Sharawy, M.; Misch, C.E.; Weller, N.; Tehemar, S. Heat generation during implant drilling: The significance of motor speed. J. Oral Maxillofac. Surg. 2002, 60, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Marzook, H.; Yousef, E.; Denewar, M.; Farahat, M. In-vitro assessment of bone viability with different implant drill speeds. Br. J. Oral Maxillofac. Surg. 2020, 58, e301–e306. [Google Scholar] [CrossRef] [PubMed]
- Aldabagh, A.H.N. The Significance of Motor Speed on Heat Generation during Implant Drilling (Experimental Study on Bovine Bone). Al-Rafidain Dent. J. 2009, 9, 303–306. [Google Scholar] [CrossRef][Green Version]
- Limmeechokchai, S.; Kan, J.Y.; Rungcharassaeng, K.; Goodacre, C.J.; Lozada, J.; Oyoyo, U. Heat and Sound Generation during Implant Osteotomy When Using Different Types of Drills in Artificial and Bovine Bone Blocks. J. Oral Implant. 2022, 48, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Yoon, J.-U.; Joo, J.-Y.; Lee, J.-Y.; Kim, H.-J. Effects of a simplified drilling protocol at 50 rpm on heat generation under water-free conditions: An in vitro study. J. Periodontal Implant. Sci. 2023, 53, 85–95. [Google Scholar] [CrossRef]
- Salomó-Coll, O.; Auriol-Muerza, B.; Lozano-Carrascal, N.; Hernández-Alfaro, F.; Wang, H.-L.; Gargallo-Albiol, J. Influence of bone density, drill diameter, drilling speed, and irrigation on temperature changes during implant osteotomies: An in vitro study. Clin. Oral Investig. 2021, 25, 1047–1053. [Google Scholar] [CrossRef]
- Chen, C.-H.; Coyac, B.R.; Arioka, M.; Leahy, B.; Tulu, U.S.; Aghvami, M.; Holst, S.; Hoffmann, W.; Quarry, A.; Bahat, O.; et al. A Novel Osteotomy Preparation Technique to Preserve Implant Site Viability and Enhance Osteogenesis. J. Clin. Med. 2019, 8, 170. [Google Scholar] [CrossRef]
- Tur, D.; Giannis, K.; Unger, E.; Mittlböck, M.; Rausch-Fan, X.; Strbac, G.D. Thermal effects of various drill materials during implant site preparation—Ceramic vs. stainless steel drills: A comparative in vitro study in a standardised bovine bone model. Clin. Oral Implant. Res. 2021, 32, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Tur, D.; Giannis, K.; Unger, E.; Mittlböck, M.; Rausch-Fan, X.; Strbac, G.D. Drilling- and withdrawing-related thermal effects of implant site preparation for ceramic and stainless steel twist drills in standardized bovine bone. Clin. Implant Dent. Relat. Res. 2023, 25, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Noumbissi, S. Infrared Thermographic Evaluation of Temperature Modifications Induced during Implant Site Preparation with Steel vs. Zirconia Implant Drill. J. Clin. Med. 2020, 9, 148. [Google Scholar] [CrossRef]
- Oliveira, N.; Alaejos-Algarra, F.; Mareque-Bueno, J.; Ferrés-Padró, E.; Hernández-Alfaro, F. Thermal changes and drill wear in bovine bone during implant site preparation. A comparative in vitro study: Twisted stainless steel and ceramic drills. Clin. Oral Implant. Res. 2012, 23, 963–969. [Google Scholar] [CrossRef]
- Hochscheidt, C.J.; Shimizu, R.H.; Andrighetto, A.R.; Moura, L.M.; Golin, A.L.; Hochscheidt, R.C. Thermal Variation during Osteotomy with Different Dental Implant Drills: A Standardized Study in Bovine Ribs. Implant Dent. 2017, 26, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.-T.; Kim, M.-H.; Kim, H.-Y.; Wikesjö, U.M.E.; Yang, J.-H.; Yeo, I.-S. Effects of implant drill wear, irrigation, and drill materials on heat generation in osteotomy sites. J. Oral Implant. 2015, 41, e19–e23. [Google Scholar] [CrossRef] [PubMed]
- Er, N.; Alkan, A.; Ilday, S.; Bengu, E. Improved Dental Implant Drill Durability and Performance Using Heat and Wear Resistant Protective Coatings. J. Oral Implant. 2018, 44, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Moufti, M.A.; Seoudi, N.; Pisani, F.; Almasri, M. The Effect of Ceramic and Conventional Implant Drill Materials on Heat Generation in Osteotomy Sites. Clin. Oral Implant. Res. 2020, 31, 134. [Google Scholar] [CrossRef]
- Chakraborty, S.; Moufti, M.-A.; Kheder, W. The Effect of Dental Implant Drills Materials on Heat Generation in Osteotomy Sites: A Systematic Review. Eur. J. Dent. 2023, 18, 065–072. [Google Scholar] [CrossRef]
- Hobkirk, J.; Rusiniak, K. Investigation of variable factors in drilling bone. J. Oral Surg. 1977, 35, 968–973. [Google Scholar] [PubMed]
- Cordioli, G.; Majzoub, Z. Heat generation during implant site preparation: An in vitro study. Int. J. Oral Maxillofac. Implant. 1997, 12, 186–193. [Google Scholar]
- Matthews, L.S.; Hirsch, C. Temperatures measured in human cortical bone when drilling. J. Bone Jt. Surg. 1972, 54, 297–308. [Google Scholar] [CrossRef]
- Bachus, K.N.; Rondina, M.T.; Hutchinson, D.T. The effects of drilling force on cortical temperatures and their duration: An in vitro study. Med. Eng. Phys. 2000, 22, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.; Kaiser, A.; Prochnow, N.; Schmitz, I.; Hoffmann, E.; Maurer, P. Heat production during different ultrasonic and conventional osteotomy preparations for dental implants. Clin. Oral Implant. Res. 2011, 22, 1361–1365. [Google Scholar] [CrossRef]
- Stelzle, F.; Frenkel, C.; Riemann, M.; Knipfer, C.; Stockmann, P.; Nkenke, E. The effect of load on heat production, thermal effects and expenditure of time during implant site preparation—An experimental ex vivo comparison between piezosurgery and conventional drilling. Clin. Oral Implant. Res. 2014, 25, e140–e148. [Google Scholar] [CrossRef]
- Abouzgia, M.B.; James, D.F. Temperature rise during drilling through bone. Int. J. Oral Maxillofac. Implant. 1997, 12, 342–353. [Google Scholar]
- Reingewirtz, Y.; Senger, B.; Szmukler-Moncler, S. Influence of different parameters on bone heating and drilling time in implantology. Clin. Oral Implant. Res. 1997, 8, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Sivolella, S.; Stocco, E.; Favero, V.; Stellini, E. Experimental Analysis of Temperature Differences during Implant Site Preparation: Continuous Drilling Technique Versus Intermittent Drilling Technique. J. Oral Implant. 2018, 44, 46–50. [Google Scholar] [CrossRef]
- Chacon, G.E.; Bower, D.L.; Larsen, P.E.; McGlumphy, E.A.; Beck, F.M. Heat production by 3 implant drill systems after repeated drilling and sterilization. J. Oral Maxillofac. Surg. 2006, 64, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, C.; Funkenbusch, P.D.; Lee, H.J.; Moss, M.E.; Graser, G.N. The influence of drill wear on cutting efficiency and heat production during osteotomy preparation for dental implants: A study of drill durability. Int. J. Oral Maxillofac. Implant. 2004, 19, 335–349. [Google Scholar]
- Harris, B.H.; Kohles, S.S. Efects of Mechanical and thermal fatigue on dental drill performance. J. Oral Maxillofac. Implant. 2001, 16, 819–826. [Google Scholar]
- Augustin, G.; Zigman, T.; Davila, S.; Udilljak, T.; Staroveski, T.; Brezak, D.; Babic, S. Cortical bone drilling and thermal osteonecrosis. Clin. Biomech. 2012, 27, 313–325. [Google Scholar] [CrossRef]
- Kalidindi, V. Optimization of Drill Design and Coolant Systems during Dental Implant Surgery. Master’s Thesis, University of Kentucky, Lexington, KT, USA, 2014. [Google Scholar]
- Lee, J.; Ozdoganlar, O.B.; Rabin, Y. An experimental investigation on thermal exposure during bone drilling. Med. Eng. Phys. 2012, 34, 1510–1520. [Google Scholar] [CrossRef]
- Strbac, G.D.; Giannis, K.; Unger, E.; Mittlböck, M.; Vasak, C.; Watzek, G.; Zechner, W. Drilling- and withdrawing-related thermal changes during implant site osteotomies. Clin. Implant Dent. Relat. Res. 2015, 17, 32–43. [Google Scholar] [CrossRef]
- Sannino, G.; Gherlone, E. Thermal Changes during Guided Flapless Implant Site Preparation: A Comparative Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Katic, Z.D.; Jukic, T.D.; Stubljar, D.B. Effects of Osteotomy Lengths on the Temperature Rise of the Crestal Bone during Implant Site Preparation. Implant Dent. 2018, 27, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Sannino, G.; Cappare, P.; Gherlone, E.F.; Barlattani, A. Influence of the implant drill design and sequence on temperature changes during site preparation. Int. J. Oral Maxillofac. Implant. 2015, 30, 351–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.; Ju, S.; Kim, M.; Park, M.; Jun, S.; Ahn, J. Direct Measurement of Heat Produced during Drilling for Implant Site Preparation. Appl. Sci. 2019, 9, 1898. [Google Scholar] [CrossRef]
- Gargallo-Albiol, J.; Salomó-Coll, O.; Lozano-Carrascal, N.; Wang, H.L.; Hernández-Alfaro, F. Intra-osseous heat generation during implant bed preparation with static navigation: Multi-factor in vitro study. Clin. Oral Implant. Res. 2021, 32, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Blaskovic, M.; Gabrić, D.; Coleman, N.J.; Slipper, I.J.; Mladenov, M.; Gjorgievska, E. Bone Healing Following Different Types of Osteotomy: Scanning Electron Microscopy (SEM) and Three-Dimensional SEM Analyses. Microsc. Microanal. 2016, 22, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, X.; Guo, J.; Wang, Y. The Stability and Survival Rate of Dental Implants after Preparation of the Site by Piezosurgery vs Conventional Drilling: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2020, 30, e51–e56. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Alsabeeha, N.H.M.; Tawse-Smith, A.; Duncan, W.J. Piezoelectric versus conventional implant site preparation: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2018, 20, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Lamazza, L.; Lollobrigida, M.; Vozza, I.; Palmieri, L.; Stacchi, C.; Lombardi, T.; De Biase, A. Piezoelectric Implant Site Preparation: Influence of Handpiece Movements on Temperature Elevation. Materials 2020, 13, 4072. [Google Scholar] [CrossRef]
- Aquilanti, L.; Antognoli, L.; Rappelli, G.; Di Felice, R.; Scalise, L. Heat Generation during Initial Osteotomy for Implant Site Preparation: An In Vitro Measurement Study. J. Maxillofac. Oral Surg. 2023, 22, 313–320. [Google Scholar] [CrossRef]
- Bhargava, N.; Perrotti, V.; Caponio, V.C.A.; Matsubara, V.H.; Patalwala, D.; Quaranta, A. Comparison of heat production and bone architecture changes in the implant site preparation with compressive osteotomes, osseodensification technique, piezoelectric devices, and standard drills: An ex vivo study on porcine ribs. Odontology 2023, 111, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Lajolo, C.; Valente, N.A.; Romandini, W.G.; Petruzzi, M.; Verdugo, F.; D’Addona, A. Bone heat generated using conventional implant drills versus piezosurgery unit during apical cortical plate perforation. J. Periodontol. 2018, 89, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Szalma, J.; Vajta, L.; Lempel, E.; Tóth, Á.; Jeges, S.; Olasz, L. Intracanal temperature changes during bone preparations close to and penetrating the inferior alveolar canal: Drills versus piezosurgery. J. Cranio-Maxillofac. Surg. 2017, 45, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Stübinger, S.; Biermeier, K.; Bächi, B.; Ferguson, S.J.; Sader, R.; von Rechenberg, B. Comparison of Er:YAG laser, piezoelectric, and drill osteotomy for dental implant site preparation: A biomechanical and histological analysis in sheep. Lasers Surg. Med. 2010, 42, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Lopes, G.R.; Samico, R.P.; Matos, J.D.; Souza, F.A.; Corat, E.J.; Nishioka, R.S. Evaluation of temperature and osteotomy speed with piezoelectric system. Minerva Dent. Oral Sci. 2021, 70, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.M.; Aoki, A.; Ichinose, S.; Ishikawa, I. Ultrastructural analysis of bone tissue irradiated by Er:YAG Laser. Lasers Surg. Med. 2002, 31, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Gabrić, D.; Aumiler, D.; Vuletić, M.; Gjorgievska, E.; Blašković, M.; Mladenov, M.; Pavlić, V. Thermal Evaluation by Infrared Thermography Measurement of Osteotomies Performed with Er:YAG Laser, Piezosurgery and Surgical Drill—An Animal Study. Materials 2021, 14, 3051. [Google Scholar] [CrossRef] [PubMed]
- Fugito, K.J.; Gonzalez Cortes, A.R.; de Carvalho Destro, R.; Yoshimoto, M. Comparative Study on the Cutting Effectiveness and Heat Generation of Rotary Instruments Versus Piezoelectric Surgery Tips Using Scanning Electron Microscopy and Thermal Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 345–350. [Google Scholar]
- Verstreken, K.; Van Cleynenbreugel, J.; Martens, K.; Marchal, G.; van Steenberghe, D.; Suetens, P. An image-guided planning system for endosseous oral implants. IEEE Trans. Med. Imaging 1998, 17, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, M.; Amorfini, L.; Signori, A.; Barberis, F.; Biavati, A.S.; Benedicenti, S. Internal bone temperature change during guided surgery preparations for dental implants: An in vitro study. Int. J. Oral Maxillofac. Implant. 2013, 28, 1464–1469. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Pereira Queiroz, T.; Margonar, R.; de Souza Carvalho, A.C.G.; Betoni, W., Jr.; Rodrigues Rezende, R.R.; dos Santos, P.H.; Garcia, R., Jr. Evaluation of Bone Heating, Drill Deformation, and Drill Roughness after Implant Osteotomy: Guided Surgery and Classic Drilling Procedure. Int. J. Oral Maxillofac. Implant. 2014, 29, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Markovic, A.; Lazic, Z.; Misic, T.; Scepanovic, M.; Todorovic, A.; Thakare, K.; Janjic, B.; Vlahovic, Z.; Glisic, M. Effect of surgical drill guide and irrigans temperature on thermal bone changes during drilling implant sites—Thermographic analysis on bovine ribs. Vojn. Pregl. 2016, 73, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Alhroob, K.; Alsabbagh, M.M.; Alsabbagh, A.Y. Effect of the use of a surgical guide on heat generation during implant placement: A comparative in vitro study. Dent. Med. Probl. 2021, 58, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Frösch, L.; Mukaddam, K.; Filippi, A.; Zitzmann, N.U.; Kühl, S. Comparison of heat generation between guided and conventional implant surgery for single and sequential drilling protocols—An in vitro study. Clin. Oral Implant. Res. 2019, 30, 121–130. [Google Scholar] [CrossRef]
- Waltenberger, L.; Wied, S.; Wolfart, S.; Tuna, T. Effect of different dental implant drilling template designs on heat generation during osteotomy—An in vitro study. Clin. Oral Implant. Res. 2022, 33, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ashry, A.; Elattar, M.S.; Elsamni, O.A.; Soliman, I.S. Effect of Guiding Sleeve Design on Intraosseous Heat Generation During Implant Site Preparation (In Vitro Study). J. Prosthodont. 2022, 31, 147–154. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Oh, J.-W.; Lee, Y.; Lee, D.-W. Thermal changes during implant site preparation with a digital surgical guide and slot design drill: An ex vivo study using a bovine rib model. J. Periodontal Implant. Sci. 2022, 52, 411–421. [Google Scholar] [CrossRef]
- Abuhajar, E.; Salim, N.A.; Sallam, M.; Jarab, F.; Satterthwaite, J.D. The impact of surgical guide design and bone quality on heat generation during pilot implant site preparation: An in vitro study. BMC Oral Health 2023, 23, 273. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, S.; Cameron, A.; Tadakamadla, S.; Figueredo, C.M.S.; Reher, P. A Novel Irrigation System to Reduce Heat Generation during Guided Implantology: An In Vitro Study. J. Clin. Med. 2023, 12, 3944. [Google Scholar] [CrossRef]
- Stocchero, M.; Sivolella, S.; Brunello, G.; Zoppello, A.; Cavallin, F.; Biasetto, L. Bone Temperature Variation Using a 3D-Printed Surgical Guide with Internal Irrigation. Appl. Sci. 2021, 11, 2588. [Google Scholar] [CrossRef]
- Boa, K.; Varga, E.; Pinter, G.; Csonka, A.; Gargyan, I.; Varga, E. External cooling efficiently controls intraosseous temperature rise caused by drilling in a drilling guide system: An in vitro study. Br. J. Oral Maxillofac. Surg. 2015, 53, 963–967. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeong, S.-M.; Yoo, J.-H.; Fang, Y.; Choi, B.-H.; Son, J.-S.; Oh, J.-H. The effect of guided flapless implant procedure on heat generation from implant drilling. J. Cranio-Maxillofac. Surg. 2014, 42, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Teich, S.; Bocklet, M.; Evans, Z.; Gutmacher, Z.; Renne, W. 3D printed implant surgical guides with internally routed irrigation for temperature reduction during osteotomy preparation: A pilot study. J. Esthet. Restor. Dent. 2022, 34, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Orgev, A.; Gonzaga, L.; Martin, W.; Morton, D.; Lin, W.-S. Addition of an irrigation channel to a surgical template to facilitate cooling during implant osteotomy. J. Prosthet. Dent. 2021, 126, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Alevizakos, V.; Mitov, G.; von See, C. Guided Implant Placement Using an Internally Cooling Surgical Template: A Technical Note. J. Oral Implant. 2020, 46, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Strbac, G.D.; Unger, E.; Donner, R.; Bijak, M.; Watzek, G.; Zechner, W. Thermal effects of a combined irrigation method during implant site drilling. A standardized in vitro study using a bovine rib model. Clin. Oral Implant. Res. 2014, 25, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Bullon, B.; Bueno, E.F.; Herrero, M.; Fernandez-Palacin, A.; Rios, J.V.; Bullon, P.; Gil, F.J. Effect of irrigation and stainless steel drills on dental implant bed heat generation. J. Mater. Sci. Mater. Med. 2015, 26, 75. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P.; Perfetti, G. Insufficient irrigation induces peri-implant bone resorption: An in vivo histologic analysis in sheep. Clin. Oral Implant. Res. 2014, 25, 696–701. [Google Scholar] [CrossRef]
- José, L.F.D.S.; Ruggeri, F.M.; Rucco, R.; Zubizarreta-Macho, A.; Pérez-Barquero, J.A.; Deglow, E.R.; Montero, S.H. Influence of Drilling Technique on the Radiographic, Thermographic, and Geomorphometric Effects of Dental Implant Drills and Osteotomy Site Preparations. J. Clin. Med. 2020, 9, 3631. [Google Scholar] [CrossRef]
- Subelza, P.H.; Kopp, G.; Sivila, M.F.H.; Sivila, H.K.H.; Francischone, C.E. Comparative Study of Conventional Drill Bits and a New Model for Low-rotation in the Surgical Bed Preparation in Bone Blocks for Installation of Dental Implants. J. Young-Pharm. 2019, 11, 429–433. [Google Scholar] [CrossRef]
- Woods, J.C.; Cook, J.L.; Bozynski, C.C.; Tegethoff, J.D.; Kuroki, K.; Crist, B.D. Does Irrigating While Drilling Decrease Bone Damage? Iowa Orthop. J. 2022, 42, 22. [Google Scholar] [PubMed] [PubMed Central]
- Sindel, A.; Dereci, Ö.; Hatipoglu, M.; Altay, A.; Ozalp, Ö.; Ozturk, A. The effects of irrigation volume to the heat generation during implant surgery. Med. Oral Patol. Oral Cirugia Bucal 2017, 22, e506–e511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brand, S.; Klotz, J.; Petri, M.; Ettinger, M.; Hassel, T.; Krettek, C.; Goesling, T.; Bach, F.-W. Temperature control with internally applied cooling in solid material drilling: An experimental, biomechanical study. Int. Orthop. 2013, 37, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Aramburú Júnior, J.S.; Pérez-Albacete Martínez, C.; Ramirez Fernandez, M.P.; Maté Sánchez de Val, J.E.; Calvo-Guirado, J.L. The influence of drill length and irrigation system on heat production during osteotomy preparation for dental implants: An ex vivo study. Clin. Oral Implant. Res. 2018, 29, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Benington, I.C.; Biagioni, P.A.; Briggs, J.; Sheridan, S.; Lamey, P. Thermal changes observed at implant sites during internal and external irrigation. Clin. Oral Implant. Res. 2002, 13, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Strbac, G.D.; Giannis, K.; Unger, E.; Mittlböck, M.; Watzek, G.; Zechner, W. A novel standardized bone model for thermal evaluation of bone osteotomies with various irrigation methods. Clin. Oral Implant. Res. 2014, 25, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Harder, S.; Egert, C.; Wenz, H.J.; Jochens, A.; Kern, M. Influence of the drill material and method of cooling on the development of intrabony temperature during preparation of the site of an implant. Br. J. Oral Maxillofac. Surg. 2013, 51, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Augustin, G.; Davila, S.; Udilljak, T.; Staroveski, T.; Brezak, D.; Babic, S. Temperature changes during cortical bone drilling with a newly designed step drill and an internally cooled drill. Int. Orthop. 2012, 36, 1449–1456. [Google Scholar] [CrossRef]
- Sener, B.C.; Dergin, G.; Gursoy, B.; Kelesoglu, E.; Slih, I. Effects of irrigation temperature on heat control in vitro at different drilling depths. Clin. Oral Implant. Res. 2009, 20, 294–298. [Google Scholar] [CrossRef]
- Reddy, S.C.; Schipper, O.N.; Li, J. The Effect of Chilled vs Room-Temperature Irrigation on Thermal Energy Dissipation during Minimally Invasive Calcaneal Osteotomy of Cadaver Specimens. Foot Ankle Orthop. 2022, 7, 24730114221136548. [Google Scholar] [CrossRef]
- Rathi, N.B.; Kapse, P.G.; Thakare, K.S.; Yeltiwar, R.K.; Parwani, S.R.; Ashtankar, M.A. Thermal Effect of Operatory Room Temperature, Surgical Drill Diameter, and Temperature of Irrigants at Different Depths of Implant Site Preparation—Thermographic Analysis on Goat Mandible. J. Indian Soc. Periodontol. 2022, 26, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Barrak, I.; Joób-Fancsaly, A.; Varga, E.; Boa, K.; Piffko, J. Effect of the Combination of Low-Speed Drilling and Cooled Irrigation Fluid on Intraosseous Heat Generation during Guided Surgical Implant Site Preparation: An In Vitro Study. Implant Dent. 2017, 26, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Kosior, P.; Pelc, A.; Mikulewicz, M. Comparative Analysis of Bone Tissue Temperature during Implant Preparation with Variable Drilling Parameters: In Vitro Study. BioMed Res. Int. 2020, 2020, 7420718. [Google Scholar] [CrossRef]
- Barrak, I.; Boa, K.; Joob-Fancsaly, A.; Varga, E.; Sculean, A.; Piffko, J. Heat generation during guided and freehand implant site preparation at drilling speeds of 1500 and 2000 RPM at different irrigation temperatures: An in vitro study. Oral Health Prev. Dent. 2019, 17, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.; Zhang, J.; Peng, W.; Liao, W. Numerical and Experimental Analyses on the Temperature Distribution in the Dental Implant Preparation Area when Using a Surgical Guide. J. Prosthodont. 2018, 27, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Mercan, U.; Sumer, M.; Kaya, O.; Keskiner, I.; Meral, D.; Erdogan, O. An In-vitro Study on Thermal Changes during Implant Drilling with Different Irrigation Volumes. Niger. J. Clin. Pract. 2019, 22, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, A. Radiographic comparisons of crestal bone levels around implants placed with low-speed drilling and standard drilling protocols: Preliminary results. Saudi Dent. J. 2021, 33, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, G.; Staas, T.; Urban, I. A Retrospective Observational Study Assessing the Clinical Outcomes of a Novel Implant System with Low-Speed Site Preparation Protocol and Tri-Oval Implant Geometry. J. Clin. Med. 2022, 11, 4859. [Google Scholar] [CrossRef] [PubMed]
- Lucchiari, N.; Frigo, A.C.; Stellini, E.; Coppe, M.; Berengo, M.; Bacci, C. Heat with Traditional and Single-Bur Drilling. Clin. Implant Dent. Relat. Res. 2016, 18, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Salimov, F.; Ozcan, M.; Ucak Turer, O.; Haytac, C.M. The effects of repeated usage of implant drills on cortical bone temperature, primary/secondary stability and bone healing: A preclinical in vivo micro-CT study. Clin. Oral Implant. Res. 2020, 31, 687–693. [Google Scholar] [CrossRef]
- Tsiagadigui, J.G.; Ndiwe, B.; Yamben, M.-A.N.; Fotio, N.; Belinga, F.E.; Njeugna, E. The effects of multiple drilling of a bone with the same drill bit: Thermal and force analysis. Heliyon 2022, 8, e08927. [Google Scholar] [CrossRef] [PubMed]
- Allan, W.; Williams, E.D.; Kerawala, C.J. Effects of repeated drill use on temperature of bone during preparation for osteo-synthesis self-tapping screws. Br. J. Oral Maxillofac. Surg. 2005, 43, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Jochum, R.M.; Reichart, P.A. Influence of multiple use of Timedur-titanium cannon drills: Thermal response and scanning electron microscopic findings. Clin. Oral Implant. Res. 2000, 11, 139–143. [Google Scholar] [PubMed]
- Scarano, A.; Carinci, F.; Quaranta, A.; Di Iorio, D.; Assenza, B.; Piattelli, A. Effects of bur wear during implant site preparation: An in vitro study. Int. J. Immunopathol. Pharmacol. 2007, 20 (Suppl. 1), 23–26. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Qamar, S.Z.; Iqbal, M.; Piya, S.; Al-Kindi, M.; Qureshi, A.; Al-Ghaithi, A.; Al-Sumri, B.; Silberschmidt, V.V. Effect of drill quality on biological damage in bone drilling. Sci. Rep. 2023, 13, 6234. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, A.D.; Andreana, S.D.; Spazzafumo, L.; Piemontese, M.M. An in vitro evaluation of heat production during osteotomy preparation for dental implants with compressive osteotomes. Implant Dent. 2013, 22, 161–164. [Google Scholar] [CrossRef]
- Allsobrook, O.F.L.; Leichter, J.; Holborow, D.; Swain, M. Descriptive study of the longevity of dental implant surgery drills. Clin. Implant Dent. Relat. Res. 2011, 13, 244–254. [Google Scholar] [CrossRef]
- Soldatos, N.; Pham, H.; Fakhouri, W.D.; Ngo, B.; Lampropoulos, P.; Tran, T.; Weltman, R. Temperature Changes during Implant Osteotomy Preparations in Human Cadaver Tibiae Comparing MIS® Straight Drills with Densah® Burs. Genes 2022, 13, 1716. [Google Scholar] [CrossRef]
- Lorusso, F.; Gehrke, S.A.; Festa, F.; Scarano, A. Wearing Effect of Implant Steel Drills and Tappers for the Preparation of the Bone Osteotomies: An Infrared Thermal Analysis and Energy Dispersive Spectroscopy-Scanning Electron Microscopy (EDS-SEM) Study. Prosthesis 2022, 4, 679–694. [Google Scholar] [CrossRef]
- Branemark, I.-P.; Zarb, G.A.; Albrektsson, T.; Harvey, R. Tissue-Integrated Prostheses. Osseointegration in Clinical Dentistry. Plast. Reconstr. Surg. 1986, 77, 496–497. [Google Scholar] [CrossRef]
- Eriksson, R.A.; Albrektsson, T. The effect of heat on bone regeneration: An experimental study in the rabbit using the bone growth chamber. J. Oral Maxillofac. Surg. 1984, 42, 705–711. [Google Scholar] [CrossRef]
- Stocchero, M.; Jinno, Y.; Toia, M.; Ahmad, M.; Papia, E.; Yamaguchi, S.; Becktor, J.P. Intraosseous Temperature Change during Installation of Dental Implants with Two Different Surfaces and Different Drilling Protocols: An In Vivo Study in Sheep. J. Clin. Med. 2019, 8, 1198. [Google Scholar] [CrossRef] [PubMed]
- Stocchero, M.; Toia, M.; Jinno, Y.; Cecchinato, F.; Becktor, J.P.; Naito, Y.; Halldin, A.; Jimbo, R. Influence of different drilling preparation on cortical bone: A biomechanical, histological, and micro-CT study on sheep. Clin. Oral Implant. Res. 2018, 29, 707–715. [Google Scholar] [CrossRef]
- Homer, D.B. A self-powered low-speed surgical drill: Prevention of thermal necrosis. Am. J. Orthop. 1961, 3, 278–283. [Google Scholar]
- Thompson, H.C. Effect of drilling into bone. J. Oral Surg. 1958, 16, 22–30. [Google Scholar] [PubMed]
- Celles, C.A.S.; Ferreira, I.; Valente, M.d.L.d.C.; dos Reis, A.C. Osseointegration in relation to drilling speed in the preparation of dental implants sites: A systematic review. J. Prosthet. Dent. 2023. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ashequl, I. Temperature measurement methods in an experimental setup during bone drilling: A brief review on the comparison of thermocouple and infrared thermography. J. Phys. Conf. Ser. 2021, 2129, 012096. [Google Scholar]
- Tai, B.; Zhang, L.; Wang, A.; Sullivan, S.; Shih, A.J. Neurosurgical Bone Grinding Temperature Monitoring. Procedia Cirp 2013, 5, 226–230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).