The Biological Impact of Residual Aluminum Particles on Sand-Blasted Dental Implant Surfaces: A Systematic Review of Animal Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- In vivo experimental animal studies evaluating the biological effects of residual Al particles on Al2O3-blasted dental implant surfaces.
- Studies published in peer-reviewed journals.
- Articles available in English.
- Studies not involving dental implants.
- Reviews, editorials, and opinion pieces without original data.
- Studies not focusing on Al particles or residual Al contamination.
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Synthesis
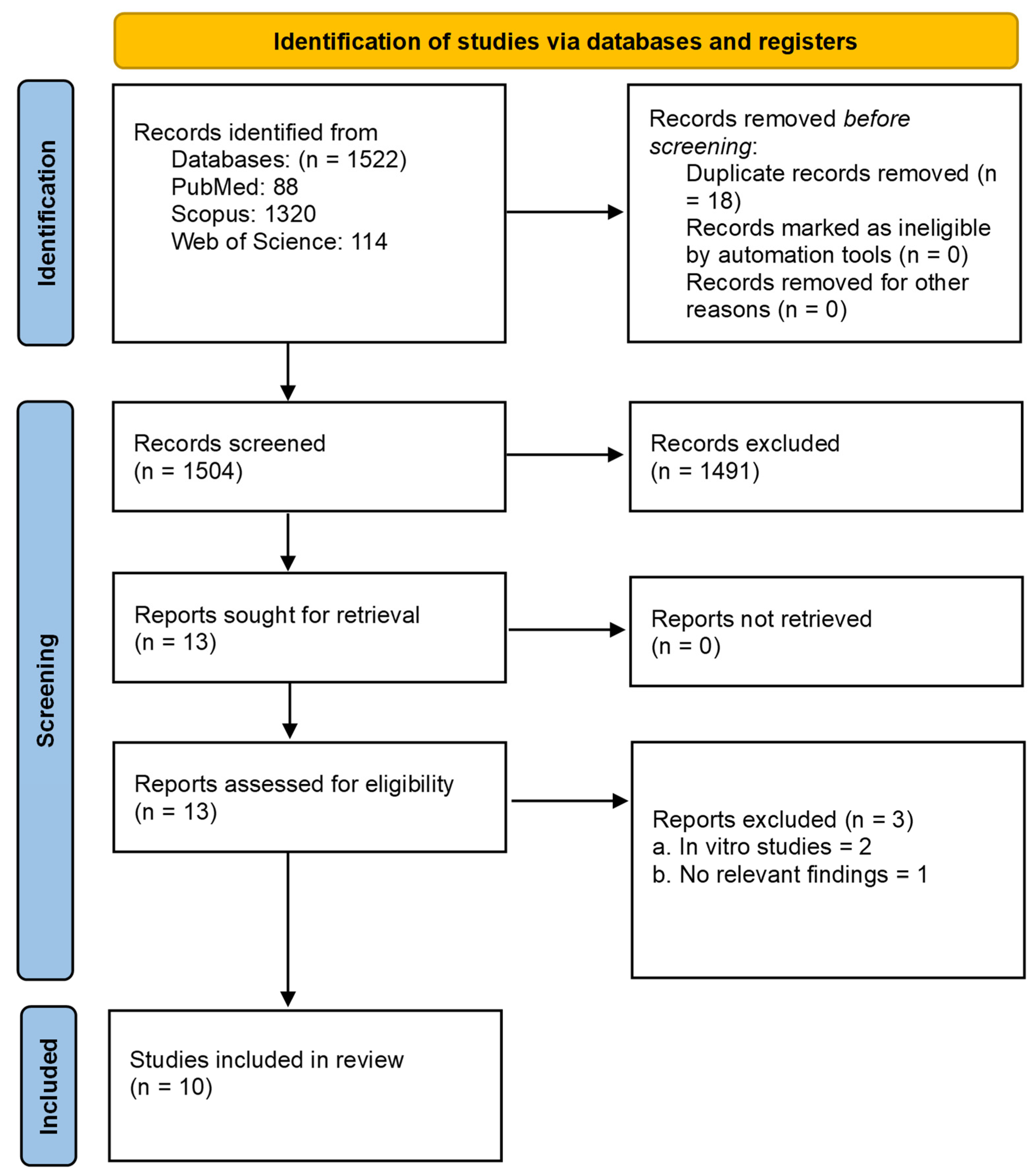
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium Toxicosis: A Review of Toxic Actions and Effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef]
- Renke, G.; Almeida, V.B.P.; Souza, E.A.; Lessa, S.; Teixeira, R.L.; Rocha, L.; Sousa, P.L.; Starling-Soares, B. Clinical Outcomes of the Deleterious Effects of Aluminum on Neuro-Cognition, Inflammation, and Health: A Review. Nutrients 2023, 15, 2221. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated Implants in the Treatment of the Edentulous Jaw. Experience from a 10-Year Period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.; Guida, L. The Effect of Titanium Surface Modifications on Dental Implant Osseointegration. In Frontiers of Oral Biology; Deb, S., Ed.; S. Karger AG: Basel, Switzerland, 2015; Volume 17, pp. 62–77. ISBN 978-3-318-02460-9. [Google Scholar]
- Sivaswamy, V.; Bahl, V. Surface Modifications of Commercial Dental Implant Systems: An Overview. J. Long-Term Eff. Med. Implant. 2023, 33, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel Surfaces and Osseointegration in Implant Dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Ortiz-Garcia, I.; Jiménez-Guerra, A.; Núñez-Márquez, E.; Moreno-Muñoz, J.; Rondón-Romero, J.L.; Cabanillas-Balsera, D.; Gil, J.; Muñoz-Guzón, F.; Monsalve-Guil, L. Osseointegration of Sandblasted and Acid-Etched Implant Surfaces. A Histological and Histomorphometric Study in the Rabbit. Int. J. Mol. Sci. 2021, 22, 8507. [Google Scholar] [CrossRef]
- Peck, M.T.; Chrcanovic, B.R. Chemical and Topographic Analysis of Eight Commercially Available Dental Implants. J. Contemp. Dent. Pract. 2016, 17, 354–360. [Google Scholar] [CrossRef]
- Aneksomboonpol, P.; Mahardawi, B.; Nan, P.N.; Laoharungpisit, P.; Kumchai, T.; Wongsirichat, N.; Aimjirakul, N. Surface Structure Characteristics of Dental Implants and Their Potential Changes Following Installation: A Literature Review. J. Korean Assoc. Oral Maxillofac. Surg. 2023, 49, 114–124. [Google Scholar] [CrossRef]
- Stea, S.; Savarino, L.; Toni, A.; Sudanese, A.; Giunti, A.; Pizzoferrato, A. Microradiographic and Histochemical Evaluation of Mineralization Inhibition at the Bone-Alumina Interface. Biomaterials 1992, 13, 664–667. [Google Scholar] [CrossRef]
- Thompson, G.J.; Puleo, D.A. Ti-6Al-4V Ion Solution Inhibition of Osteogenic Cell Phenotype as a Function of Differentiation Timecourse in Vitro. Biomaterials 1996, 17, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Bushinsky, D.A.; Sprague, S.M.; Hallegot, P.; Girod, C.; Chabala, J.M.; Levi-Setti, R. Effects of Aluminum on Bone Surface Ion Composition. J. Bone Miner. Res. 1995, 10, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Nimb, L.; Jensen, J.S.; Gotfredsen, K. Interface Mechanics and Histomorphometric Analysis of Hydroxyapatite-coated and Porous Glass-ceramic Implants in Canine Bone. J. Biomed. Mater. Res. 1995, 29, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Degidi, M.; Paolantonio, M.; Mangano, C.; Scarano, A. Residual Aluminum Oxide on the Surface of Titanium Implants Has No Effect on Osseointegration. Biomaterials 2003, 24, 4081–4089. [Google Scholar] [CrossRef]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human Health Risk Assessment for Aluminium, Aluminium Oxide, and Aluminium Hydroxide. J. Toxicol. Environ. Health Part B 2007, 10, 1–269. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Abdulla, M.A.; Hasan, R.H.; Al-Hyani, O.H. Radiographic and Histologic Assessment of Osseointegration for Surface-Treated Titanium Dental Implants: An Experimental Study in Dogs. J. Dent. Res. Dent. Clin. Dent. Prospect. 2024, 18, 44–54. [Google Scholar] [CrossRef]
- Gil, F.J.; Pérez, R.A.; Olmos, J.; Herraez-Galindo, C.; Gutierrez-Pérez, J.L.; Torres-Lagares, D. The Effect of Using Al2O3 and TiO2 in Sandblasting of Titanium Dental Implants. J. Mater. Res. 2022, 37, 2604–2613. [Google Scholar] [CrossRef]
- Gil, J.; Pérez, R.; Herrero-Climent, M.; Rizo-Gorrita, M.; Torres-Lagares, D.; Gutierrez, J.L. Benefits of Residual Aluminum Oxide for Sand Blasting Titanium Dental Implants: Osseointegration and Bactericidal Effects. Materials 2022, 15, 178. [Google Scholar] [CrossRef]
- Ríos-Carrasco, B.; Lemos, B.F.; Herrero-Climent, M.; Gil Mur, F.J.; Ríos-Santos, J.V. Effect of the Acid-Etching on Grit-Blasted Dental Implants to Improve Osseointegration: Histomorphometric Analysis of the Bone-Implant Contact in the Rabbit Tibia Model. Coatings 2021, 11, 1426. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Ramírez-Fernandez, M.P.; Granero Marín, J.M.; Barbosa Salles, M.; Del Fabbro, M.; Calvo Guirado, J.L. A Comparative Evaluation between Aluminium and Titanium Dioxide Microparticles for Blasting the Surface Titanium Dental Implants: An Experimental Study in Rabbits. Clin. Oral Implants Res. 2018, 29, 802–807. [Google Scholar] [CrossRef]
- Yurttutan, M.E.; Keskin, A. Evaluation of the Effects of Different Sand Particles That Used in Dental Implant Roughened for Osseointegration. BMC Oral Health 2018, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Rüger, M.; Gensior, T.J.; Herren, C.; Walter, M.V.; Ocklenburg, C.; Marx, R.; Erli, H.-J. The Removal of Al2O3 Particles from Grit-Blasted Titanium Implant Surfaces: Effects on Biocompatibility, Osseointegration and Interface Strength in Vivo. Acta Biomater. 2010, 6, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Müeller, W.-D.; Gross, U.; Fritz, T.; Voigt, C.; Fischer, P.; Berger, G.; Rogaschewski, S.; Lange, K.-P. Evaluation of the Interface between Bone and Titanium Surfaces Being Blasted by Aluminium Oxide or Bioceramic Particles. Clin. Oral Implants Res. 2003, 14, 349–356. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T.; Johansson, C.; Andersson, B. Experimental Study of Turned and Grit-Blasted Screw-Shaped Implants with Special Emphasis on Effects of Blasting Material and Surface Topography. Biomaterials 1996, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Hallgren, C.; Johansson, C.; Danelli, S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin. Oral Implants Res. 1998, 9, 11–19. [Google Scholar] [CrossRef]
- Hansson, S.; Norton, M. The relation between surface roughness and interfacial shear strength for bone-anchored implants. A mathematical model. J. Biomech. 1999, 32, 829–836. [Google Scholar] [CrossRef]
- Scarano, A.; Degidi, M.; Iezzi, G.; Petrone, G.; Piattelli, A. Correlation between implant stability quotient and bone-implant contact: A retrospective histological and histomorphometrical study of seven titanium implants retrieved from humans. Clin. Implant Dent. Relat. Res. 2006, 8, 218–222. [Google Scholar] [CrossRef]
- Degidi, M.; Perrotti, V.; Piattelli, A.; Iezzi, G. Mineralized bone-implant contact and implant stability quotient in 16 human implants retrieved after early healing periods: A histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implants 2010, 25, 45–48. [Google Scholar]
- Chambrone, L.; Shibli, J.A.; Mercúrio, C.E.; Cardoso, B.; Preshaw, P.M. Efficacy of Standard (SLA) and Modified Sandblasted and Acid-Etched (SLActive) Dental Implants in Promoting Immediate and/or Early Occlusal Loading Protocols: A Systematic Review of Prospective Studies. Clin. Oral Implants Res. 2015, 26, 359–370. [Google Scholar] [CrossRef]
- Karabuda, Z.C.; Abdel-Haq, J.; Arisan, V. Stability, Marginal Bone Loss and Survival of Standard and Modified Sand-Blasted, Acid-Etched Implants in Bilateral Edentulous Spaces: A Prospective 15-Month Evaluation. Clin. Oral Implants Res. 2011, 22, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Sayin Ozel, G.; Inan, O.; Secilmis Acar, A.; Alniacik Iyidogan, G.; Dolanmaz, D.; Yildirim, G. Stability of Dental Implants with Sandblasted and Acid-Etched (SLA) and Modified (SLActive) Surfaces during the Osseointegration Period. J. Dent. Res. Dent. Clin. Dent. Prospects 2021, 15, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Kaushik, M.; Wadhawan, A.; Agarwal, M.; Arun, A. Comparative Evaluation of Osseointegration between Sandblasted Large Grit, Acid Etched (SLA) and Calcium Phosphate Coated Implants. A Randomized Controlled Clinical Trial. J. Osseointegration 2022, 14, 112–121. [Google Scholar] [CrossRef]
- Duddeck, D.U.; Albrektsson, T.; Wennerberg, A.; Larsson, C.; Beuer, F. On the Cleanliness of Different Oral Implant Systems: A Pilot Study. J. Clin. Med. 2019, 8, 1280. [Google Scholar] [CrossRef] [PubMed]
- Schupbach, P.; Glauser, R.; Bauer, S. Al2O3 Particles on Titanium Dental Implant Systems Following Sandblasting and Acid-Etching Process. Int. J. Biomater. 2019, 2019, 6318429. [Google Scholar] [CrossRef]
| Database | Search String | Records |
|---|---|---|
| PubMed | (“Dental Implants” [MeSH Terms] OR “Dental Implants” [Title/Abstract] OR “Titanium Implants” [Title/Abstract]) AND (“Aluminum Particles” [Title/Abstract] OR “Alumina Oxide” [Title/Abstract]) AND (“Surface Properties” [MeSH Terms] OR “Surface Contamination” [Title/Abstract] OR “Surface Modification” [Title/Abstract]) AND (“Osseointegration” [MeSH Terms] OR Biocompatibility OR “Biological Effects” [Title/Abstract]) | 88 |
| Scopus | (TITLE-ABS-KEY (“Dental Implants”) OR TITLE-ABS-KEY (“Titanium Implants”)) AND (TITLE-ABS-KEY (“Aluminum Particles”) OR TITLE-ABS-KEY (“Alumina Oxide”)) AND (TITLE-ABS-KEY (“Surface Properties”) OR TITLE-ABS-KEY (“Surface Contamination”) OR TITLE-ABS-KEY (“Surface Modification”)) AND (TITLE-ABS-KEY (“Osseointegration”) OR TITLE-ABS-KEY (“Biocompatibility”) OR TITLE-ABS-KEY (“Biological Effects”) | 1320 |
| Web of Science | (TS = (“Dental Implants” OR “Titanium Implants”)) AND (TS = (“Aluminum” OR “Aluminum Particles” OR “Alumina Oxide”)) AND (TS = (“Surface Properties” OR “Surface Contamination” OR “Surface Modification”)) AND (TS = (“Osseointegration” OR “Biocompatibility” OR “Biological Effects” OR “)) | 114 |
| Study Author | Study Year | Study Type (Animal Model) | Number and Features of Animal Used | Dental Implant Number and Type; Location of Placement | Groups | SLA Technique Used | Residual Al | Biological Effects | Study Findings | Comments/Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdulla et al. [18] | 2024 | Experimental (Dogs) | 24 healthy, mature adult, local breed male dogs, aged 1–1.5 years and weighing 22 ± 3 kg | 72 commercially pure, standard screw-type Titanium dental implants; mandible | G1: SLA surface G2: 50 µm Al2O3-blasted G3: Laser-treated G4: Propolis-coated | Air-abraded with 50 μm Al2O3 particles for 15 s at 0.6 MPa, 6 bars of pressure | Present | Good bone response with sufficient new bone development along the implant surface. | Uniform and ongoing pattern of bone growth and many osteoblasts, with few osteocytes within lacunae in new bone trabeculae. | Al2O3 showed no negative effect on osseointegration. |
| Gil et al. [19] | 2022 | Experimental (Minipig) | 20 twelve-year-old female minipigs | 240 commercially pure grade 3 titanium dental implants (3.8 mm-wide, 12 mm-long); mandible and maxilla | G1: As-received (CTR) G2: TiO2-blasted G3: Al2O3-blasted | G2–G3: Grit-blasted with 600-μm size particles and 0.25 MPa blast pressure. All samples AE with HCl/H2SO4 for 15 s | Present | The presence of residual blasting of Al2O3 and TiO2 did not affect the osseointegration of titanium dental implants. | Osseointegration of Al2O3-blasted implants is higher than the control and TiO2-blasted implants, with BIC values twice as high (63% vs. 38%) at 6 weeks follow-up. | Blasting with Al2O3 favors the osseointegration of titanium dental implants compared with TiO2. |
| Gil et al. [20] | 2022 | Experimental (Minipig) | 8 twelve-year-old female minipigs | 110 cylindrical, commercially pure grade 3 titanium dental implants (3.8 mm-wide, 12 mm-long); mandible | G1: No blast G2: Al2O3-blasted G3: Al2O3-blasted and special cleaning | Shot-blasted with Al2O3 particles with a size range of 212–300 mm at a pressure of 2.5 MPa until saturation | Present and removed | Residual Al accelerated bone growth and reduced bacterial adhesion. | In vivo studies demonstrated the beneficial effects of residual Al on bone growth and bactericidal properties | Presence of residual blasting of Al2O3 favors the osseointegration of titanium dental implants. |
| Ríos-Carrasco et al. [21] | 2021 | Experimental (Rabbit) | 6 New Zealand white rabbits of 6 months of age weighing 3 to 4 kg | 24 commercially pure titanium grade 4 dental implants, 4.0 mm-wide × 8.0 mm-long and a neck section of 1.5 mm in height; tibia | G1: Al2O3-blasted G2: Al2O3-blasted, and acid-etched | Shot-blasted with Al2O3 particles of 425 to 600 µm | Present | No negative impact on osseointegration. | The bone-to-implant contact ratio (BIC%) showed a similar tendency, with 55.18 ± 15.67 and 59.9 ± 13.15 for SB and SB + AE implants. | Both surfaces of implants studied showed high osseointegration. |
| Gehrke et al. [22] | 2018 | Experimental (Rabbit) | 8 New Zealand white adult rabbits weighing approximately 4 kg | 48 cylindrical titanium dental implants; tibia | G1: Al2O3 microparticles G2: TiO2 microparticles | Sandblasting with Al2O3 and TiO2 | Present | No significant differences in osseointegration between Al2O3 and TiO2. | Histological analysis showed a complete bone organization and mineralization at 8 weeks in both groups. The BIC% did not show statistical differences. | Both Al2O3 and TiO2 can be used effectively for surface blasting. |
| Yurttutan et al. [23] | 2018 | Experimental (Sheep) | 4 two-year-old female sheep, weighing 45 kg | 64 cylindrical titanium dental implants, 4.0 mm-wide and 10 mm-long; tibia | G1: Al2O3-blasted G2: TiO2-blasted G3: SiO2-blasted G4: Machined | Sand-blasted with Al2O3 particles of 180 to 200 µm | Present | Better osseointegration with implants blasted with Al2O3 particles. | Although there were no statistically significant differences between the groups, the implants sandblasted with Al2O3 showed a higher Implant Stability Quotient (ISQ) and removal torque value at the end of the 1st and 3rd months. | The results demonstrate that Al2O3 is superior to other sand particles. |
| Rüger et al. [24] | 2010 | Experimental (Rabbit) | 38 adult female New Zealand white rabbits | Cylindrical implants of either commercial pure titanium (ISO5832-2) or Ti6Al7Nb (ISO5832-11), 10 mm, the outer diameter 5 mm, and the inner diameter 2 mm, with a threaded bore; distal femoral metaphysis | G1: 20 µm Al2O3 grit blasting G2: Al2O3 free (acid-etched) | Grit-blasting with 20 µm Al2O3, followed by a special cleaning procedure rinse in ammonium bifluoride and nitric acid (<1 min each) | Present and removed | Improved biocompatibility and increased bone–implant contact, and lower shear resistance. | Al2O3-free implants exhibited increased bone–implant contact but lower shear resistance. | Indicates that removing residual Al particles may enhance bone–implant contact. |
| Piattelli et al. [15] | 2003 | Experimental (Rabbit) | 12 New Zealand white mature male rabbits | 24 threaded screw-shaped, machined grade 3 commercially pure titanium dental implants; tibia | G1: Al2O3-blasted G2: Saline-treated | Sandblasted with 100–120 µm Al2O3 particles at a 5 atm pressure for 1 min | Present | Newly formed bone was found in contact with the implant surface. Bone trabeculae were in close contact with the implant surface. Newly formed blood vessels were observed. Some osteoblasts were actively secreting osteoid matrix directly on the implant surface. | Found no statistically significant differences in bone–implant contact, number of multinucleated cells, and osteoclasts between groups. | Residual Al oxide particles on implant surface do not affect osseointegration. |
| Mueller et al. [25] | 2003 | Experimental (Rabbit) | 27 female adult Chinchilla rabbits, strain CH bb-CH, body weight 3–4 kg | 63 commercially pure titanium cylinders (8 mm in length and 4 mm in diameter); distal femur epiphysis | G1: Untreated G2: 110 µm Al2O3-blasted G3: 50 µm bio-ceramic-blasted | Blasted with Al2O3 particles (carborundum) with a particle size of 110 mm | Present | No untoward or negative effects from Al ions found on implant surfaces. | A dense ring of bone was developed, similar to cortical bone regarding density and development of Haversian canals with all surfaces after 84 days. | The MBC showed a tendency for more bone after bio-ceramics were used as a blasting material, compared to Al2O3. |
| Wennerberg et al. [26] | 1996 | Experimental (Rabbit) | 10 adult (9–11 months old) male New Zealand white rabbits | 60 screw-shaped, commercially pure titanium implants of length 6mm, diameter 3.75 mm, and pitch height 0.6 mm; tibia | G1: Machined surface G2: TiO2-blasted G3: 25 µm Al2O3-blasted G4: 75 µm Al2O3-blasted | Sandblasting with 25 µm and 75 µm Al2O3 particles | Present | No untoward or negative effects from Al ions found on implant surfaces. | Comparing implants blasted with 75 µm Al2O3 particles to as-machined implants, the blasted specimens exhibited a statistically significant higher bone-to-metal contact after 12 weeks in the rabbit bone. | Implants blasted with 75 µm Al2O3 particles showed better osseointegration than 25 µm particles. |
| Study Author(s) | Year | Sequence Generation (Selection Bias) | Baseline Characteristics (Selection Bias) | Allocation Concealment (Selection Bias) | Random Housing (Performance Bias) | Blinding (Performance Bias) | Random Outcome Assessment (Detection Bias) | Blinding (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Outcome Reporting (Reporting Bias) | Other Sources of Bias (Other) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdulla et al. [18] | 2024 | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Gil et al. [19] | 2022 | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Gil et al. [20] | 2022 | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Ríos-Carrasco et al. [21] | 2021 | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Gehrke et al. [22] | 2018 | Low Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Yurttutan et al. [23] | 2018 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk |
| Rüger et al. [24] | 2010 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Piattelli et al. [15] | 2003 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Mueller et al. [25] | 2003 | Low Risk | Low Risk | Unclear Risk | Unclear Risk | Unclear Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Wennerberg et al. [26] | 1996 | Low Risk | Low Risk | Unclear Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, S.; Tumedei, M.; Panda, S.; Goker, F.; Depalma, C.M.; Pande, T.; Del Fabbro, M. The Biological Impact of Residual Aluminum Particles on Sand-Blasted Dental Implant Surfaces: A Systematic Review of Animal Studies. Appl. Sci. 2024, 14, 7745. https://doi.org/10.3390/app14177745
Panda S, Tumedei M, Panda S, Goker F, Depalma CM, Pande T, Del Fabbro M. The Biological Impact of Residual Aluminum Particles on Sand-Blasted Dental Implant Surfaces: A Systematic Review of Animal Studies. Applied Sciences. 2024; 14(17):7745. https://doi.org/10.3390/app14177745
Chicago/Turabian StylePanda, Sourav, Margherita Tumedei, Sital Panda, Funda Goker, Cristina Maria Depalma, Tejas Pande, and Massimo Del Fabbro. 2024. "The Biological Impact of Residual Aluminum Particles on Sand-Blasted Dental Implant Surfaces: A Systematic Review of Animal Studies" Applied Sciences 14, no. 17: 7745. https://doi.org/10.3390/app14177745
APA StylePanda, S., Tumedei, M., Panda, S., Goker, F., Depalma, C. M., Pande, T., & Del Fabbro, M. (2024). The Biological Impact of Residual Aluminum Particles on Sand-Blasted Dental Implant Surfaces: A Systematic Review of Animal Studies. Applied Sciences, 14(17), 7745. https://doi.org/10.3390/app14177745








